

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337102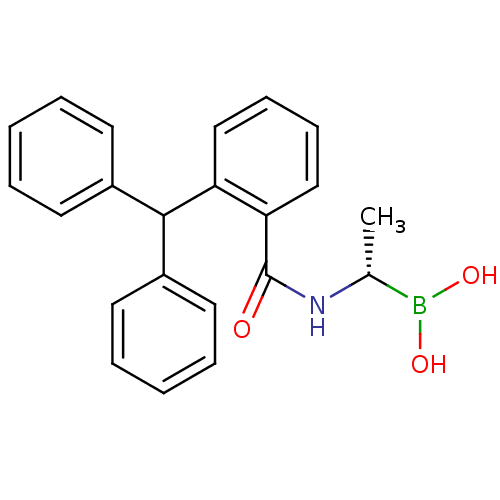 ((S)-1-(2-(Diphenylmethyl)benzamido)ethaneboronate ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337101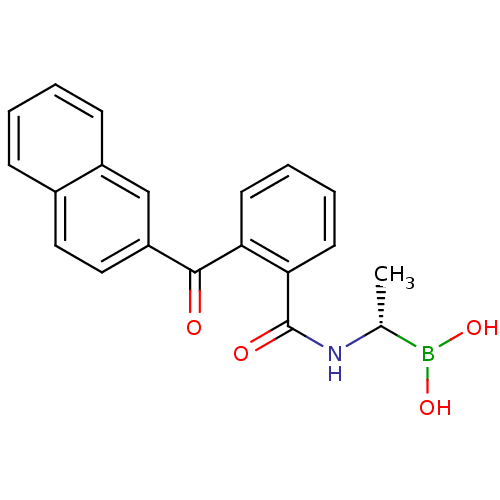 ((S)-1-(2-(Naphthalen-2-ylcarbonyl)benzamido)ethane...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337101 ((S)-1-(2-(Naphthalen-2-ylcarbonyl)benzamido)ethane...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337094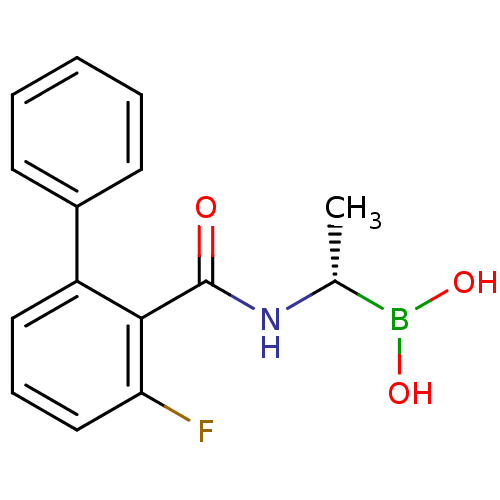 ((S)-1-(2-Fluoro-6-phenylbenzamido)ethaneboronic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337100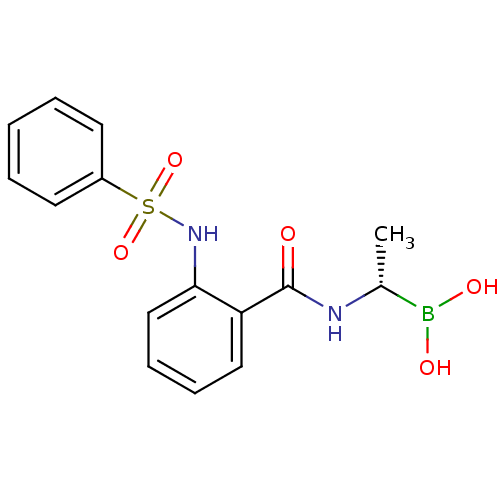 ((S)-1-(2-[(Phenylsulfonyl)amino]benzamido)ethanebo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337095 ((S)-1-(2,6-Difluorobenzamido)ethaneboronic acid | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337093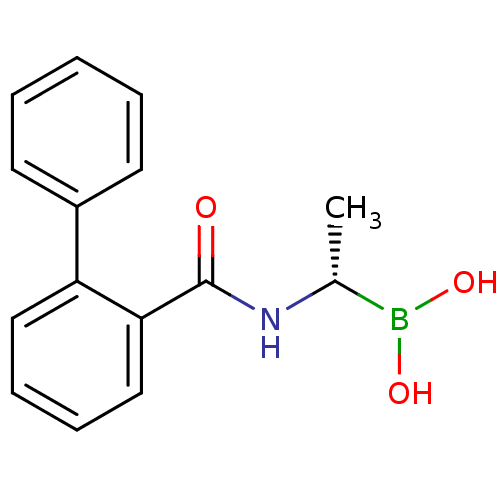 ((S)-1-(2-Phenylbenzamido)ethaneboronic acid | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337092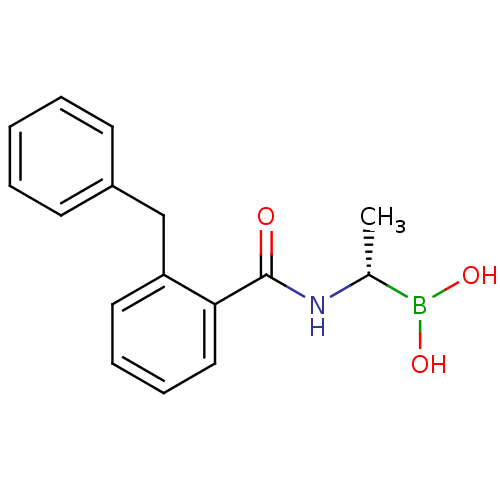 ((S)-1-(2-Benzylbenzamido)ethaneboronic acid | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337091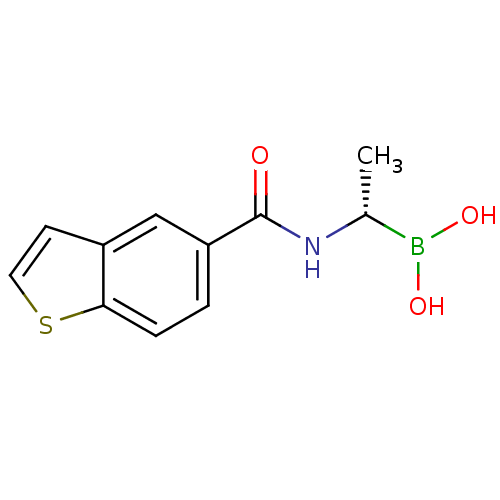 ((S)-1-(1-Benzothiophene-5-carboxamido)ethaneboroni...