

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085483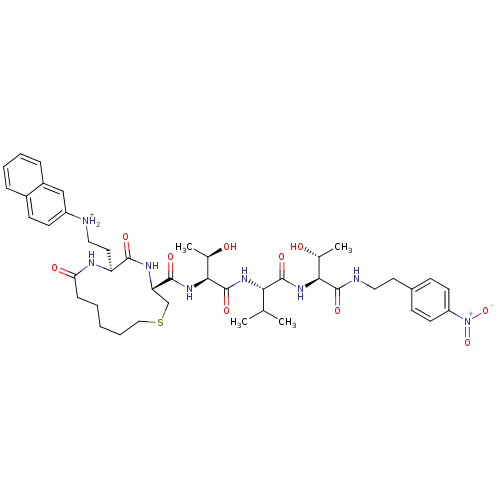 ((2-{(3R,6S)-3-[(1S,2R)-2-Hydroxy-1-((S)-1-{(1S,2R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085481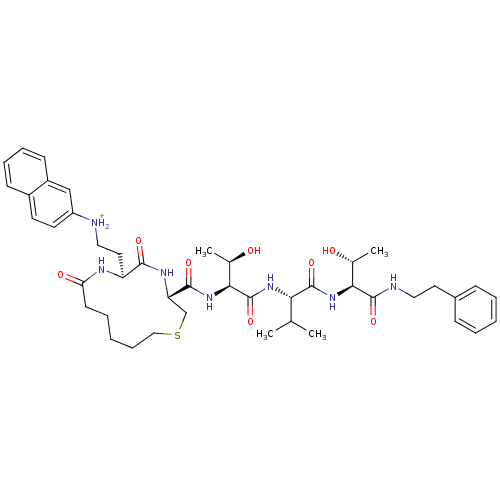 (CID44328462 | [2-((3R,6S)-3-{(1S,2R)-2-Hydroxy-1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085488 (2-{(3R,6S)-3-[(1S,2R)-2-Hydroxy-1-((S)-1-{(1S,2R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085489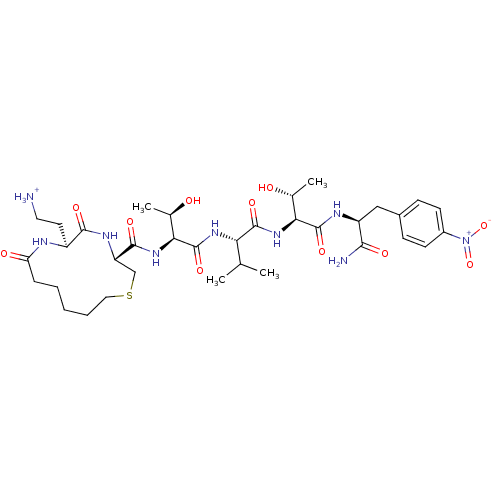 (2-{(3R,6S)-3-[(1S,2R)-1-((S)-1-{(1S,2R)-1-[(S)-1-C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085491 (c[Hex-gamma-{N-2-naphthyl}-Dab-Cys]-Thr-val-Thr-Np...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43866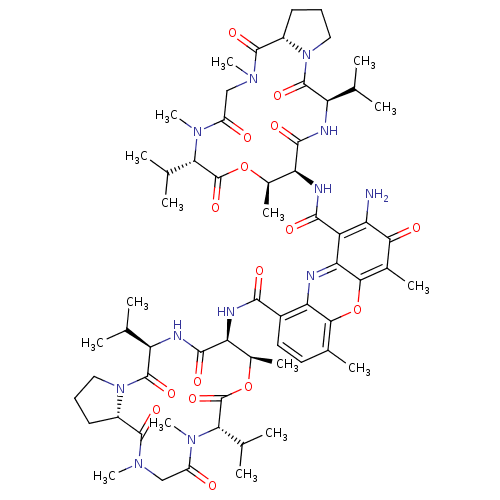 (2-amino-4,6-dimethyl-3-oxo-1-N,9-N-bis[(3R,6S,7R,1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 41 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085487 (2-((3R,6S)-3-{(1S,2R)-2-Hydroxy-1-[(S)-1-((1S,2R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085482 (2-[(3R,6S)-3-((1S,2R)-2-Hydroxy-1-{(S)-1-[(1S,2R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085490 ((2-{(3R,6S)-3-[(1S,2R)-1-((S)-1-{(1S,2R)-1-[(S)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43865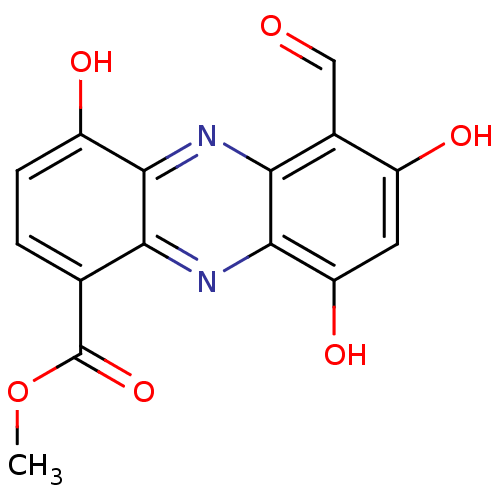 (6-formyl-4,7-dihydroxy-9-keto-5H-phenazine-1-carbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 62 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085486 (2-((3R,6S)-3-{(1S,2R)-1-[(S)-1-((1S,2R)-1-Carbamoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085493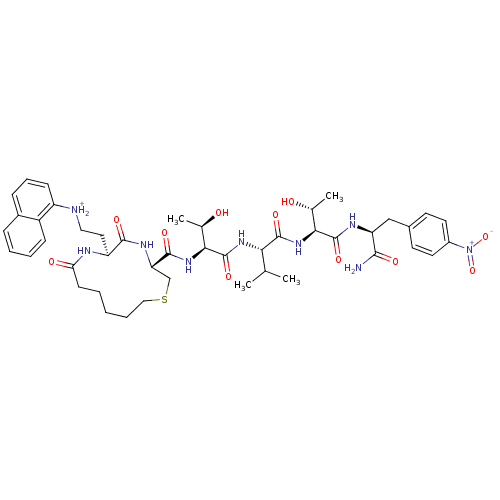 (c[Hex-gamma-{N-1-naphthyl}-Dab-Cys]-Thr-val-Thr-Np...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085485 (2-Benzylamino-5-(4-nitro-benzylamino)-pentanoic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085484 (2-Benzylamino-5-(4-methoxy-benzylamino)-pentanoic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43863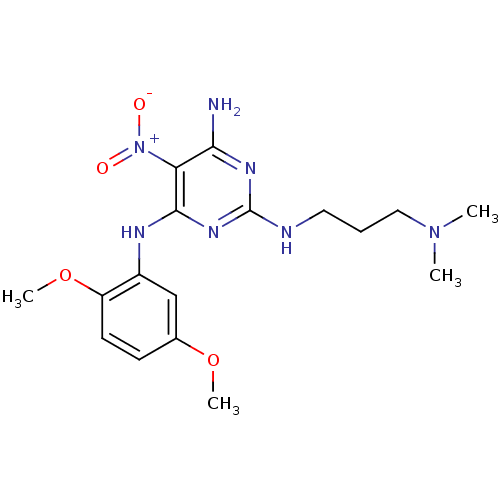 (3-[[4-amino-6-(2,5-dimethoxyanilino)-5-nitro-pyrim...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | -31.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43860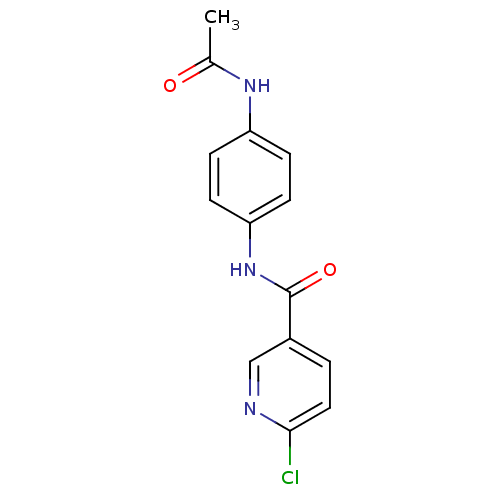 (Glycopeptide, 2 | MLS000517385 | N-(4-acetamidophe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -29.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43864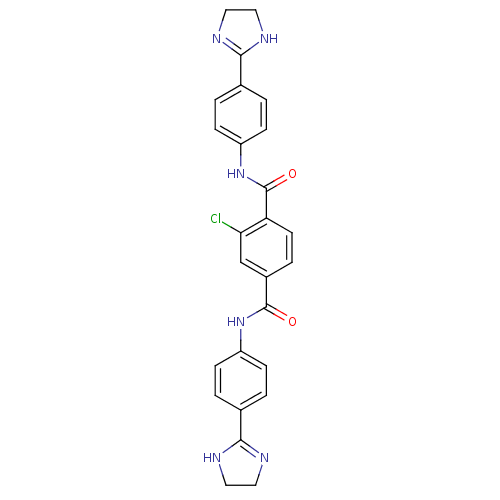 (2-chloranyl-N1,N4-bis[4-(4,5-dihydro-1H-imidazol-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | -28.