 Found 443 hits with Last Name = 'irie' and Initial = 't'
Found 443 hits with Last Name = 'irie' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(MOUSE) | BDBM50609010
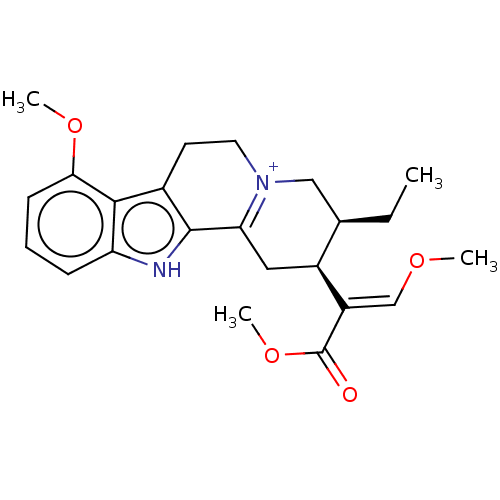
(CHEMBL5276728) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609010

(CHEMBL5276728) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50519927
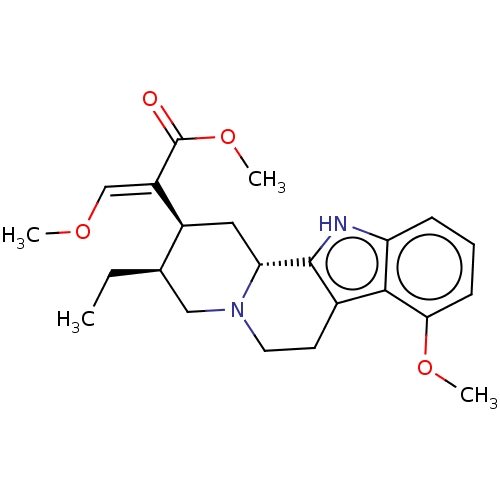
(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609010

(CHEMBL5276728) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 578 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50519927

(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 649 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50566317
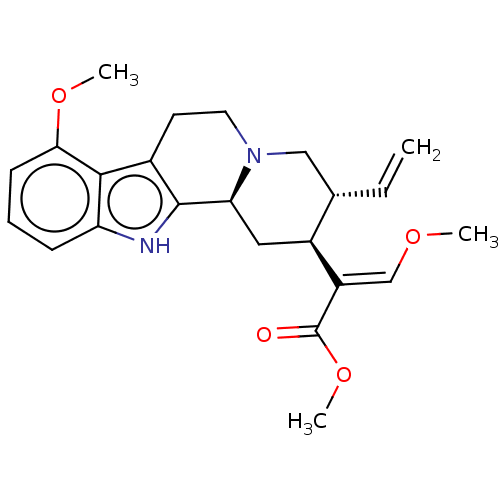
(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 666 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50566317

(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 883 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50519927

(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609011
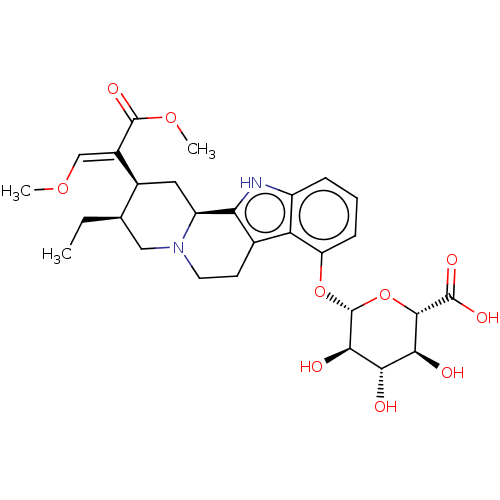
(CHEMBL5287921) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609011

(CHEMBL5287921) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609011

(CHEMBL5287921) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50566317

(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609009
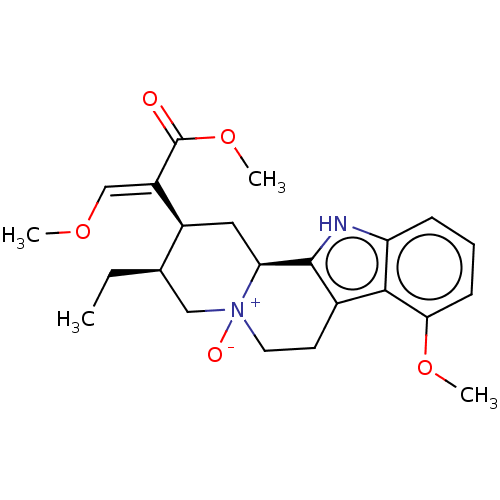
(CHEMBL56717) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 4.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609009

