

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433027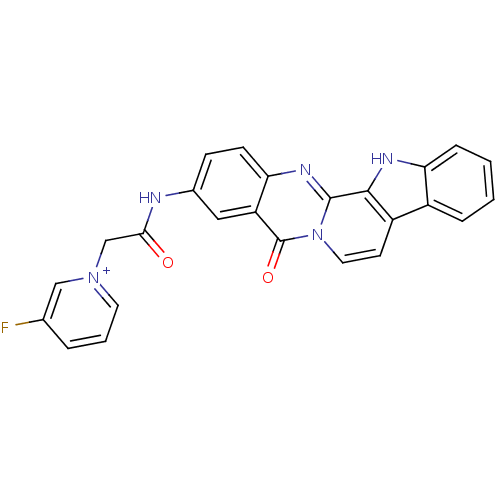 (CHEMBL2375941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433028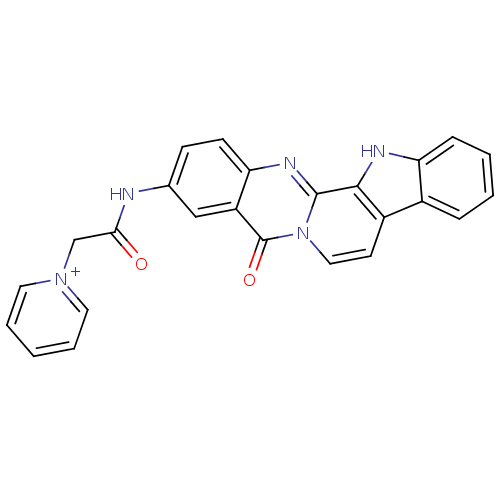 (CHEMBL2375940) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433018 (CHEMBL2375923) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433019 (CHEMBL2375922) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433022 (CHEMBL2375919) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433005 (CHEMBL2375936) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433026 (CHEMBL2375942) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433017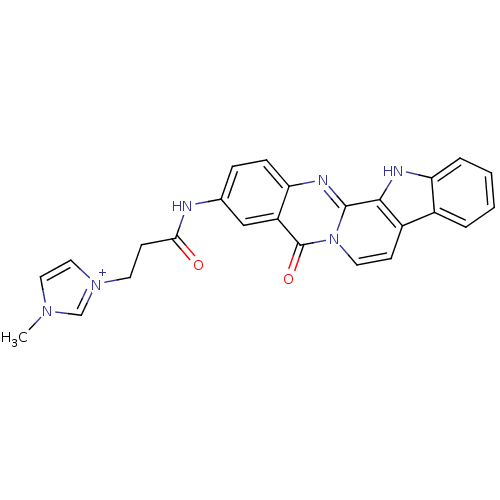 (CHEMBL2375924) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433004 (CHEMBL2375937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433010 (CHEMBL2375931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433014 (CHEMBL2375927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433013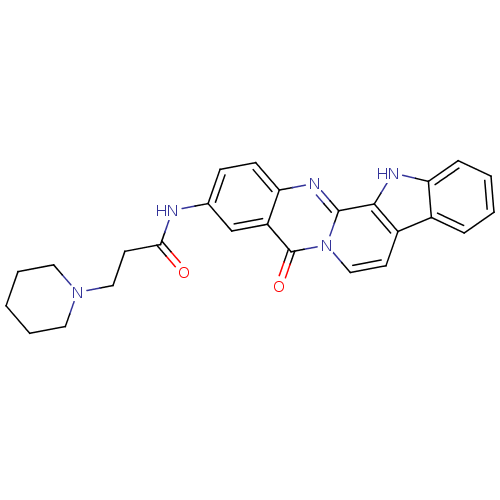 (CHEMBL2375928) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433016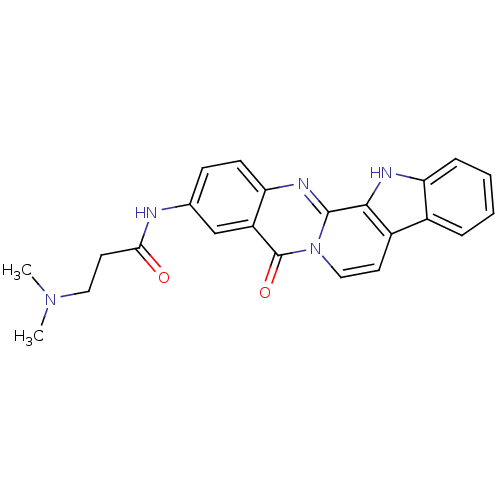 (CHEMBL2375925) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316368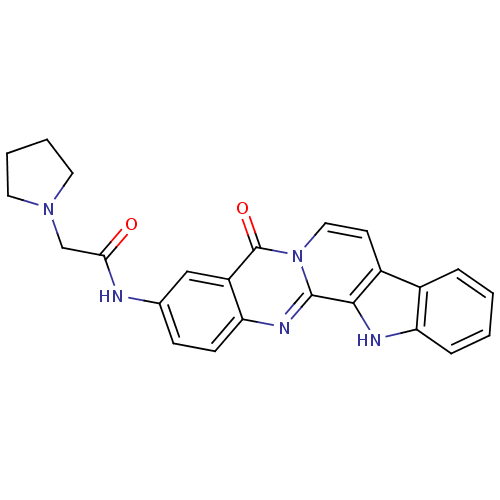 (3-(2-N-Pyrrolyl-acetamino)-7,8-dehydrorutaecarpine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433015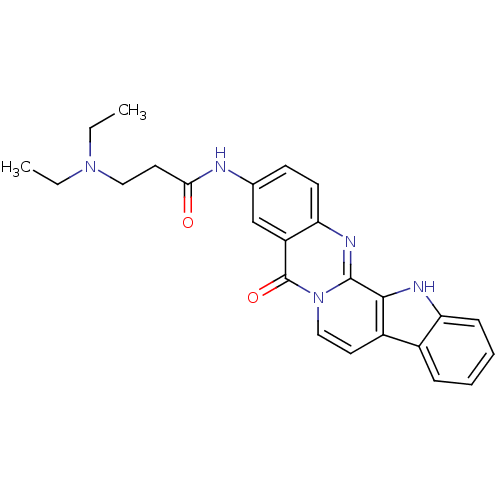 (CHEMBL2375926) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433025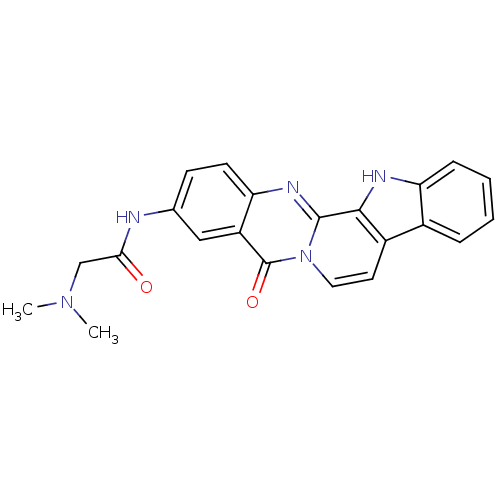 (CHEMBL2375943) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316367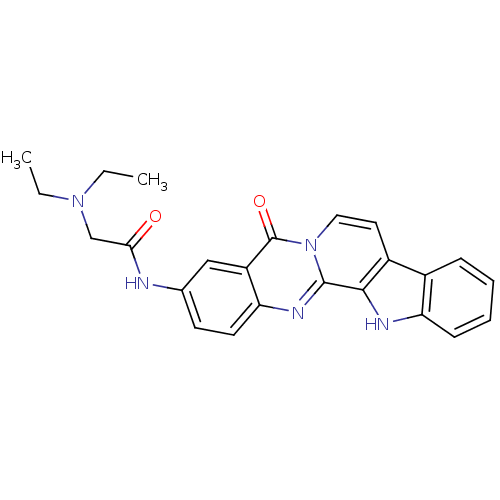 (3-(2-Diethylamino-acetamino)-7,8-dehydrorutaecarpi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316369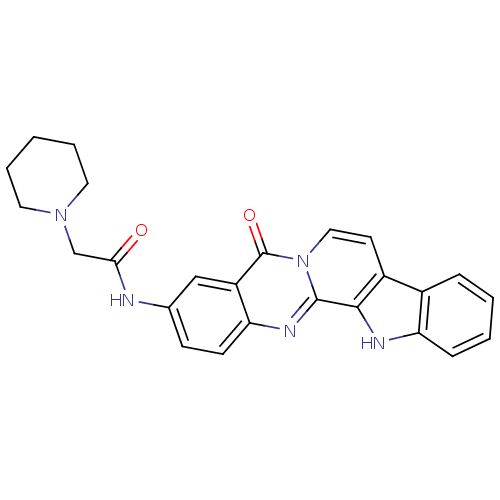 (3-(2-N-Piperidyl-acetamino)-7,8-dehydrorutaecarpin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433021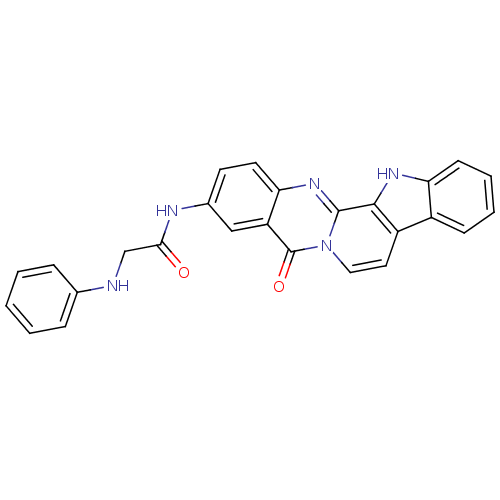 (CHEMBL2375920) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433012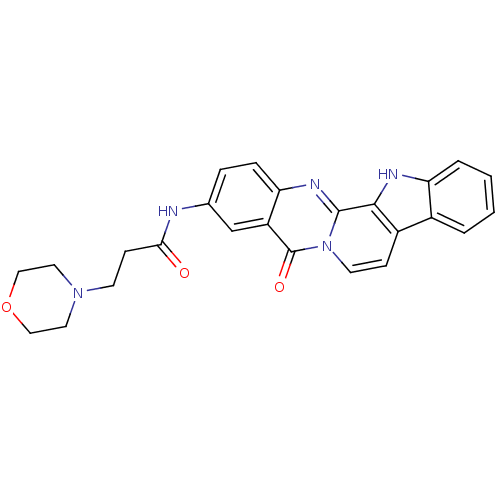 (CHEMBL2375929) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433003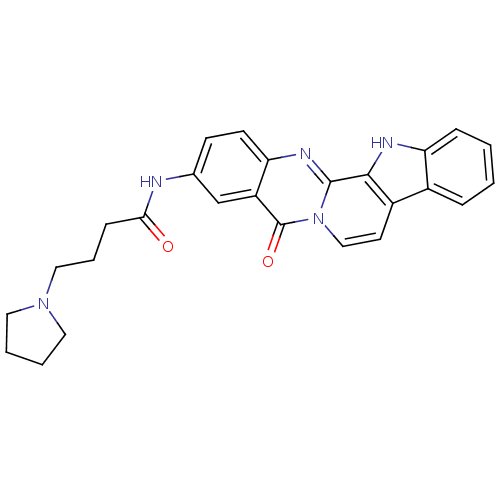 (CHEMBL2375938) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433009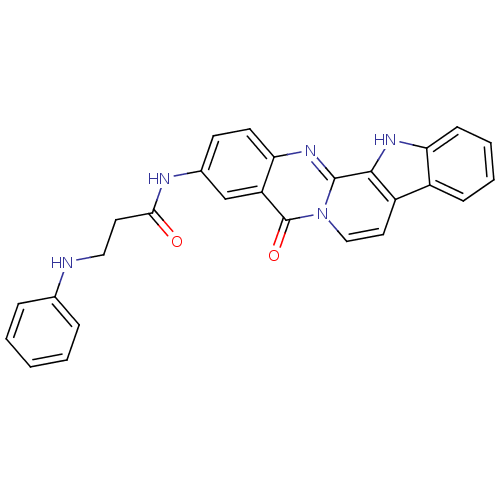 (CHEMBL2375932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433024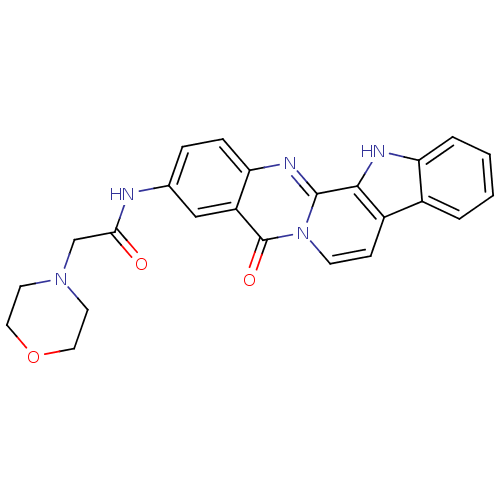 (CHEMBL2375944) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