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43861 (4-[7-[4-[diethyl(methyl)ammonio]butoxy]-9-keto-flu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit (Homo sapiens (Human)) | BDBM50085492 (2,5-Bis-benzylamino-pentanoic acid ({1-[1-(1-carba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of asparagine-linked glycosylation by oligosaccharyl transferase | Bioorg Med Chem Lett 10: 281-4 (2000) BindingDB Entry DOI: 10.7270/Q2FJ2H95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43862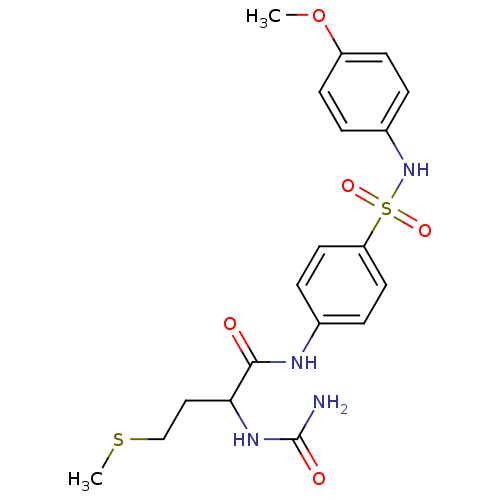 (2-(aminocarbonylamino)-N-[4-[(4-methoxyphenyl)sulf...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.40E+4 | -23.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43859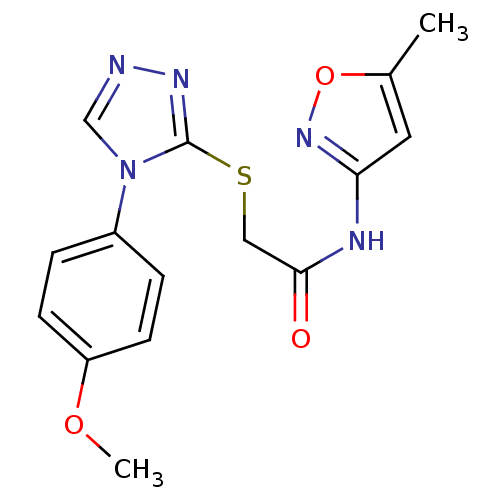 (2-[[4-(4-methoxyphenyl)-1,2,4-triazol-3-yl]sulfany...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | -20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50326697 ((3S,6S,9S,12S,15S,18S,21S,24S,27S,30S,33S,36S)-18-...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50287632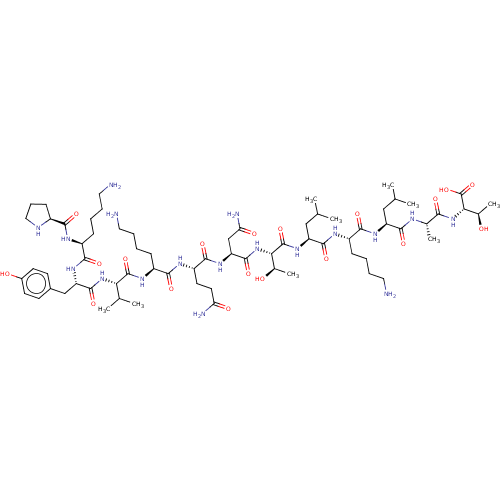 (CHEMBL1253325 | PKYVKQNTLKLAT (HA306-318 peptide)) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50326699 ((2S,5S,8S,11S,14S,17S,20S,23S,26S)-2-amino-1-(5-(d...