(CHEMBL56717) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609009

(CHEMBL56717) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 7.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465744
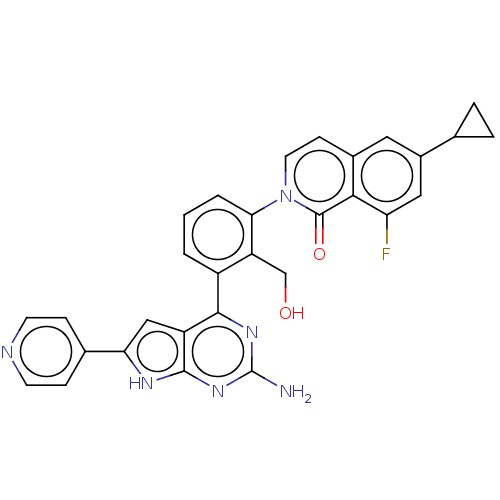
(2-{3-[2-amino-6-(pyridin-4-yl)-7H- pyrrolo[2,3-d]p...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)-c1ccncc1 Show InChI InChI=1S/C30H23FN6O2/c31-23-13-19(16-4-5-16)12-18-8-11-37(29(39)26(18)23)25-3-1-2-20(22(25)15-38)27-21-14-24(17-6-9-33-10-7-17)34-28(21)36-30(32)35-27/h1-3,6-14,16,38H,4-5,15H2,(H3,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by colorimetric analysis |
Bioorg Med Chem Lett 22: 591-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.076
BindingDB Entry DOI: 10.7270/Q2JQ11GW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50452720
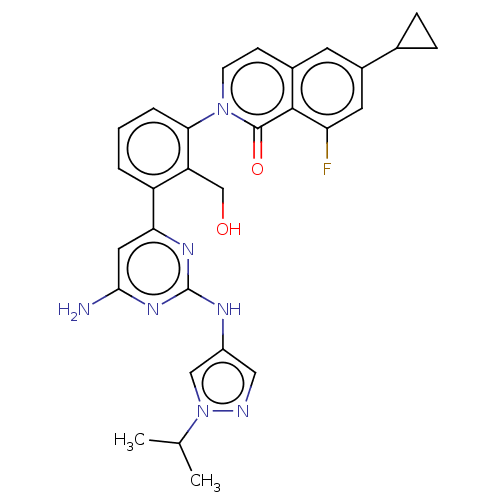
(CHEMBL4212285)Show SMILES CC(C)n1cc(Nc2nc(N)cc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C29H28FN7O2/c1-16(2)37-14-20(13-32-37)33-29-34-24(12-26(31)35-29)21-4-3-5-25(22(21)15-38)36-9-8-18-10-19(17-6-7-17)11-23(30)27(18)28(36)39/h3-5,8-14,16-17,38H,6-7,15H2,1-2H3,(H3,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged lambda phosphatase pre-treated N-terminal DYKDDDDK tagged and biotinylated BTK unactivated form (unknown origin) using FITC-... |
Bioorg Med Chem Lett 28: 145-151 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.037
BindingDB Entry DOI: 10.7270/Q2X069M2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50452701
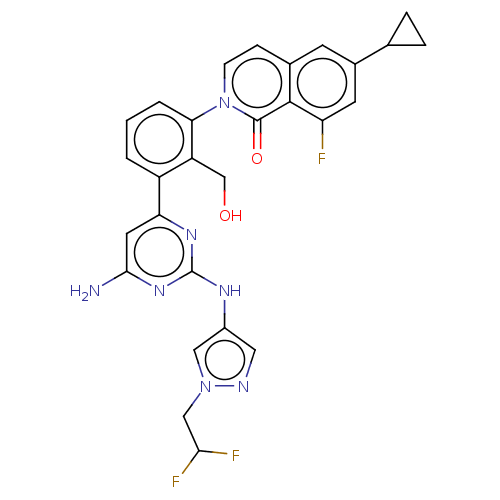
(CHEMBL4211960)Show SMILES Nc1cc(nc(Nc2cnn(CC(F)F)c2)n1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C28H24F3N7O2/c29-21-9-17(15-4-5-15)8-16-6-7-38(27(40)26(16)21)23-3-1-2-19(20(23)14-39)22-10-25(32)36-28(35-22)34-18-11-33-37(12-18)13-24(30)31/h1-3,6-12,15,24,39H,4-5,13-14H2,(H3,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged lambda phosphatase pre-treated N-terminal DYKDDDDK tagged and biotinylated BTK unactivated form (unknown origin) using FITC-... |
Bioorg Med Chem Lett 28: 145-151 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.037
BindingDB Entry DOI: 10.7270/Q2X069M2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468314