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337098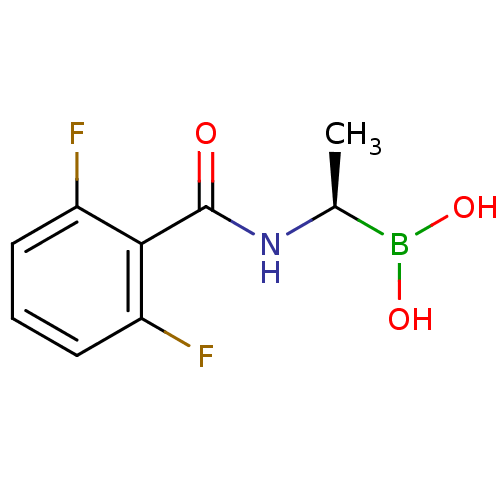 ((R)-1-(2,6-Difluorobenzamido)ethaneboronic acid | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337096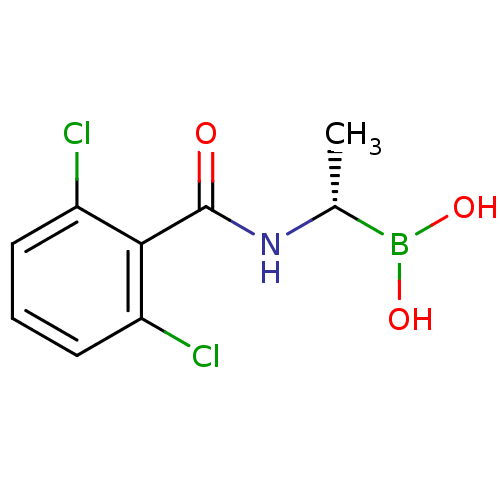 ((S)-1-(2,6-Dichlorobenzamido)ethaneboronic acid | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337099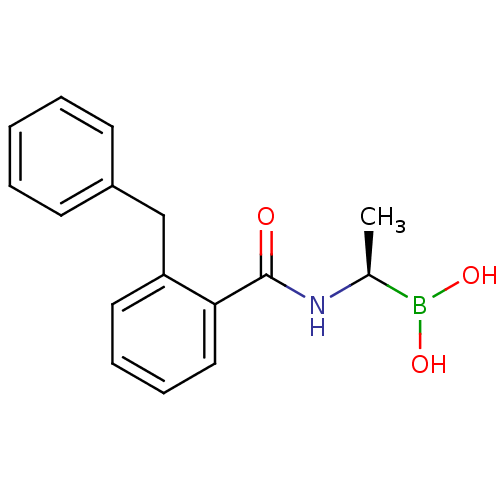 ((R)-1-(2-Benzylbenzamido)ethaneboronic acid | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337090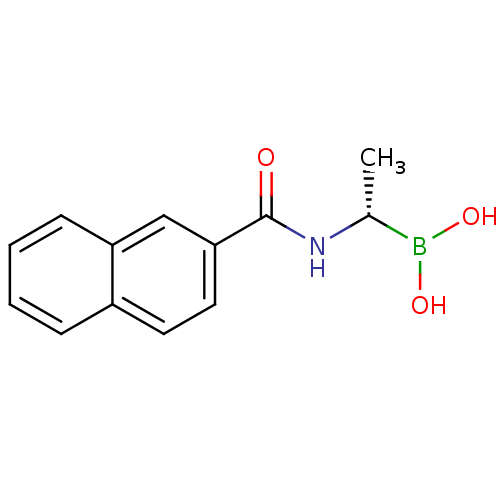 ((S)-1-(Naphthalene-2-carboxamido)ethaneboronic aci...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50300661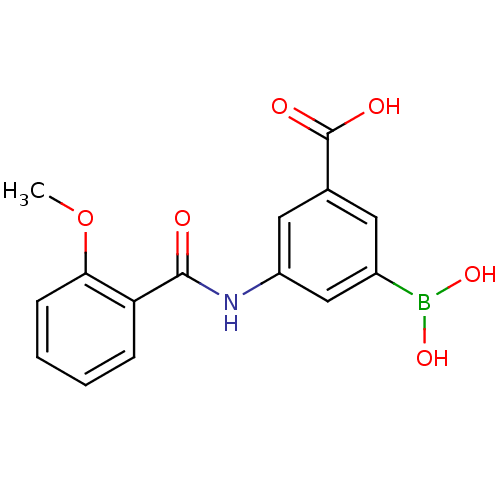 (3-borono-5-(2-methoxybenzamido)benzoic acid | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... | J Med Chem 52: 6097-106 (2009) Article DOI: 10.1021/jm9009718 BindingDB Entry DOI: 10.7270/Q2G73DSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50300664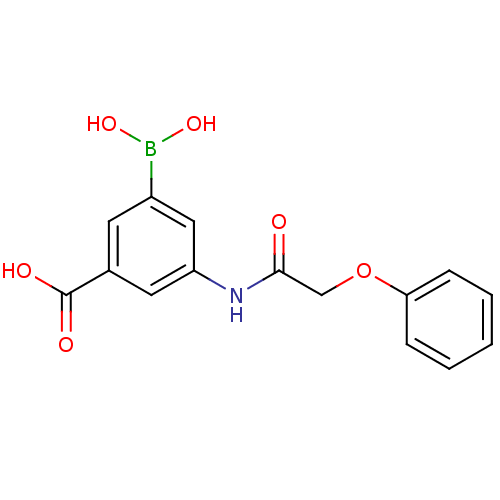 (3-borono-5-(2-phenoxyacetamido)benzoic acid | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... | J Med Chem 52: 6097-106 (2009) Article DOI: 10.1021/jm9009718 BindingDB Entry DOI: 10.7270/Q2G73DSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50300662 (3-borono-5-(thiophene-2-carboxamido)benzoic acid |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... | J Med Chem 52: 6097-106 (2009) Article DOI: 10.1021/jm9009718 BindingDB