China-Japan Union Hospital of Jilin University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 by ELISA | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127173 BindingDB Entry DOI: 10.7270/Q2D79FZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433008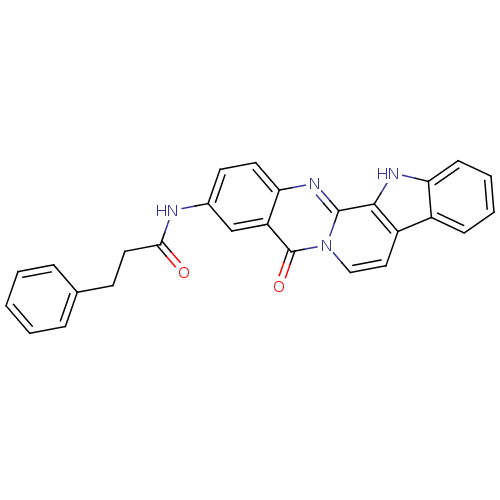 (CHEMBL2375933) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433020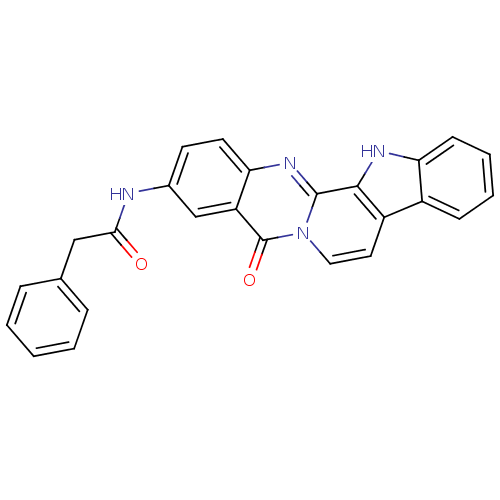 (CHEMBL2375921) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433002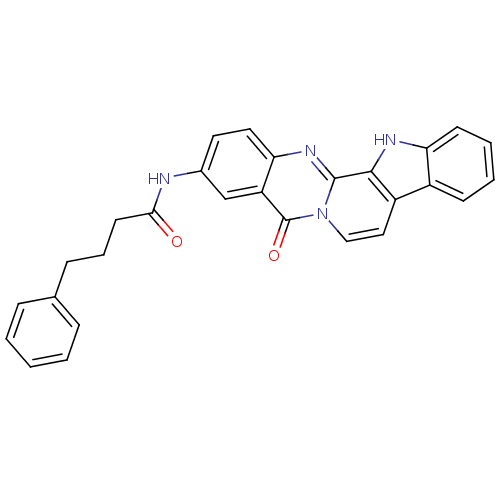 (CHEMBL2375939) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433006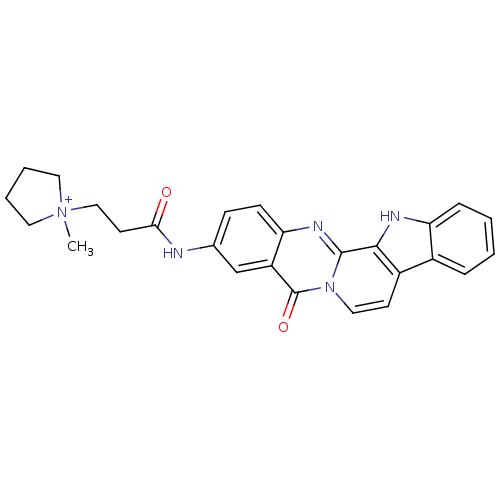 (CHEMBL2375935) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433011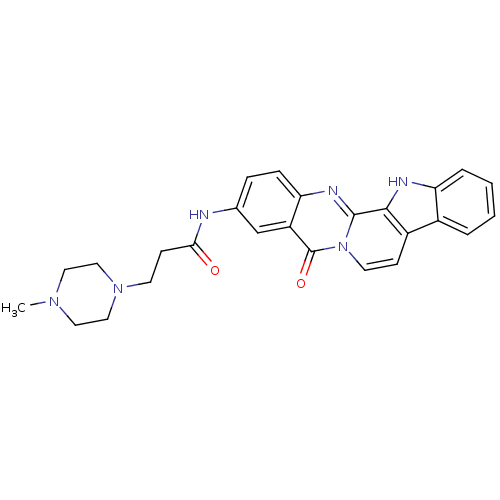 (CHEMBL2375930) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433023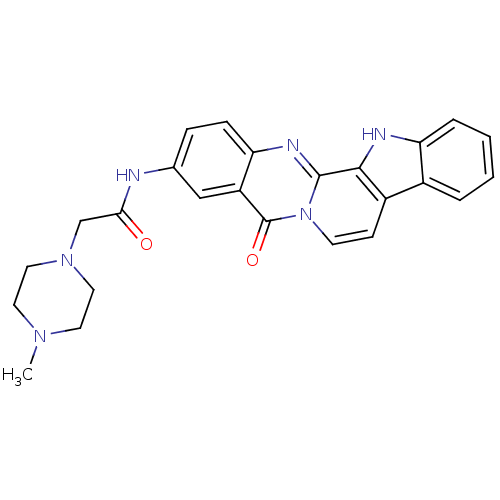 (CHEMBL2375945) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50542436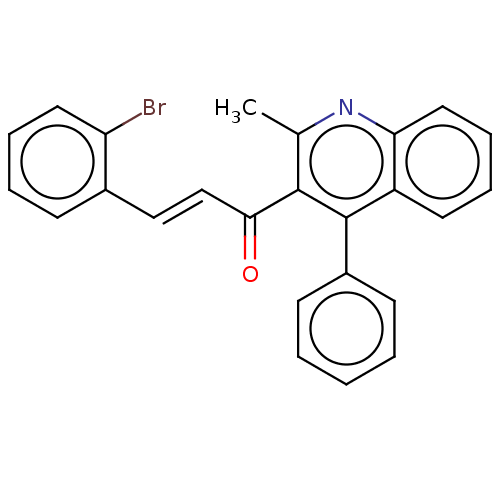 (CHEMBL4644802) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