(2-{3-[2-amino-6- (1,1-dioxido-3,6- dihydro-2H- thi...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCS(=O)(=O)CC1 |t:39| Show InChI InChI=1S/C30H26FN5O4S/c31-23-13-19(16-4-5-16)12-18-6-9-36(29(38)26(18)23)25-3-1-2-20(22(25)15-37)27-21-14-24(33-28(21)35-30(32)34-27)17-7-10-41(39,40)11-8-17/h1-3,6-7,9,12-14,16,37H,4-5,8,10-11,15H2,(H3,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
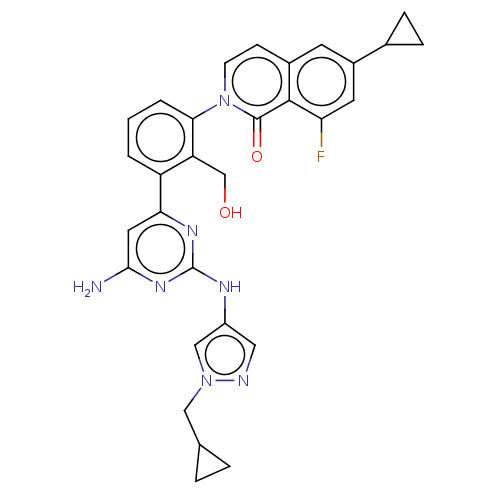
(Homo sapiens (Human)) | BDBM50452722
(CHEMBL4215160)Show SMILES Nc1cc(nc(Nc2cnn(CC3CC3)c2)n1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C30H28FN7O2/c31-24-11-20(18-6-7-18)10-19-8-9-38(29(40)28(19)24)26-3-1-2-22(23(26)16-39)25-12-27(32)36-30(35-25)34-21-13-33-37(15-21)14-17-4-5-17/h1-3,8-13,15,17-18,39H,4-7,14,16H2,(H3,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged lambda phosphatase pre-treated N-terminal DYKDDDDK tagged and biotinylated BTK unactivated form (unknown origin) using FITC-... |
Bioorg Med Chem Lett 28: 145-151 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.037
BindingDB Entry DOI: 10.7270/Q2X069M2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50452714

(CHEMBL4213985)Show SMILES Nc1cc(nc(Nc2cnn(c2)C2CC2)n1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C29H26FN7O2/c30-23-11-18(16-4-5-16)10-17-8-9-36(28(39)27(17)23)25-3-1-2-21(22(25)15-38)24-12-26(31)35-29(34-24)33-19-13-32-37(14-19)20-6-7-20/h1-3,8-14,16,20,38H,4-7,15H2,(H3,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged lambda phosphatase pre-treated N-terminal DYKDDDDK tagged and biotinylated BTK unactivated form (unknown origin) using FITC-... |
Bioorg Med Chem Lett 28: 145-151 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.037
BindingDB Entry DOI: 10.7270/Q2X069M2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459581