Entry DOI: 10.7270/Q2G73DSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50300660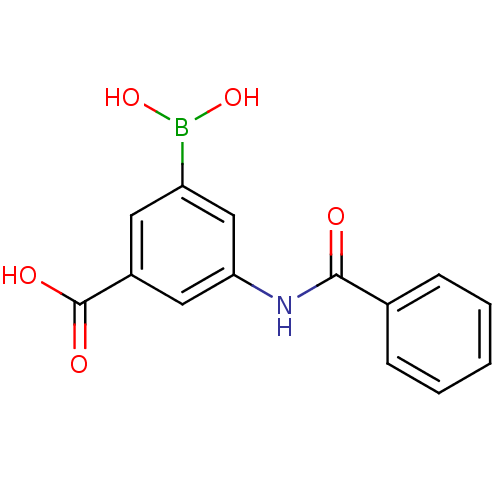 (3-benzamido-5-boronobenzoic acid | CHEMBL575719) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... | J Med Chem 52: 6097-106 (2009) Article DOI: 10.1021/jm9009718 BindingDB Entry DOI: 10.7270/Q2G73DSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50337097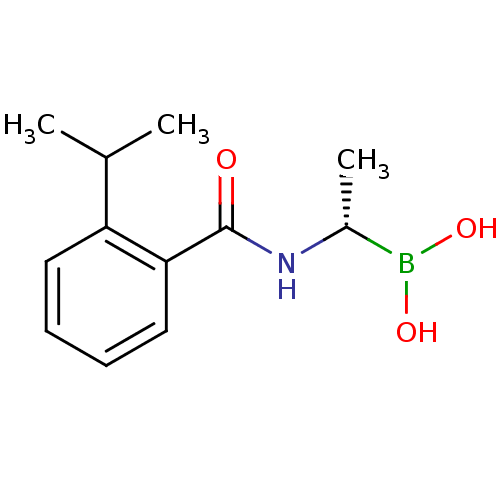 ((S)-1-(2-(Propan-2-yl)benzamido)ethaneboronic acid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Actinomadura R39 PBP after 60 mins | ACS Med Chem Lett 2: 219-223 (2011) Article DOI: 10.1021/ml100260x BindingDB Entry DOI: 10.7270/Q25H7GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50300663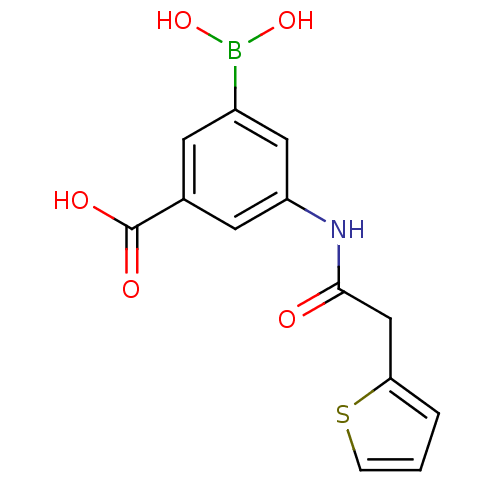 (3-borono-5-(2-(thiophen-2-yl)acetamido)benzoic aci...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... | J Med Chem 52: 6097-106 (2009) Article DOI: 10.1021/jm9009718 BindingDB Entry DOI: 10.7270/Q2G73DSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50300659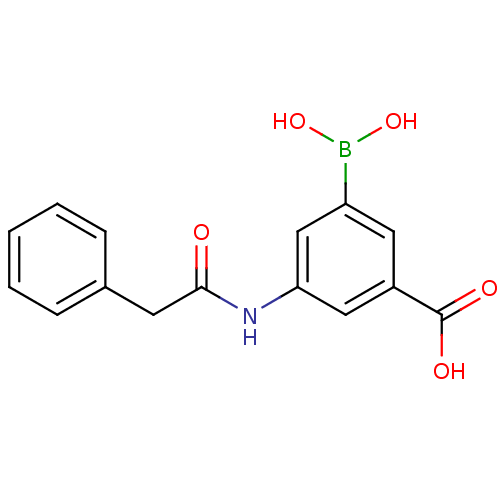 (3-borono-5-(2-phenylacetamido)benzoic acid | CHEMB...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... | J Med Chem 52: 6097-106 (2009) Article DOI: 10.1021/jm9009718 BindingDB Entry DOI: 10.7270/Q2G73DSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanyl-D-alanine