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells at acidic pH by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50326699 ((2S,5S,8S,11S,14S,17S,20S,23S,26S)-2-amino-1-(5-(d...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50326698 ((6S,9S,12S,15S,18S,21S,24S,27S)-1-amino-6-((S)-2-a...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells at acidic pH by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50326698 ((6S,9S,12S,15S,18S,21S,24S,27S)-1-amino-6-((S)-2-a...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50326704 ((4S,7S,10S,13S,16S,19S,22S,25S,28S)-4-acetamido-1-...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells at acidic pH by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50326703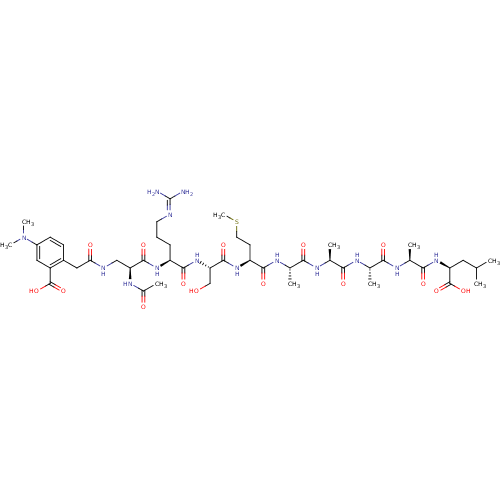 ((5S,8S,11S,14S,17S,20S,23S,26S,29S)-5-acetamido-1-...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells at acidic pH by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501676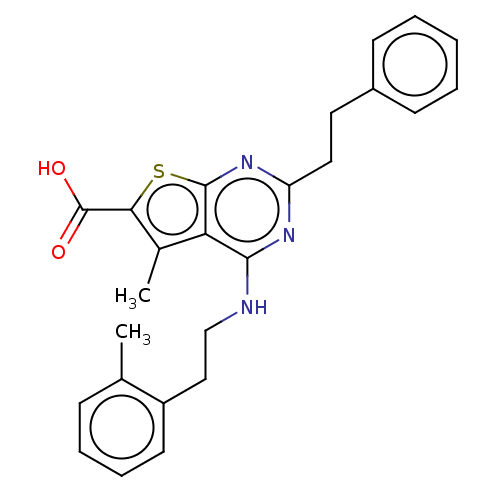 (CHEMBL4064985) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501686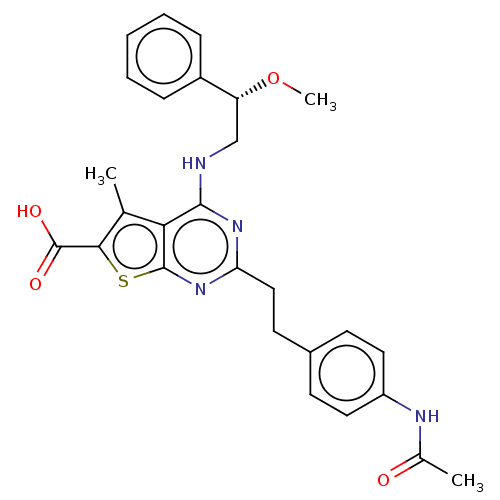 (CHEMBL4065331) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501689 (CHEMBL4104598) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501668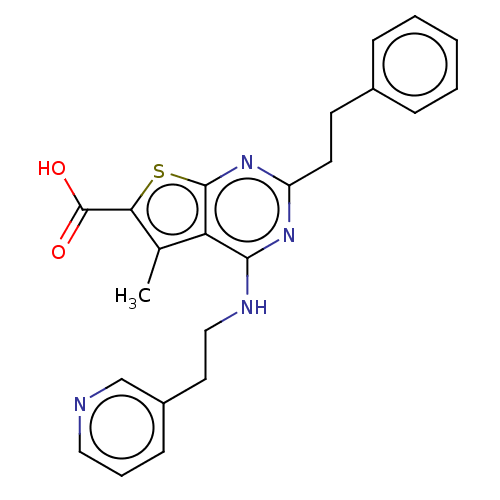 (CHEMBL4102869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501692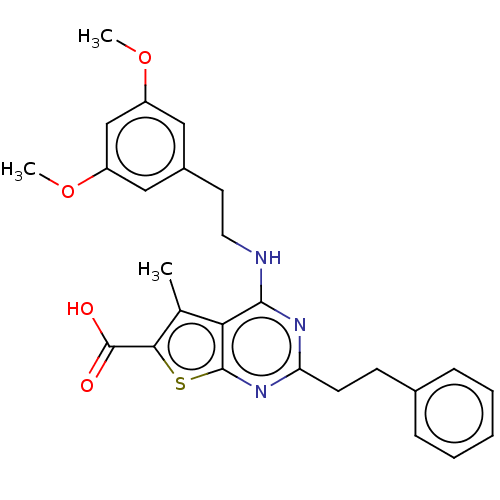 (CHEMBL4095254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501667 (CHEMBL4086446) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501681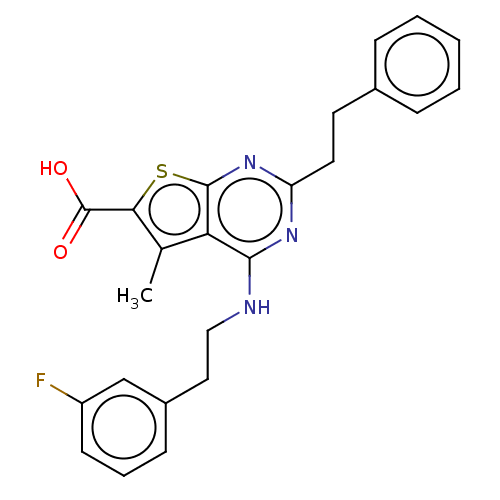 (CHEMBL4083946) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501675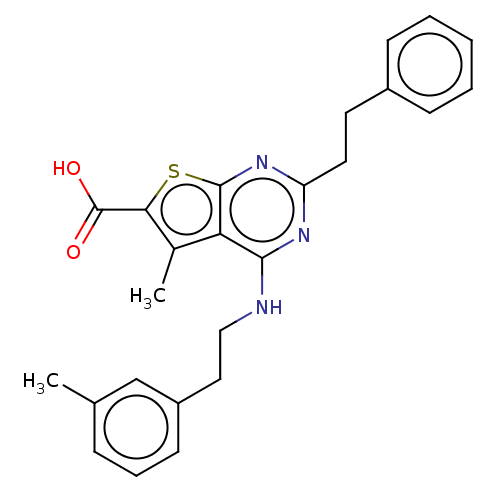 (CHEMBL4094203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501655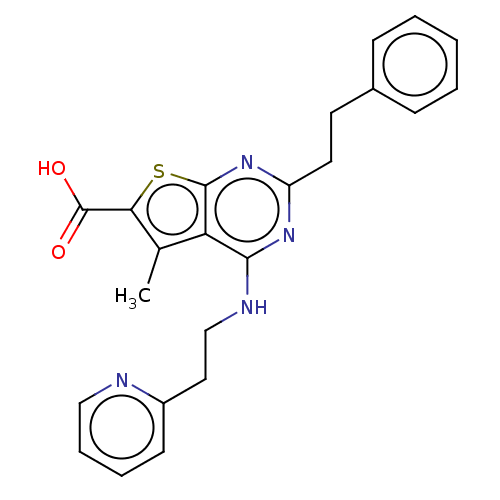 (CHEMBL4078589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501685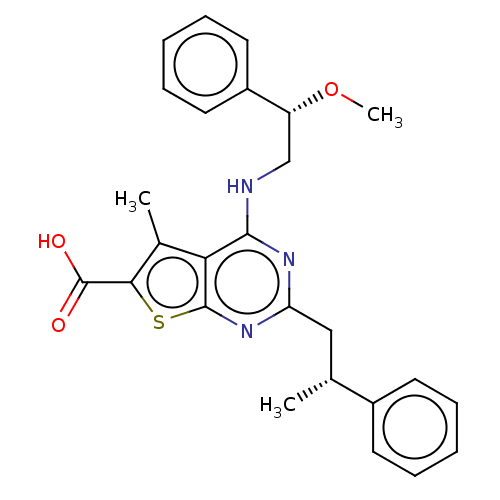 (CHEMBL4091581) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501660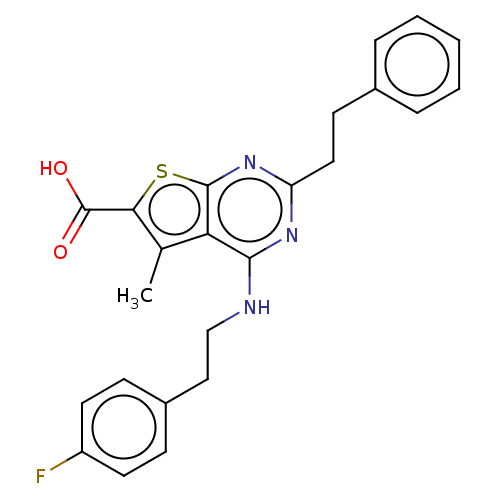 (CHEMBL4087428) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501684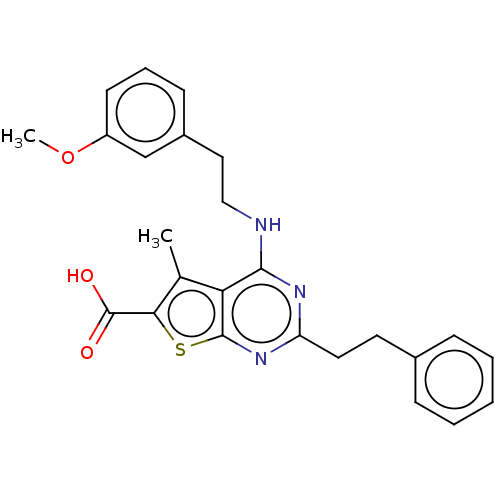 (CHEMBL4099461) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501687 (CHEMBL4068884) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501682 (CHEMBL4101939) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501666 (CHEMBL4105281) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501654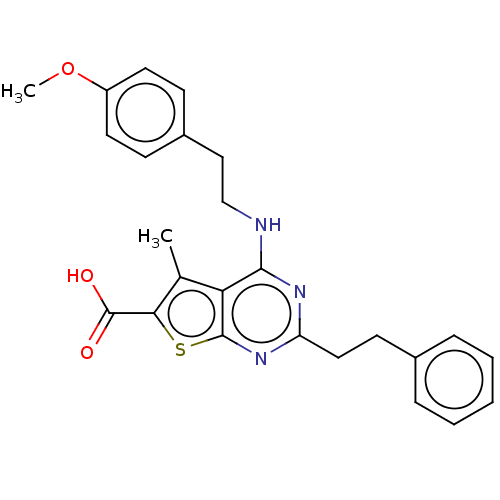 (CHEMBL4072547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501693 (CHEMBL4074801) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501692 (CHEMBL4095254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assesse... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501674 (CHEMBL4073574) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylbacillosamine N-acetyltransferase (Campylobacter jejuni subsp. jejuni serotype O:2 (s...) | BDBM50501680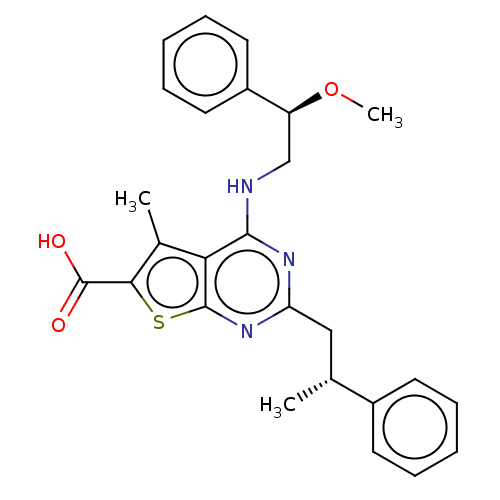 (CHEMBL4070686) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Campylobacter jejuni NCTC 11168 PglD acetyltransferase expressed in Escherichia coli BL-21(DE3) strain (Stratagene) assessed as release... | J Med Chem 60: 2099-2118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01869 BindingDB Entry DOI: 10.7270/Q2JD50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50326701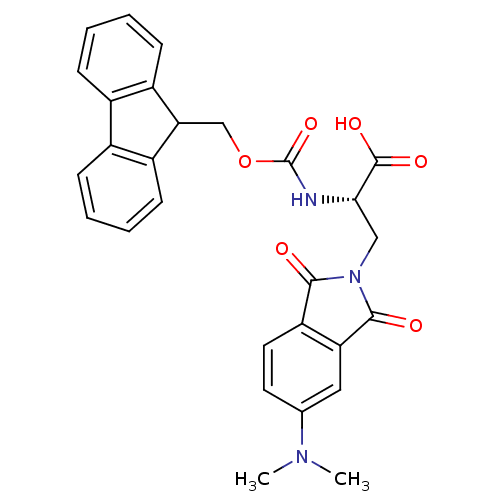 ((S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-3-...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Displacement of biotin-HA from recombinant soluble HLA-DR1 expressed in human T2 cells by ELISA | Nat Chem Biol 3: 222-8 (2007) Article DOI: 10.1038/nchembio868 BindingDB Entry DOI: 10.7270/Q2HT2PJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77 total ) | Next | Last >> |