(CHEMBL4218481)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1cccc(c1CO)-c1nc(N)nc(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C32H35N7O4/c1-32(2,3)22-11-7-20(8-12-22)28(41)35-26-6-4-5-24(25(26)19-40)27-36-30(33)38-31(37-27)34-23-13-9-21(10-14-23)29(42)39-15-17-43-18-16-39/h4-14,40H,15-19H2,1-3H3,(H,35,41)(H3,33,34,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465733
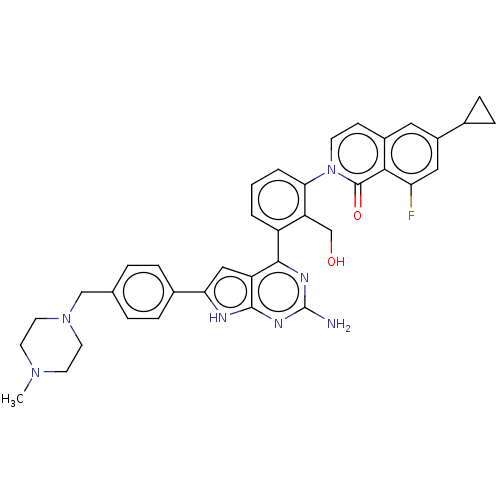
(2-[3-(2-amino-6-{4-[(4-methylpiperazin- 1-yl)methy...)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cc3c(nc(N)nc3[nH]2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)CC1 Show InChI InChI=1S/C37H36FN7O2/c1-43-13-15-44(16-14-43)20-22-5-7-24(8-6-22)31-19-28-34(41-37(39)42-35(28)40-31)27-3-2-4-32(29(27)21-46)45-12-11-25-17-26(23-9-10-23)18-30(38)33(25)36(45)47/h2-8,11-12,17-19,23,46H,9-10,13-16,20-21H2,1H3,(H3,39,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468318
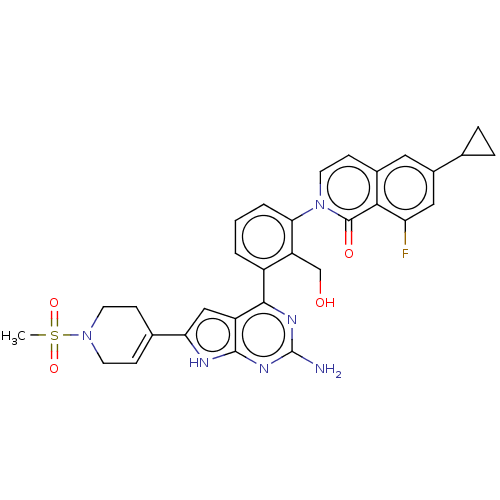
(2-(3-{2-amino-6-[1- (methylsulfonyl)- 1,2,3,6- tet...)Show SMILES CS(=O)(=O)N1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:7| Show InChI InChI=1S/C31H29FN6O4S/c1-43(41,42)37-10-7-18(8-11-37)25-15-22-28(35-31(33)36-29(22)34-25)21-3-2-4-26(23(21)16-39)38-12-9-19-13-20(17-5-6-17)14-24(32)27(19)30(38)40/h2-4,7,9,12-15,17,39H,5-6,8,10-11,16H2,1H3,(H3,33,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468311