China-Japan Union Hospital of Jilin University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 by ELISA | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127173 BindingDB Entry DOI: 10.7270/Q2D79FZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433007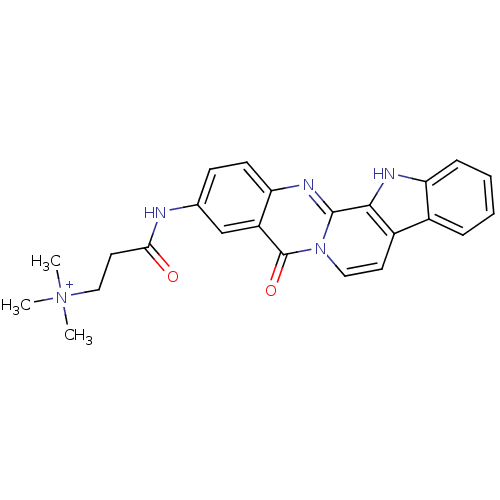 (CHEMBL2375934) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins followed by substrate addition measured fo... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50542435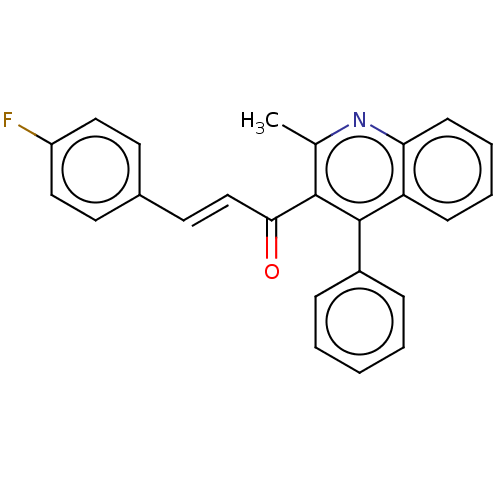 (CHEMBL4642218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
China-Japan Union Hospital of Jilin University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 by ELISA | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127173 BindingDB Entry DOI: 10.7270/Q2D79FZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50542437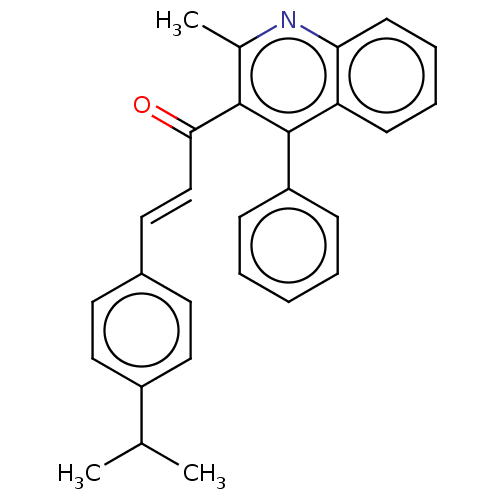 (CHEMBL4641093) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