carboxypeptidase (Actinomadura sp. (strain R39)) | BDBM50067893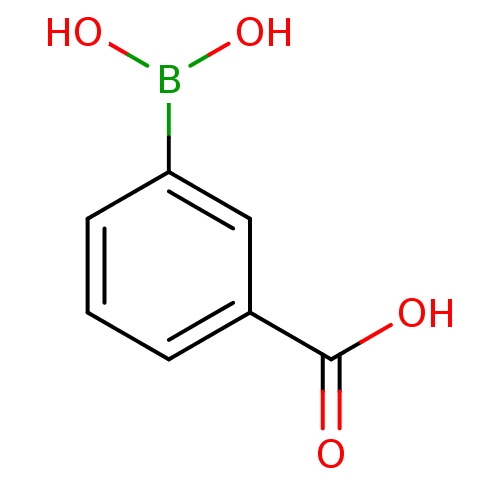 (3-Carboxyphenylboronicacid | 3-boronobenzoic acid ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... | J Med Chem 52: 6097-106 (2009) Article DOI: 10.1021/jm9009718 BindingDB Entry DOI: 10.7270/Q2G73DSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 2x (Streptococcus pneumoniae) | BDBM50300665 (5-boronothiophene-2-carboxylic acid | CHEMBL573906) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of penicillin-resistant Streptococcus pneumoniae 5204 PBP2X preincubated for 4 hrs before addition of substrate mixture of (R)-[2-(benzoyl... | J Med Chem 52: 6097-106 (2009) Article DOI: 10.1021/jm9009718 BindingDB Entry DOI: 10.7270/Q2G73DSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM50176311 (CHEMBL201720 | N-benzylquinolin-2-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.93E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity to Tec SH3 domain | Bioorg Med Chem Lett 16: 387-90 (2005) Article DOI: 10.1016/j.bmcl.2005.09.073 BindingDB Entry DOI: 10.7270/Q2GF0T28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM50176312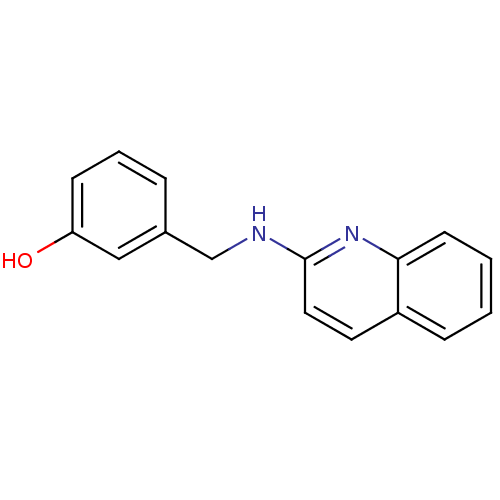 (3-((quinolin-2-ylamino)methyl)phenol | CHEMBL38299...