(2-{3-[2-amino-6-(4- {[4-(2-hydroxy- ethyl)piperazi...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)-c1ccc(CN2CCN(CCO)CC2)cc1 Show InChI InChI=1S/C38H38FN7O3/c39-31-19-27(24-8-9-24)18-26-10-11-46(37(49)34(26)31)33-3-1-2-28(30(33)22-48)35-29-20-32(41-36(29)43-38(40)42-35)25-6-4-23(5-7-25)21-45-14-12-44(13-15-45)16-17-47/h1-7,10-11,18-20,24,47-48H,8-9,12-17,21-22H2,(H3,40,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553
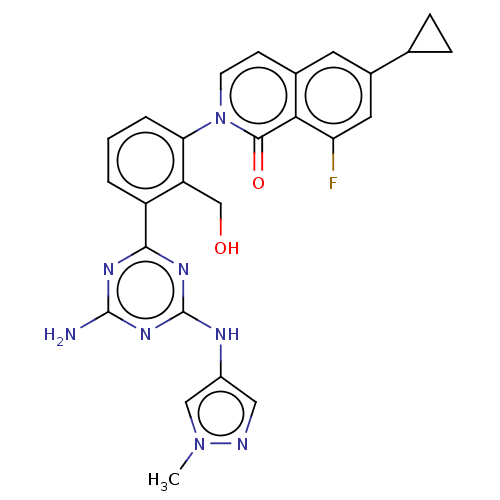
(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468313
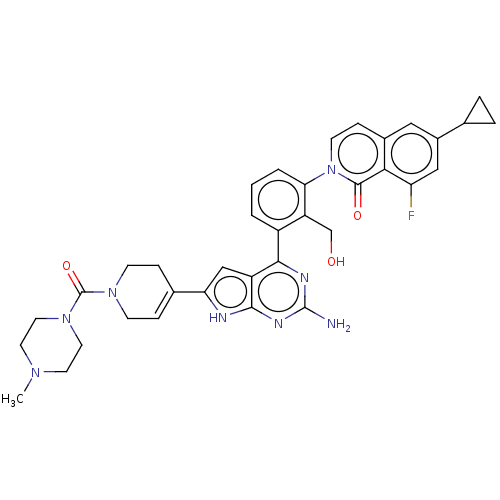
(2-(3-{2-amino-6- [1-(4-methylpiperazine-1- carbony...)Show SMILES CN1CCN(CC1)C(=O)N1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:13| Show InChI InChI=1S/C36H37FN8O3/c1-42-13-15-44(16-14-42)36(48)43-10-7-22(8-11-43)29-19-26-32(40-35(38)41-33(26)39-29)25-3-2-4-30(27(25)20-46)45-12-9-23-17-24(21-5-6-21)18-28(37)31(23)34(45)47/h2-4,7,9,12,17-19,21,46H,5-6,8,10-11,13-16,20H2,1H3,(H3,38,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459578
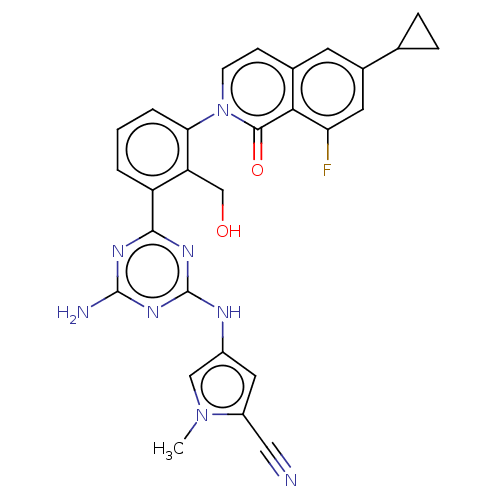
(CHEMBL4204675)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cc1C#N Show InChI InChI=1S/C28H23FN8O2/c1-36-13-18(11-19(36)12-30)32-28-34-25(33-27(31)35-28)20-3-2-4-23(21(20)14-38)37-8-7-16-9-17(15-5-6-15)10-22(29)24(16)26(37)39/h2-4,7-11,13,15,38H,5-6,14H2,1H3,(H3,31,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50452707
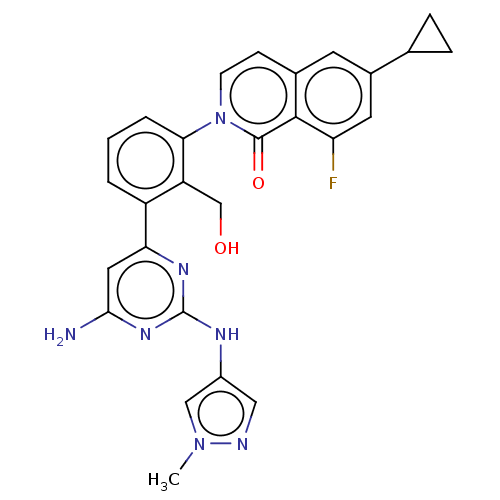
