

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50091652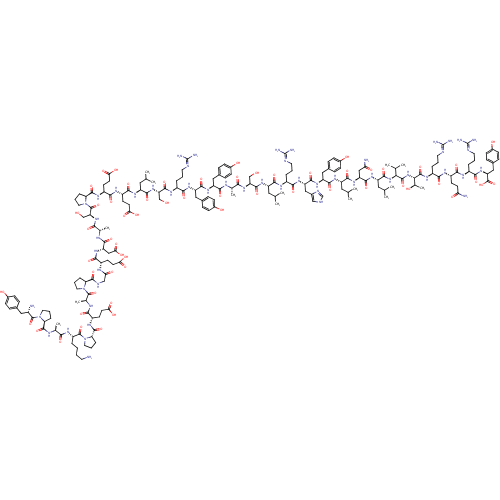 (CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50091652 (CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50091652 (CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM86732 (3-(5,6,7,8-tetrahydro-9-isopropyl-carbazol-3-yl)-1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM86732 (3-(5,6,7,8-tetrahydro-9-isopropyl-carbazol-3-yl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM86733 (5,5-DIMETHYL-2-(2,3,4,9-TETRAHYDRO-3,3-DIMETHYL-1O...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 562-70 (2006) Article DOI: 10.1124/jpet.105.099705 BindingDB Entry DOI: 10.7270/Q25Q4TPT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25052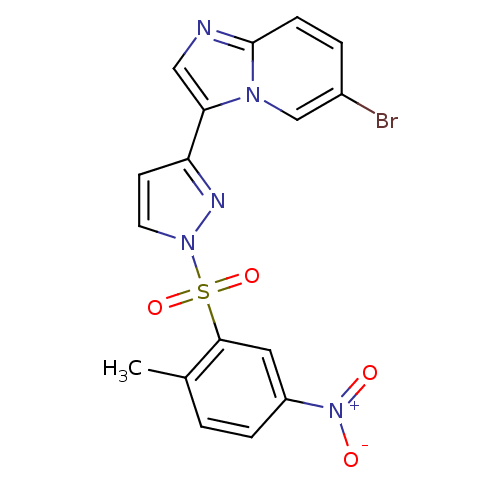 (3-{6-bromoimidazo[1,2-a]pyridin-3-yl}-1-[(2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 403-12 (2007) Article DOI: 10.1016/j.bmc.2006.09.047 BindingDB Entry DOI: 10.7270/Q2SJ1HXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25056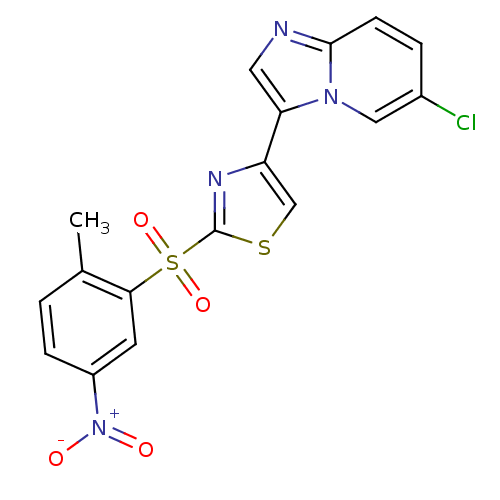 (4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 403-12 (2007) Article DOI: 10.1016/j.bmc.2006.09.047 BindingDB Entry DOI: 10.7270/Q2SJ1HXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25050 (3-{6-chloro-2-methylimidazo[1,2-a]pyridin-3-yl}-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 403-12 (2007) Article DOI: 10.1016/j.bmc.2006.09.047 BindingDB Entry DOI: 10.7270/Q2SJ1HXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428851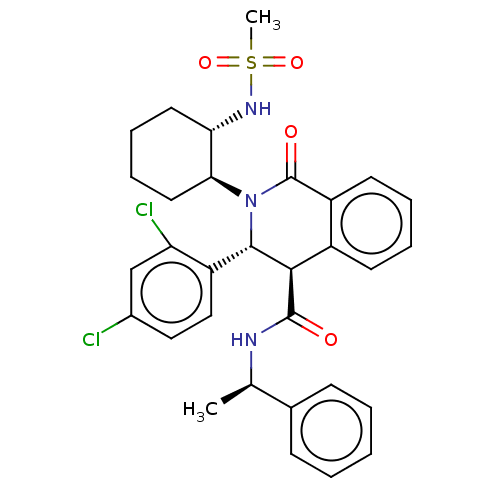 (US10532048, Example 236) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25030 (3-{6-bromo-2-methylimidazo[1,2-a]pyridin-3-yl}-1-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 403-12 (2007) Article DOI: 10.1016/j.bmc.2006.09.047 BindingDB Entry DOI: 10.7270/Q2SJ1HXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428855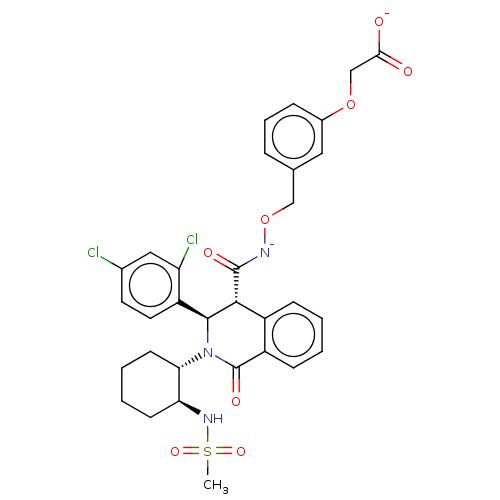 (US10532048, Example 631) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428852 (US10532048, Example 542) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428853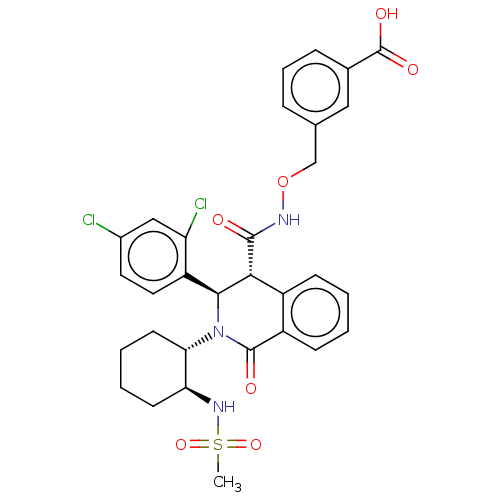 (US10532048, Example 560) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428854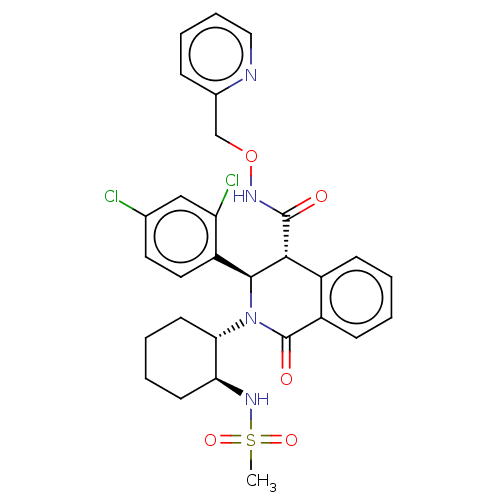 (US10532048, Example 589) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428856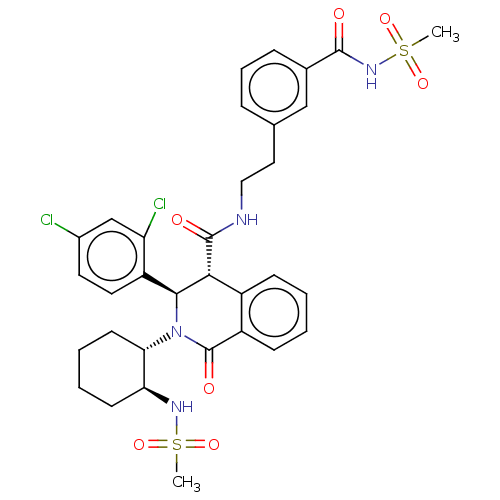 (US10532048, Example 700) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428859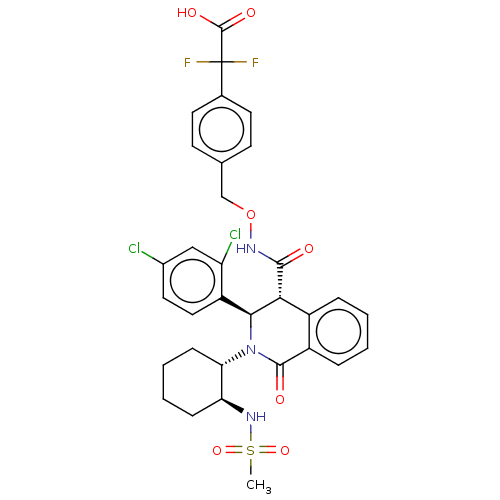 (US10532048, Example 712) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428860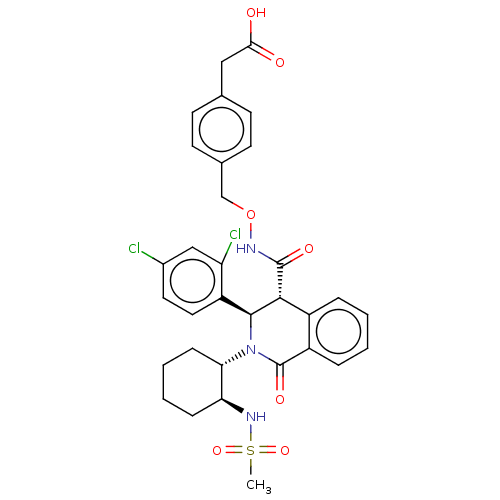 (US10532048, Example 856) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428857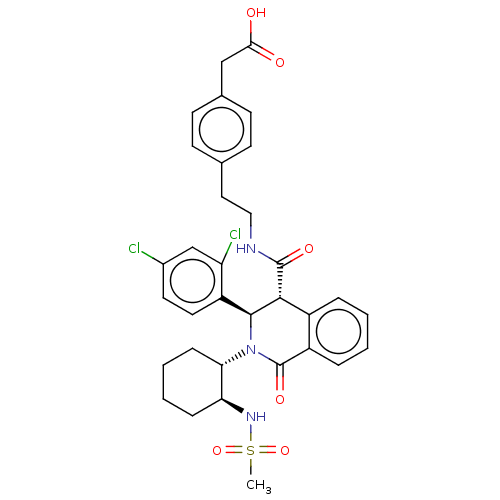 (US10532048, Example 701) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428858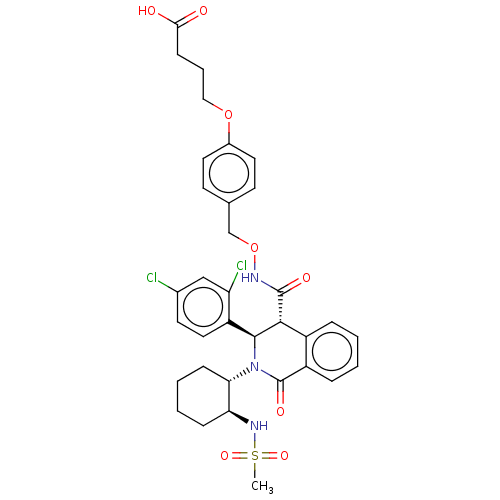 (US10532048, Example 709) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428841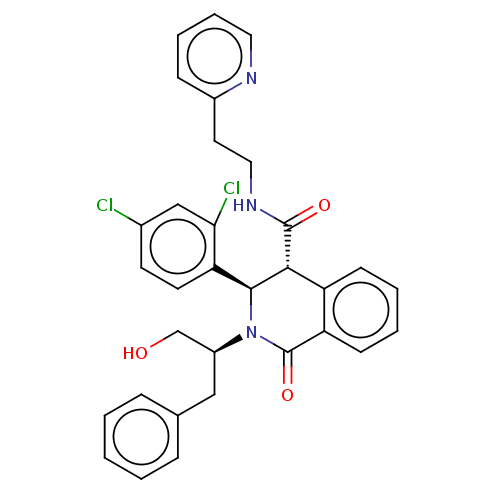 (US10532048, Example 61) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244126 (CHEMBL459464 | N-cycloheptyl-7-methoxy-2-(4-(pyrro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7021-32 (2008) Article DOI: 10.1016/j.bmc.2008.05.036 BindingDB Entry DOI: 10.7270/Q2TD9X51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244050 (C-014C | CHEMBL509396 | N-Cycloheptyl-2-[4-(cyclop...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7021-32 (2008) Article DOI: 10.1016/j.bmc.2008.05.036 BindingDB Entry DOI: 10.7270/Q2TD9X51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50243945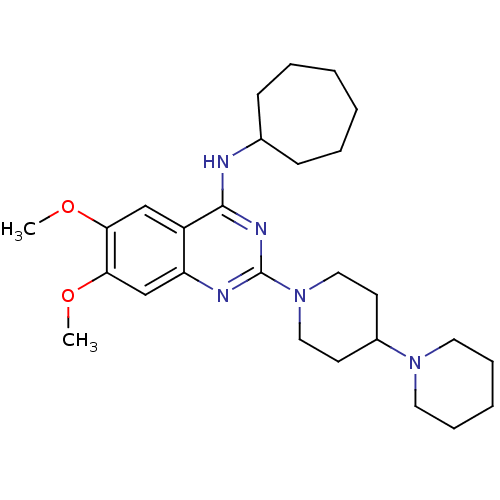 (2-(1,4'-Bipiperidine-1'-yl)-N-cycloheptyl-6,7-dime...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50243945 (2-(1,4'-Bipiperidine-1'-yl)-N-cycloheptyl-6,7-dime...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7021-32 (2008) Article DOI: 10.1016/j.bmc.2008.05.036 BindingDB Entry DOI: 10.7270/Q2TD9X51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244083 (CHEMBL451783 | N-cycloheptyl-2-(4-(cyclopentyl(met...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7021-32 (2008) Article DOI: 10.1016/j.bmc.2008.05.036 BindingDB Entry DOI: 10.7270/Q2TD9X51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM428850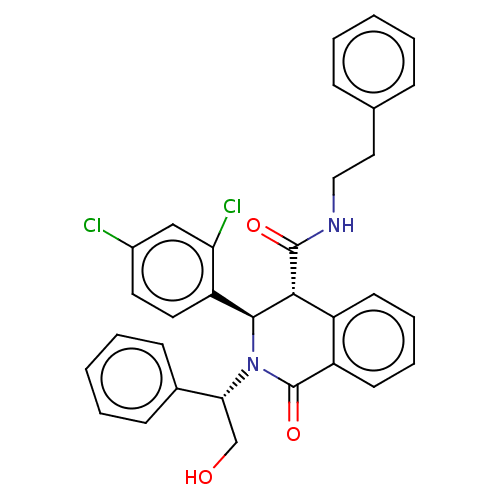 (US10532048, Example 62) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc. US Patent | Assay Description A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... | US Patent US10532048 (2020) BindingDB Entry DOI: 10.7270/Q22R3V1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244380 (CHEMBL487635 | N-Cycloheptyl-6,7-dimethoxy-2-(4-py...