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.34E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity to Tec SH3 domain | Bioorg Med Chem Lett 16: 387-90 (2005) Article DOI: 10.1016/j.bmcl.2005.09.073 BindingDB Entry DOI: 10.7270/Q2GF0T28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM50176313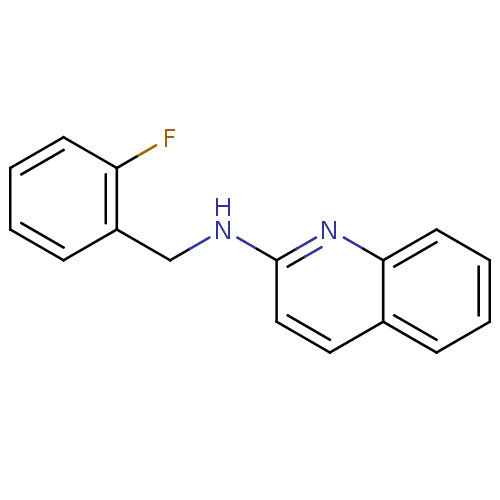 (CHEMBL381361 | N-(2-fluorobenzyl)quinolin-2-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.08E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity to Tec SH3 domain | Bioorg Med Chem Lett 16: 387-90 (2005) Article DOI: 10.1016/j.bmcl.2005.09.073 BindingDB Entry DOI: 10.7270/Q2GF0T28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM50176314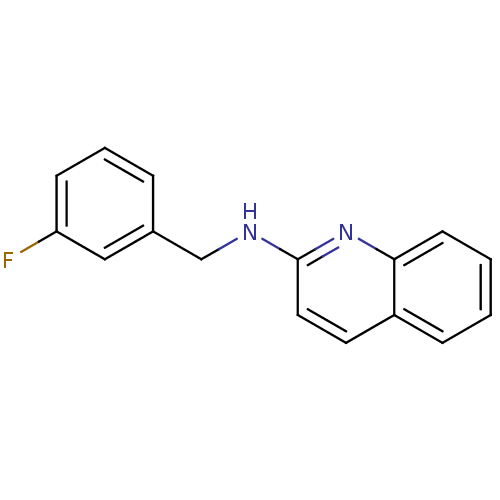 (CHEMBL201328 | N-(3-fluorobenzyl)quinolin-2-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.77E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity to Tec SH3 domain | Bioorg Med Chem Lett 16: 387-90 (2005) Article DOI: 10.1016/j.bmcl.2005.09.073 BindingDB Entry DOI: 10.7270/Q2GF0T28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM50176316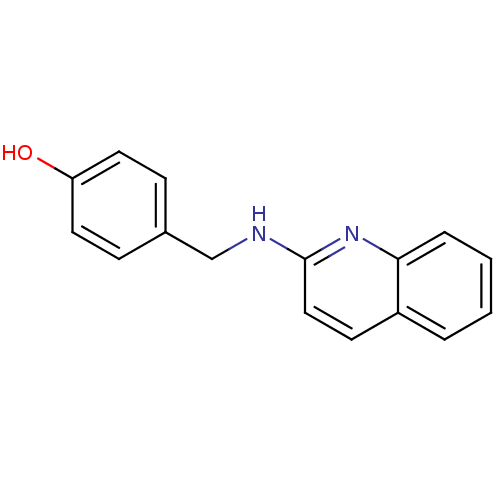 (4-((quinolin-2-ylamino)methyl)phenol | CHEMBL20298...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.92E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity to Tec SH3 domain | Bioorg Med Chem Lett 16: 387-90 (2005) Article DOI: 10.1016/j.bmcl.2005.09.073 BindingDB Entry DOI: 