(CHEMBL4216609)Show SMILES Cn1cc(Nc2nc(N)cc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C27H24FN7O2/c1-34-13-18(12-30-34)31-27-32-22(11-24(29)33-27)19-3-2-4-23(20(19)14-36)35-8-7-16-9-17(15-5-6-15)10-21(28)25(16)26(35)37/h2-4,7-13,15,36H,5-6,14H2,1H3,(H3,29,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged lambda phosphatase pre-treated N-terminal DYKDDDDK tagged and biotinylated BTK unactivated form (unknown origin) using FITC-... |
Bioorg Med Chem Lett 28: 145-151 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.037
BindingDB Entry DOI: 10.7270/Q2X069M2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50452709
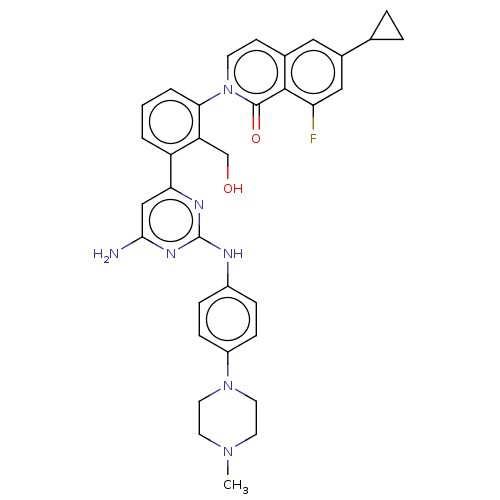
(CHEMBL4218643)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(N)cc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cc1 Show InChI InChI=1S/C34H34FN7O2/c1-40-13-15-41(16-14-40)25-9-7-24(8-10-25)37-34-38-29(19-31(36)39-34)26-3-2-4-30(27(26)20-43)42-12-11-22-17-23(21-5-6-21)18-28(35)32(22)33(42)44/h2-4,7-12,17-19,21,43H,5-6,13-16,20H2,1H3,(H3,36,37,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged lambda phosphatase pre-treated N-terminal DYKDDDDK tagged and biotinylated BTK unactivated form (unknown origin) using FITC-... |
Bioorg Med Chem Lett 28: 145-151 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.037
BindingDB Entry DOI: 10.7270/Q2X069M2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50452700
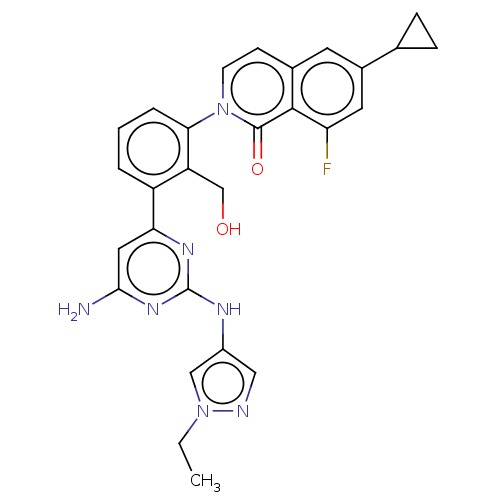
(CHEMBL4204719)Show SMILES CCn1cc(Nc2nc(N)cc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C28H26FN7O2/c1-2-35-14-19(13-31-35)32-28-33-23(12-25(30)34-28)20-4-3-5-24(21(20)15-37)36-9-8-17-10-18(16-6-7-16)11-22(29)26(17)27(36)38/h3-5,8-14,16,37H,2,6-7,15H2,1H3,(H3,30,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged lambda phosphatase pre-treated N-terminal DYKDDDDK tagged and biotinylated BTK unactivated form (unknown origin) using FITC-... |