China-Japan Union Hospital of Jilin University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 by ELISA | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127173 BindingDB Entry DOI: 10.7270/Q2D79FZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493329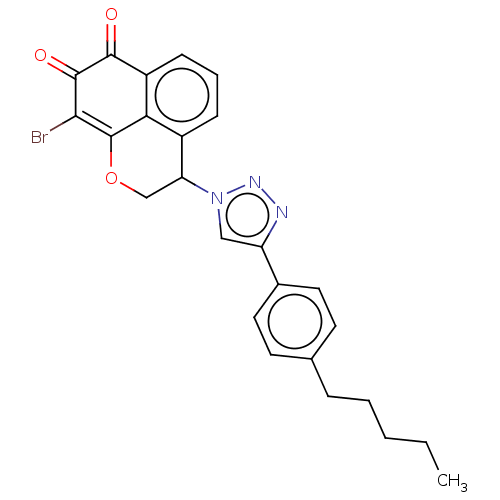 (CHEMBL2424901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2alpha using pBR322 DNA after 30 mins by agarose gel electrophoresis | Eur J Med Chem 68: 58-71 (2013) Article DOI: 10.1016/j.ejmech.2013.07.011 BindingDB Entry DOI: 10.7270/Q21Z47CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM7781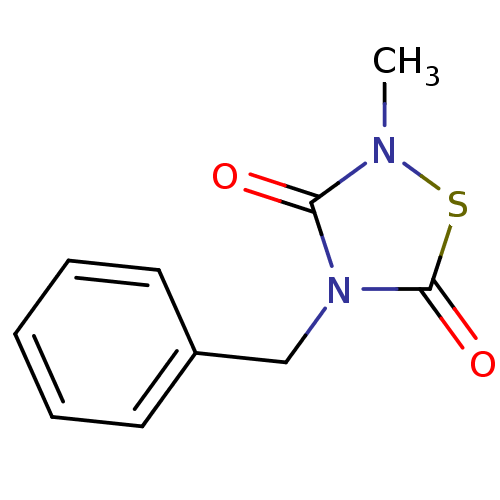 (4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of GSK3beta by kinase-glo assay method | Bioorg Med Chem Lett 22: 7232-6 (2012) Article DOI: 10.1016/j.bmcl.2012.09.043 BindingDB Entry DOI: 10.7270/Q2KD2034 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM7781 (4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using 650HSSPHQ(pS)EDEEE as substrate after 30 mins by luminescence assay | Eur J Med Chem 61: 95-103 (2013) Article DOI: 10.1016/j.ejmech.2012.09.021 BindingDB Entry DOI: 10.7270/Q24J0GF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50349933 (CHEMBL1812144) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2 