10.7270/Q2GF0T28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM50176315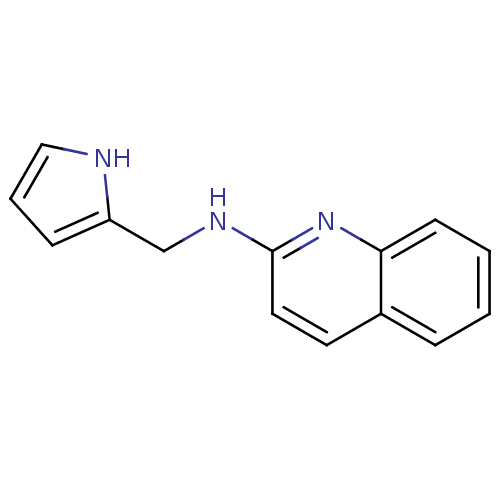 (CHEMBL199638 | N-((1H-pyrrol-2-yl)methyl)quinolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.85E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Binding affinity to Tec SH3 domain | Bioorg Med Chem Lett 16: 387-90 (2005) Article DOI: 10.1016/j.bmcl.2005.09.073 BindingDB Entry DOI: 10.7270/Q2GF0T28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154591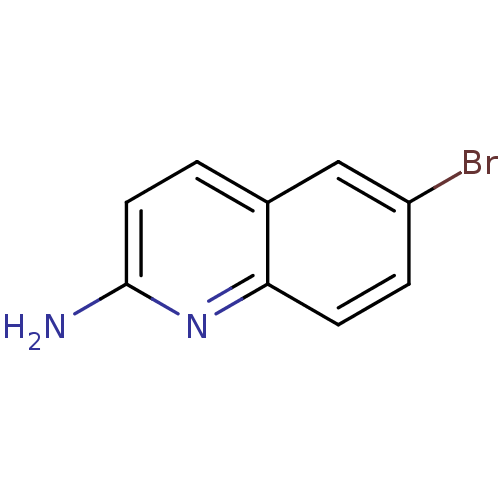 (6-Bromo-quinolin-2-ylamine | CHEMBL189197) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154582 (6-[1,3]Dioxan-2-yl-quinolin-2-ylamine | CHEMBL4266...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154587 (6-[1,3]Dioxolan-2-yl-quinolin-2-ylamine | CHEMBL36...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154589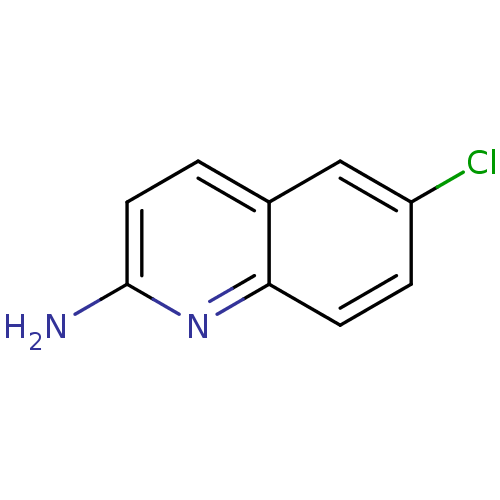 (6-Chloro-quinolin-2-ylamine | CHEMBL189514) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154590 (6-Methoxy-quinolin-2-ylamine | CHEMBL188730) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154588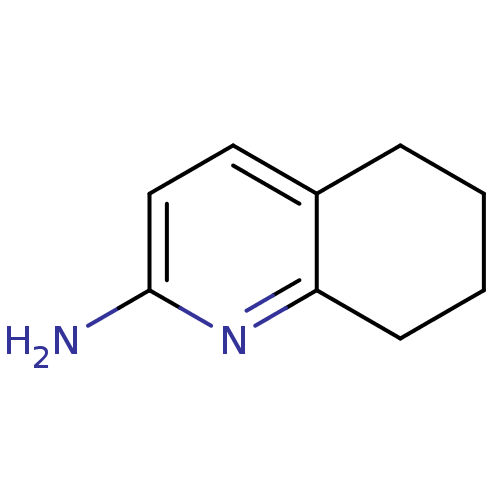 (5,6,7,8-Tetrahydro-quinolin-2-ylamine | CHEMBL1888...