Bioorg Med Chem Lett 28: 145-151 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.037
BindingDB Entry DOI: 10.7270/Q2X069M2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459577
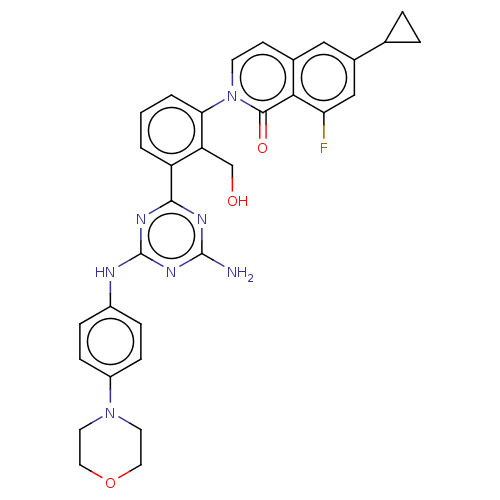
(CHEMBL4217816)Show SMILES Nc1nc(Nc2ccc(cc2)N2CCOCC2)nc(n1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C32H30FN7O3/c33-26-17-21(19-4-5-19)16-20-10-11-40(30(42)28(20)26)27-3-1-2-24(25(27)18-41)29-36-31(34)38-32(37-29)35-22-6-8-23(9-7-22)39-12-14-43-15-13-39/h1-3,6-11,16-17,19,41H,4-5,12-15,18H2,(H3,34,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468320
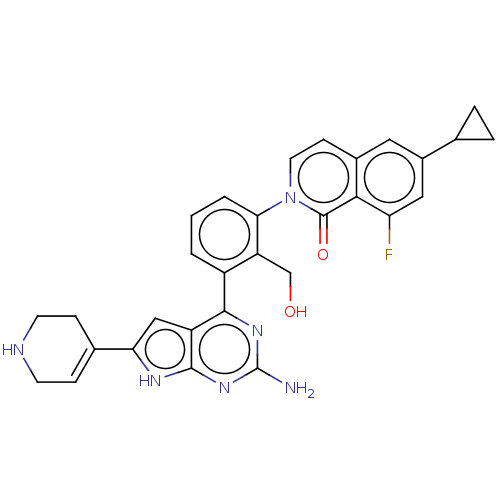
(2-{3-[2-amino-6-(1,2,3,6- tetrahydropyridin-4-yl)-...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCNCC1 |t:39| Show InChI InChI=1S/C30H27FN6O2/c31-23-13-19(16-4-5-16)12-18-8-11-37(29(39)26(18)23)25-3-1-2-20(22(25)15-38)27-21-14-24(17-6-9-33-10-7-17)34-28(21)36-30(32)35-27/h1-3,6,8,11-14,16,33,38H,4-5,7,9-10,15H2,(H3,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468315
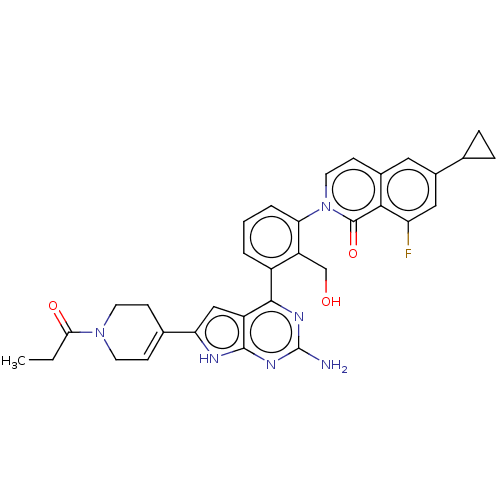
(2-{3-[2-amino-6- (1-propionyl-1,2,3,6- tetrahydrop...)Show SMILES CCC(=O)N1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:7| Show InChI InChI=1S/C33H31FN6O3/c1-2-28(42)39-11-8-19(9-12-39)26-16-23-30(37-33(35)38-31(23)36-26)22-4-3-5-27(24(22)17-41)40-13-10-20-14-21(18-6-7-18)15-25(34)29(20)32(40)43/h3-5,8,10,13-16,18,41H,2,6-7,9,11-12,17H2,1H3,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468308