alpha-mediated relaxation of supercoiled pBR322 DNA after 30 mins by agarose gel electrophoresis | Eur J Med Chem 46: 3339-47 (2011) Article DOI: 10.1016/j.ejmech.2011.04.059 BindingDB Entry DOI: 10.7270/Q2513ZJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433027 (CHEMBL2375941) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50349934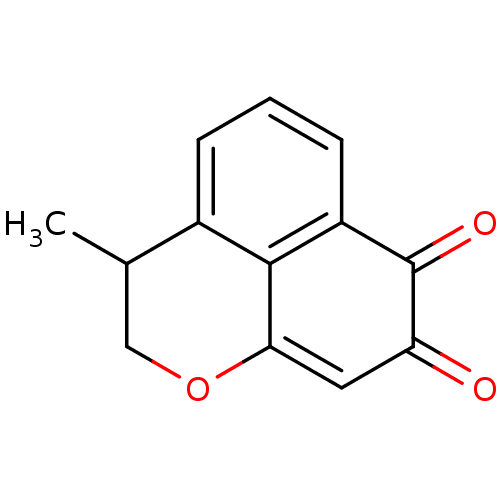 (CHEMBL1812139) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2 alpha-mediated relaxation of supercoiled pBR322 DNA after 30 mins by agarose gel electrophoresis | Eur J Med Chem 46: 3339-47 (2011) Article DOI: 10.1016/j.ejmech.2011.04.059 BindingDB Entry DOI: 10.7270/Q2513ZJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433028 (CHEMBL2375940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433018 (CHEMBL2375923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433005 (CHEMBL2375936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433019 (CHEMBL2375922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50401965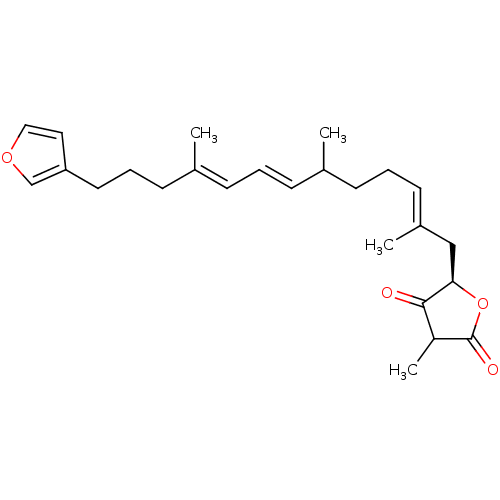 (PALINURIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) | Eur J Med Chem 61: 95-103 (2013) Article