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.15E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50084506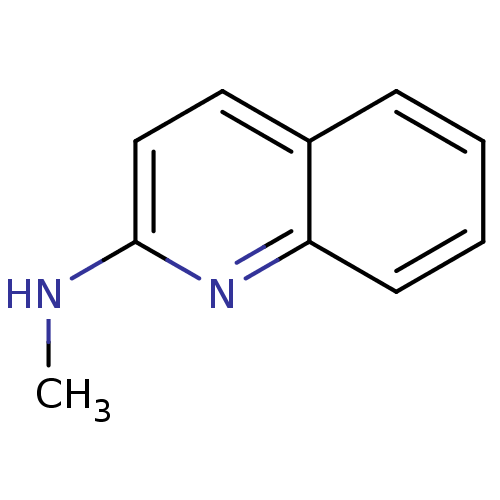 (CHEMBL319543 | Methyl-quinolin-2-yl-amine) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.80E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154587 (6-[1,3]Dioxolan-2-yl-quinolin-2-ylamine | CHEMBL36...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50013712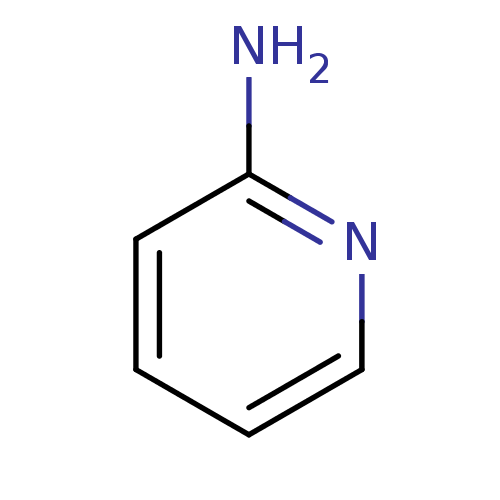 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >4.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic protein NCK1 (Homo sapiens (Human)) | BDBM50154587 (6-[1,3]Dioxolan-2-yl-quinolin-2-ylamine | CHEMBL36...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from human Nck kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM14320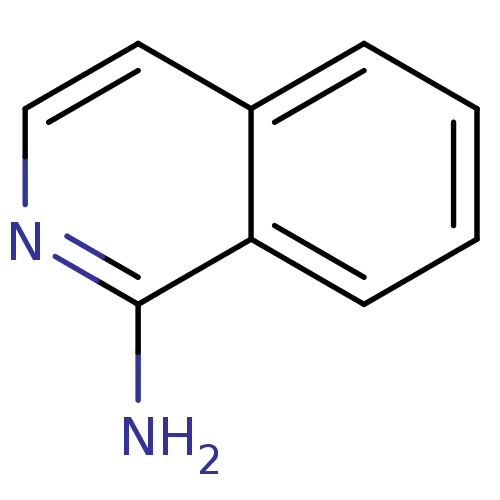 (1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.50E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM14322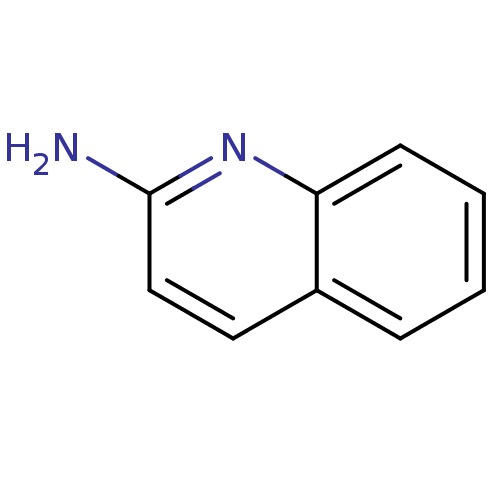 (2-Aminoquinoline 1 | 2-aminoquinoline | CHEMBL6123...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic protein NCK1 (Homo sapiens (Human)) | BDBM50154583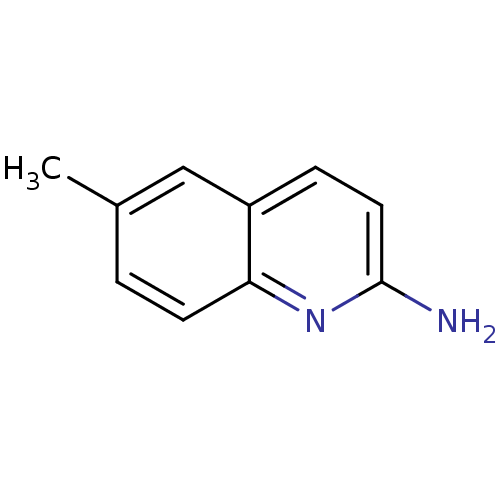 (6-Methyl-quinolin-2-ylamine | CHEMBL361660) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from human Nck kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic protein NCK1 (Homo sapiens (Human)) | BDBM50154582 (6-[1,3]Dioxan-2-yl-quinolin-2-ylamine | CHEMBL4266...