(2-[3-(2-amino-6-{4- [(diethyl- amino)methyl]phenyl...)Show SMILES CCN(CC)Cc1ccc(cc1)-c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C36H35FN6O2/c1-3-42(4-2)19-21-8-10-23(11-9-21)30-18-27-33(40-36(38)41-34(27)39-30)26-6-5-7-31(28(26)20-44)43-15-14-24-16-25(22-12-13-22)17-29(37)32(24)35(43)45/h5-11,14-18,22,44H,3-4,12-13,19-20H2,1-2H3,(H3,38,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468307

(2-[3-(2-amino-6-{4- [(dimethyl- amino)methyl]pheny...)Show SMILES CN(C)Cc1ccc(cc1)-c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C34H31FN6O2/c1-40(2)17-19-6-8-21(9-7-19)28-16-25-31(38-34(36)39-32(25)37-28)24-4-3-5-29(26(24)18-42)41-13-12-22-14-23(20-10-11-20)15-27(35)30(22)33(41)43/h3-9,12-16,20,42H,10-11,17-18H2,1-2H3,(H3,36,37,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK [C481S]
(Homo sapiens (Human)) | BDBM465733

(2-[3-(2-amino-6-{4-[(4-methylpiperazin- 1-yl)methy...)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cc3c(nc(N)nc3[nH]2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)CC1 Show InChI InChI=1S/C37H36FN7O2/c1-43-13-15-44(16-14-43)20-22-5-7-24(8-6-22)31-19-28-34(41-37(39)42-35(28)40-31)27-3-2-4-32(29(27)21-46)45-12-11-25-17-26(23-9-10-23)18-30(38)33(25)36(45)47/h2-8,11-12,17-19,23,46H,9-10,13-16,20-21H2,1H3,(H3,39,40,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trademark) MSA (commercially available kit manufactured by Carna Biosciences, Inc... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468312
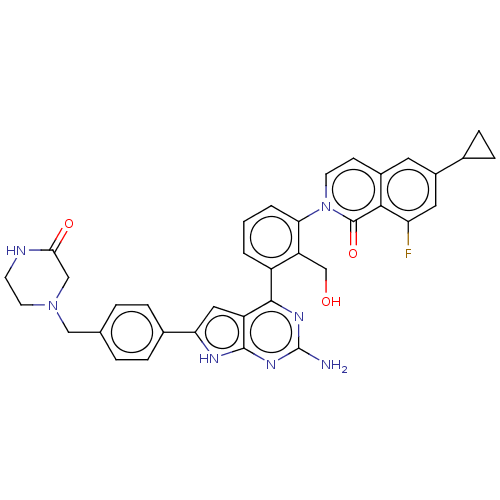
(2-[3-(2-amino-6-{4- [(3-oxopiperazin-1- yl)methyl]...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)-c1ccc(CN2CCNC(=O)C2)cc1 Show InChI InChI=1S/C36H32FN7O3/c37-28-15-24(21-8-9-21)14-23-10-12-44(35(47)32(23)28)30-3-1-2-25(27(30)19-45)33-26-16-29(40-34(26)42-36(38)41-33)22-6-4-20(5-7-22)17-43-13-11-39-31(46)18-43/h1-7,10,12,14-16,21,45H,8-9,11,13,17-19H2,(H,39,46)(H3,38,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468319
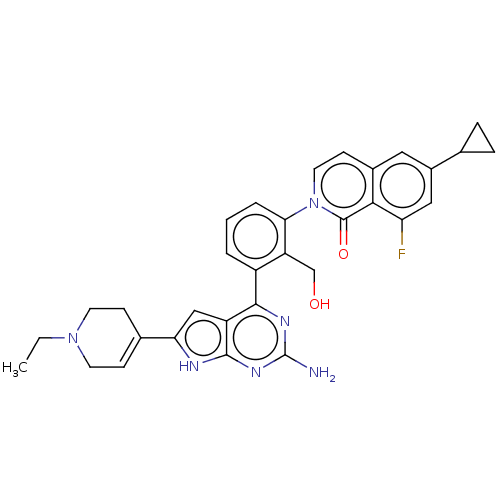
(2-{3-[2-amino-6-(1- ethyl-1,2,3,6- tetrahydropyrid...)Show SMILES CCN1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:5| Show InChI InChI=1S/C32H31FN6O2/c1-2-38-11-8-19(9-12-38)26-16-23-29(36-32(34)37-30(23)35-26)22-4-3-5-27(24(22)17-40)39-13-10-20-14-21(18-6-7-18)15-25(33)28(20)31(39)41/h3-5,8,10,13-16,18,40H,2,6-7,9,11-12,17H2,1H3,(H3,34,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465738
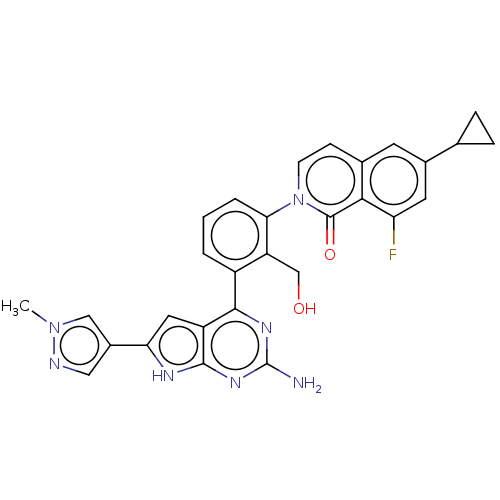
(2-{3-[2-amino-6-(1-methyl-1H-pyrazol- 4-yl)-7H-pyr...)Show SMILES Cn1cc(cn1)-c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C29H24FN7O2/c1-36-13-18(12-32-36)23-11-20-26(34-29(31)35-27(20)33-23)19-3-2-4-24(21(19)14-38)37-8-7-16-9-17(15-5-6-15)10-22(30)25(16)28(37)39/h2-4,7-13,15,38H,5-6,14H2,1H3,(H3,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
CARNA BIOSCIENCES, INC.
US Patent
| Assay Description
The kinase activity was measured using QuickScout Screening Assist (trade mark) MSA (commercially available kit manufactured by Carna Biosciences, In... |
US Patent US10793575 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0RSG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data