DOI: 10.1016/j.ejmech.2012.09.021 BindingDB Entry DOI: 10.7270/Q24J0GF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50401965 (PALINURIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Non-ATP competitive inhibition of GSK3beta | Bioorg Med Chem Lett 22: 7232-6 (2012) Article DOI: 10.1016/j.bmcl.2012.09.043 BindingDB Entry DOI: 10.7270/Q2KD2034 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50349932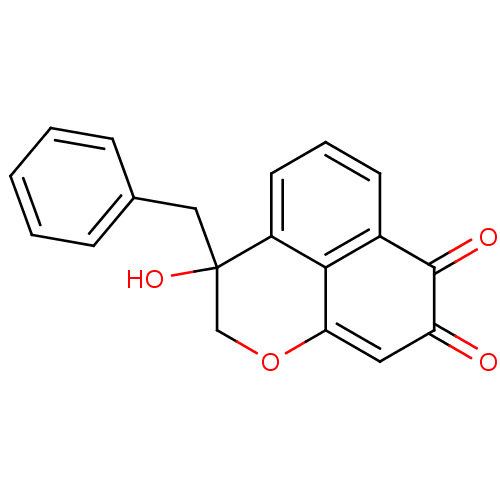 (CHEMBL1812137) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2 alpha-mediated relaxation of supercoiled pBR322 DNA after 30 mins by agarose gel electrophoresis | Eur J Med Chem 46: 3339-47 (2011) Article DOI: 10.1016/j.ejmech.2011.04.059 BindingDB Entry DOI: 10.7270/Q2513ZJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433010 (CHEMBL2375931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butylthiocholine as substrate incubated for 15 mins followed by substrate addition measured for... | Eur J Med Chem 63: 299-312 (2013) Article DOI: 10.1016/j.ejmech.2013.02.014 BindingDB Entry DOI: 10.7270/Q2SQ91RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 121 total ) | Next | Last >> |