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from human Nck kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154583 (6-Methyl-quinolin-2-ylamine | CHEMBL361660) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM14322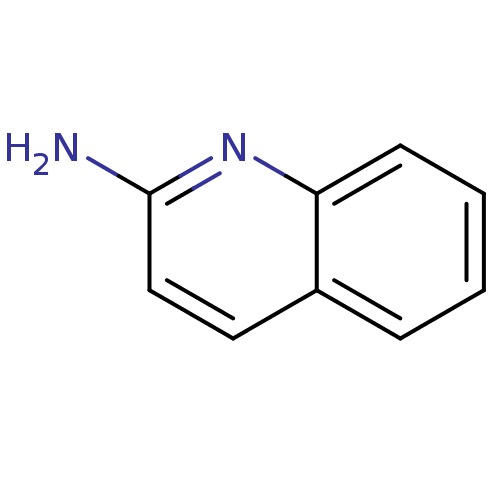 (2-Aminoquinoline 1 | 2-aminoquinoline | CHEMBL6123...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-2 peptide from human Hck kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154586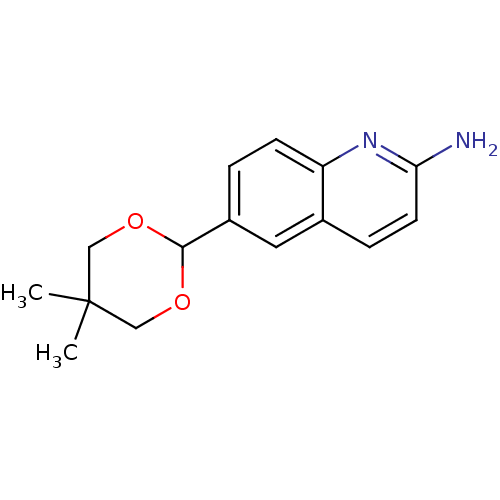 (6-(5,5-Dimethyl-[1,3]dioxan-2-yl)-quinolin-2-ylami...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154585 (CHEMBL187951 | Quinazolin-2-ylamine) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154584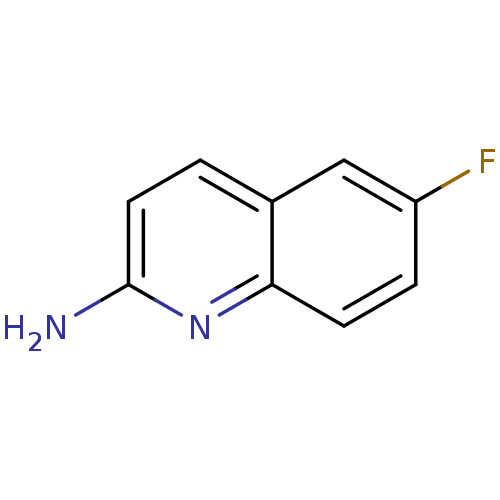 (6-Fluoro-quinolin-2-ylamine | CHEMBL185917) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM50154583 (6-Methyl-quinolin-2-ylamine | CHEMBL361660) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase (Mus musculus) | BDBM14322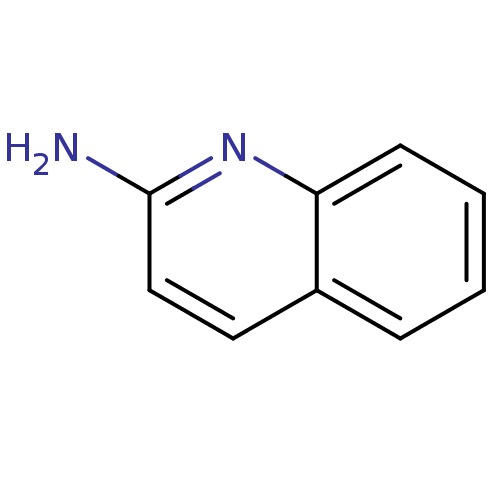 (2-Aminoquinoline 1 | 2-aminoquinoline | CHEMBL6123...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic protein NCK1 (Homo sapiens (Human)) | BDBM14322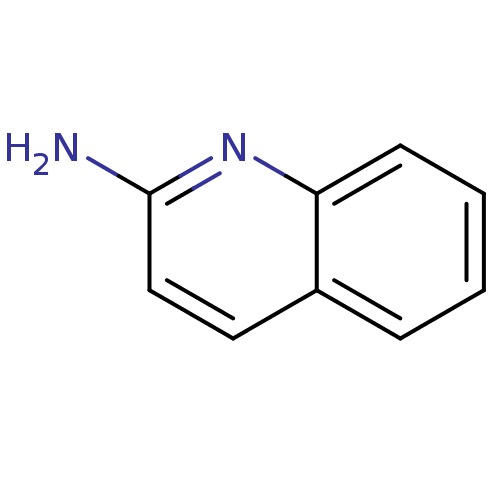 (2-Aminoquinoline 1 | 2-aminoquinoline | CHEMBL6123...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from human Nck kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |