

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM597927 (US11608347, Example 55 | [(7R,9aR)-7-(3-chloro-4- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In one embodiment, the present invention provides compounds of formula (Ie) and their pharmaceutically acceptable salts as described herein, wherein ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HX1HKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496984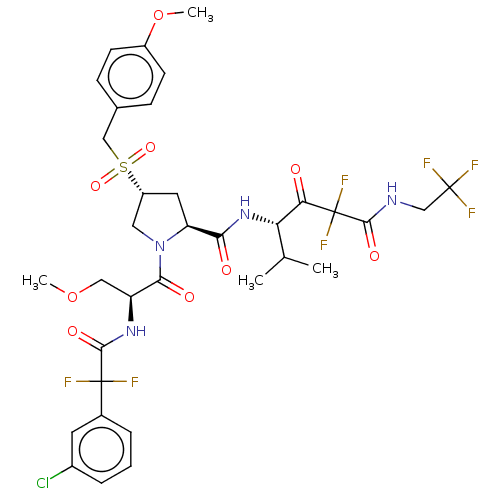 (US11001555, Example 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.262 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM475923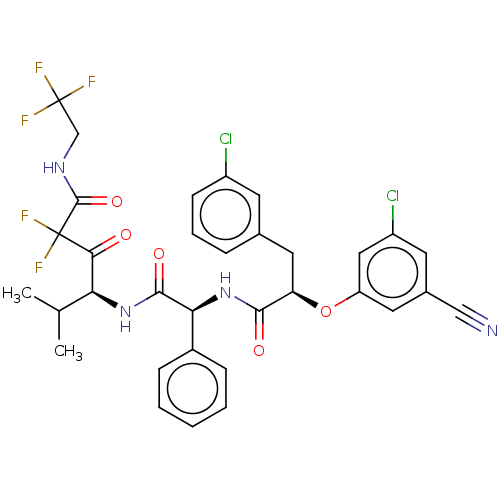 ((4S)-4-[[(2S)-2-[[(2R)-2- (3-chloro-5- cyanophenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.293 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10865182 (2020) BindingDB Entry DOI: 10.7270/Q2MC9330 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514094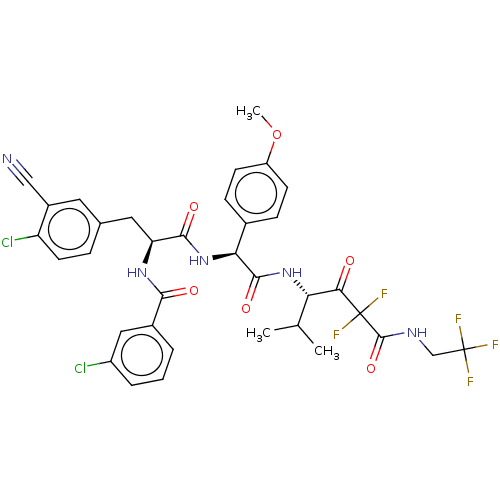 (US11059858, Example 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514076 (US11059858, Example 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514085 (US11059858, Example 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496983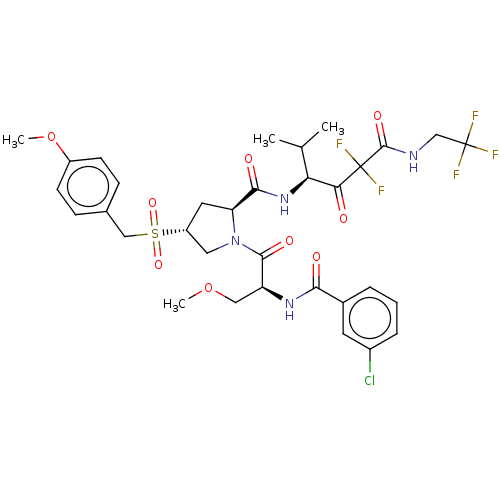 (US11001555, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.351 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514104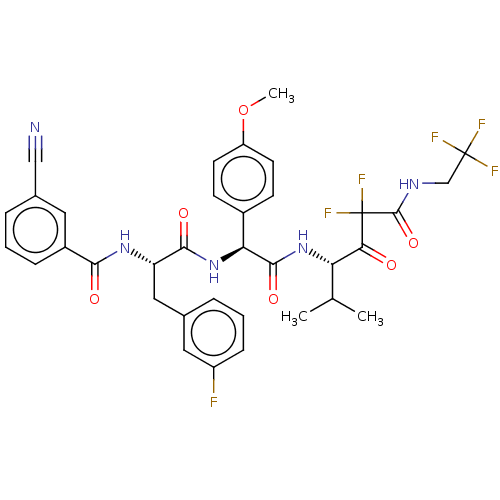 (US11059858, Example 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514098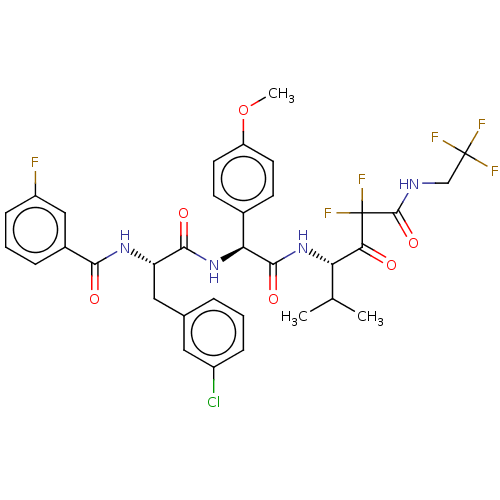 (N-[(2S)-3-(3-Chlorophenyl)-1-[[(1S)-2-[[(3S)-5,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514090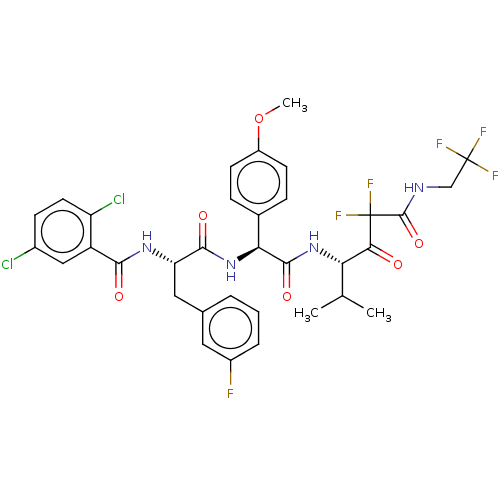 (US11059858, Example 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514091 (US11059858, Example 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514056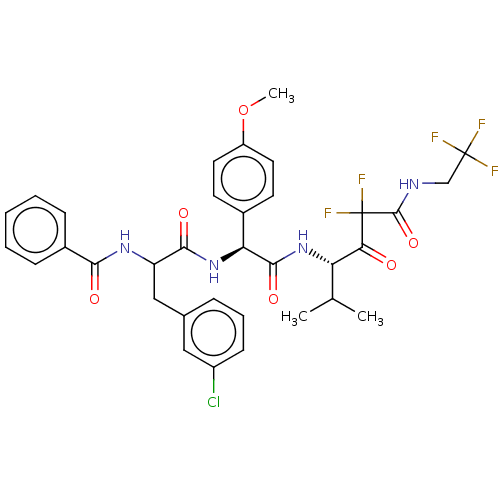 (US11059858, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499311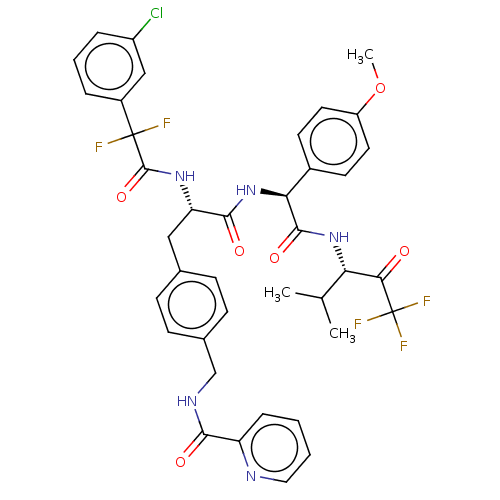 (US11014963, Example 216) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50569654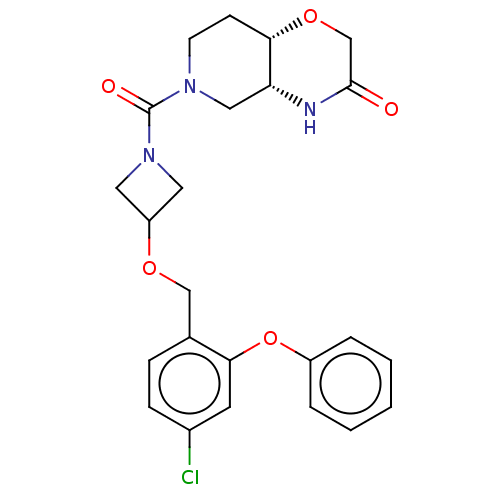 (CHEMBL4875383 | US11802133, Example 239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM475927 ((4S)-4-[[(2S)-2-[[(2R)-2- (3-chlorophenoxy)-3-(3- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.461 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10865182 (2020) BindingDB Entry DOI: 10.7270/Q2MC9330 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496981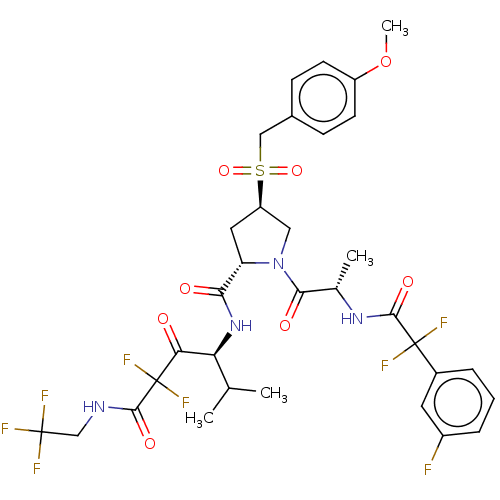 (US11001555, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.471 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499107 (US11014963, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514086 (US11059858, Example 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50541792 (CHEMBL4635011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay | J Med Chem 63: 6876-6897 (2020) Article DOI: 10.1021/acs.jmedchem.0c00233 BindingDB Entry DOI: 10.7270/Q2H998RZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514058 (US11059858, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514075 (US11059858, Example 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514061 (US11059858, Example 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514057 (US11059858, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514077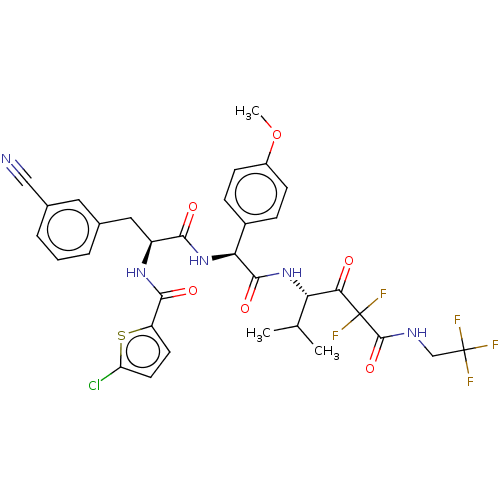 (US11059858, Example 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514078 (US11059858, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514102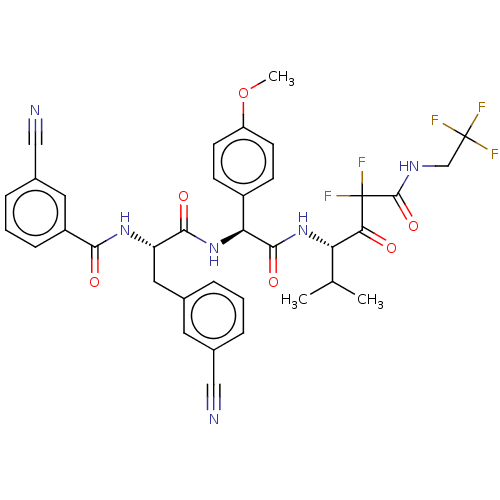 (US11059858, Example 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499266 (US11014963, Example 166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514079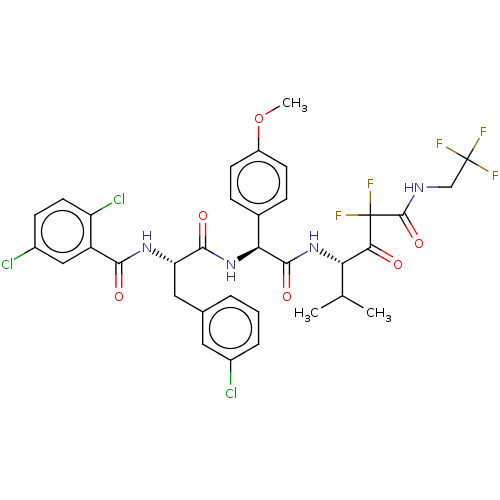 (US11059858, Example 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496990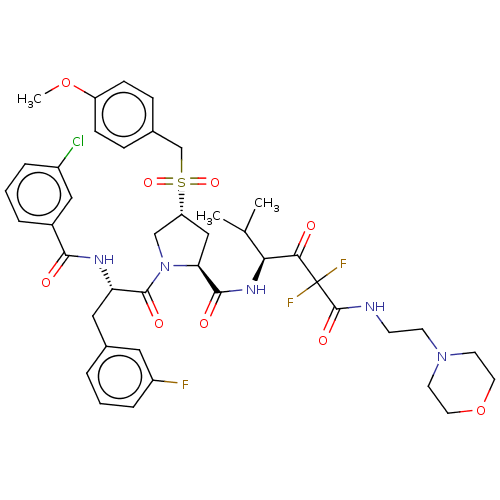 (US11001555, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.538 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM476636 (US10870623, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10870623 (2020) BindingDB Entry DOI: 10.7270/Q2CN7704 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496997 (N-[(2S)-3-(3,4-Dichlorophenyl)-1-[(2S,4R)-2-[[(3S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.574 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496982 (US11001555, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.579 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514063 (US11059858, Example 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514084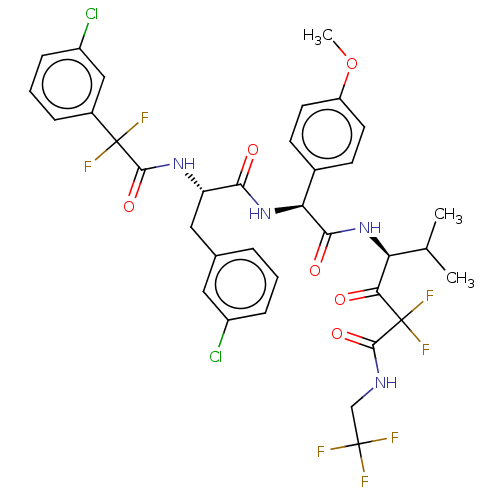 ((4S)-4-[[(2S)-2-[[(2S)-3-(3-Chlorophenyl)-2-[[2-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514099 (3,5-Dichloro-N-[(2S)-3-(3-chlorophenyl)-1-[[(1S)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514101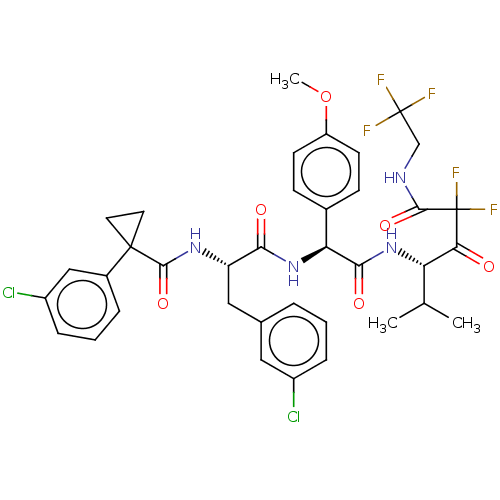 (US11059858, Example 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514103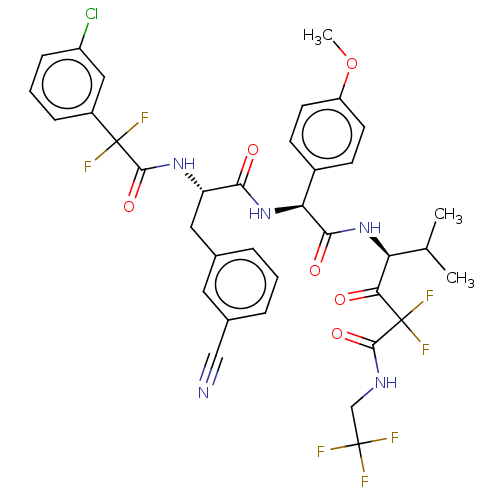 (US11059858, Example 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499267 (US11014963, Example 170) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514059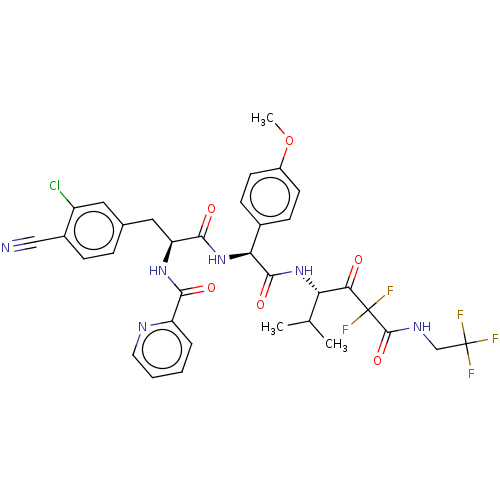 (US11059858, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499264 (US11014963, Example 163) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.602 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499095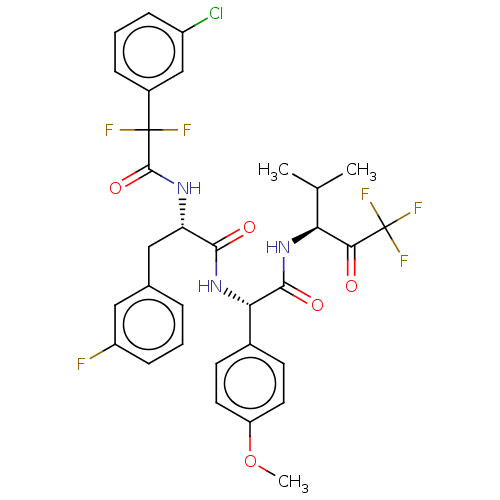 (US11014963, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499097 (US11014963, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496994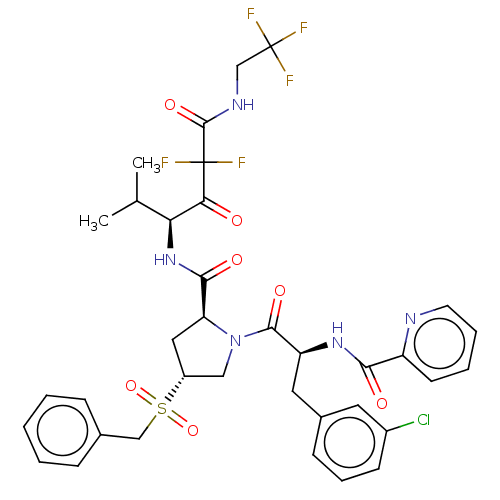 (US11001555, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.667 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM475926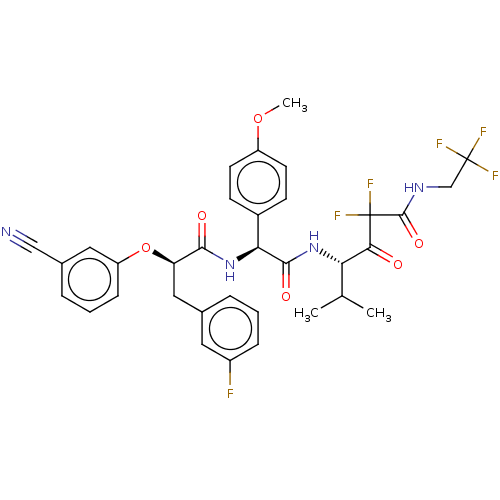 ((4S)-4-[[(2S)-2-[[(2R)-2- (3-cyanophenoxy)-3-(3- f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.686 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10865182 (2020) BindingDB Entry DOI: 10.7270/Q2MC9330 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM496988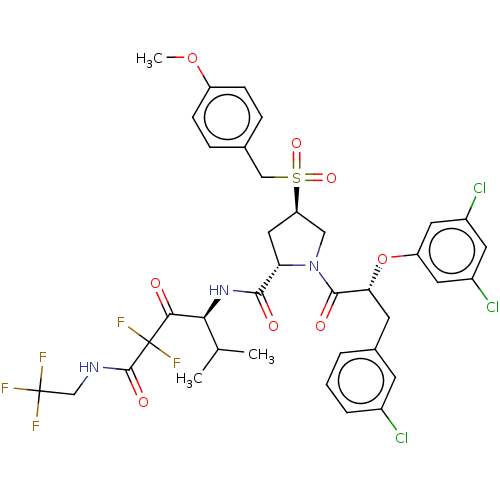 (US11001555, Example 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.694 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11001555 (2021) BindingDB Entry DOI: 10.7270/Q2G44TD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499222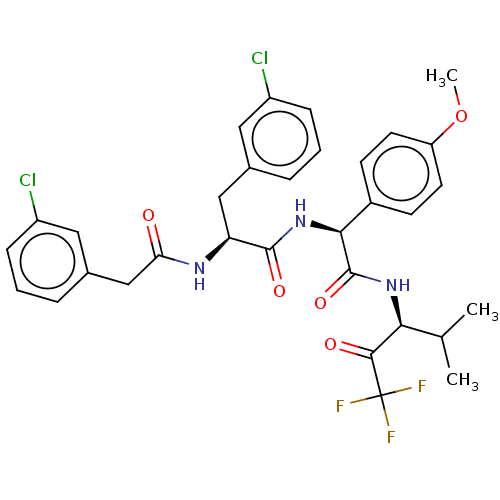 ((2S)-3-(3-Chlorophenyl)-2-[[2-(3-chlorophenyl)acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.695 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM514093 (US11059858, Example 47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499291 ((2S)-2-[[2-(2,5-Dichlorophenyl)-2,2-difluoroacetyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50569652 (CHEMBL4874643 | US11802133, Example 221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM499179 (US11014963, Example 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US11014963 (2021) BindingDB Entry DOI: 10.7270/Q22R3VSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2225 total ) | Next | Last >> |