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50265742 (CHEMBL521737 | {1'-[4-(Cycloheptylamino)-6,7-dimet...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at mouse CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50265669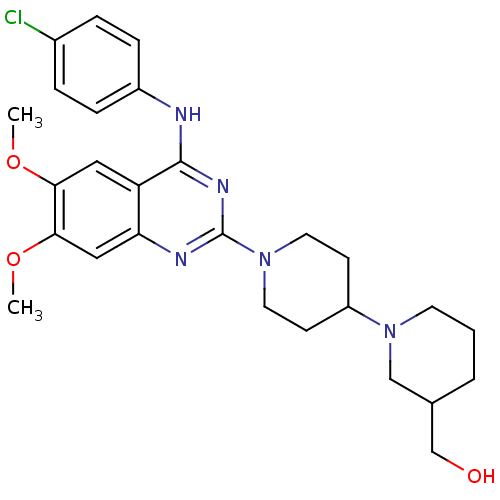 ((1'-{4-[(4-Chlorophenyl)amino]-6,7-dimethoxyquinaz...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244380 (CHEMBL487635 | N-Cycloheptyl-6,7-dimethoxy-2-(4-py...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7021-32 (2008) Article DOI: 10.1016/j.bmc.2008.05.036 BindingDB Entry DOI: 10.7270/Q2TD9X51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25057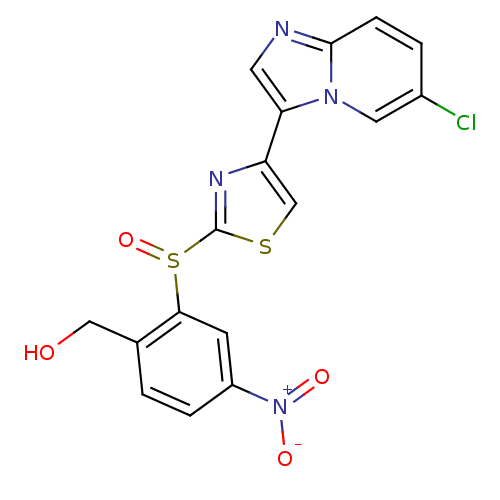 (imidazo[1,2-a]pyridine derivative, 14b | {2-[(4-{6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 403-12 (2007) Article DOI: 10.1016/j.bmc.2006.09.047 BindingDB Entry DOI: 10.7270/Q2SJ1HXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50265669 ((1'-{4-[(4-Chlorophenyl)amino]-6,7-dimethoxyquinaz...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50245036 (CHEMBL486840 | N-(4-Chlorophenyl)-6,7-dimethoxy-2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7968-74 (2008) Article DOI: 10.1016/j.bmc.2008.07.062 BindingDB Entry DOI: 10.7270/Q2QJ7H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50245036 (CHEMBL486840 | N-(4-Chlorophenyl)-6,7-dimethoxy-2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50245034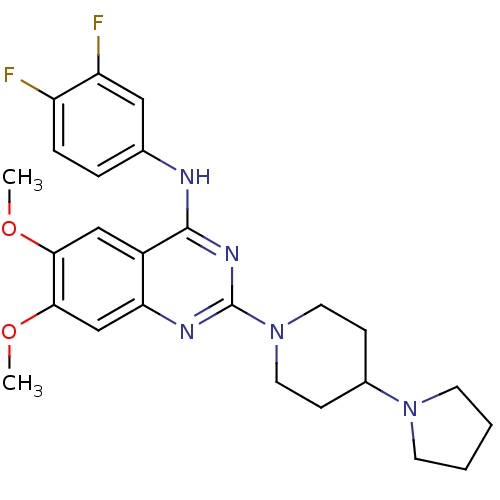 (CHEMBL488018 | N-(3,4-Difluorophenyl)-6,7-dimethox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7968-74 (2008) Article DOI: 10.1016/j.bmc.2008.07.062 BindingDB Entry DOI: 10.7270/Q2QJ7H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50265742 (CHEMBL521737 | {1'-[4-(Cycloheptylamino)-6,7-dimet...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244125 (CHEMBL516936 | N-cycloheptyl-6-methoxy-2-(4-(pyrro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7021-32 (2008) Article DOI: 10.1016/j.bmc.2008.05.036 BindingDB Entry DOI: 10.7270/Q2TD9X51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50245069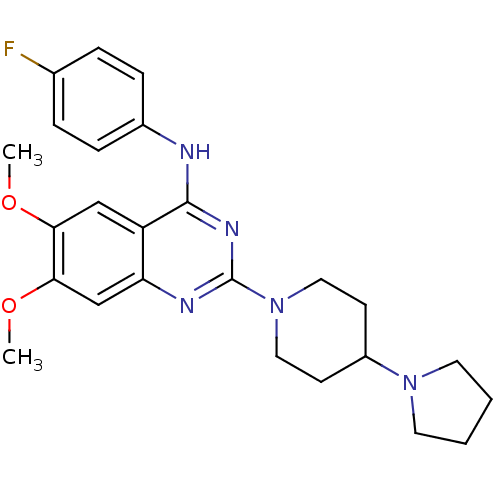 (CHEMBL487835 | N-(4-Fluorophenyl)-6,7-dimethoxy-2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7968-74 (2008) Article DOI: 10.1016/j.bmc.2008.07.062 BindingDB Entry DOI: 10.7270/Q2QJ7H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25055 (4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 403-12 (2007) Article DOI: 10.1016/j.bmc.2006.09.047 BindingDB Entry DOI: 10.7270/Q2SJ1HXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50243946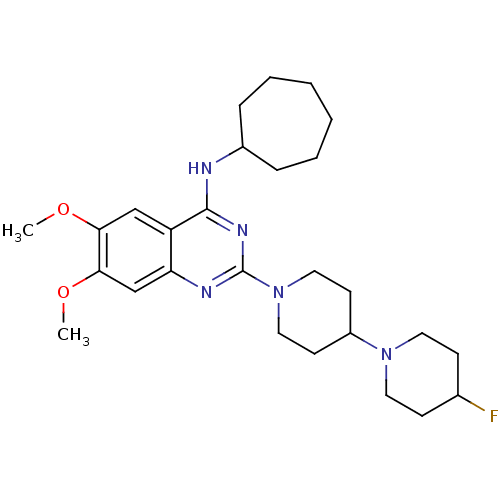 (CHEMBL453594 | N-cycloheptyl-2-(4-(4-fluoropiperid...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7021-32 (2008) Article DOI: 10.1016/j.bmc.2008.05.036 BindingDB Entry DOI: 10.7270/Q2TD9X51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50243945 (2-(1,4'-Bipiperidine-1'-yl)-N-cycloheptyl-6,7-dime...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at mouse CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50245071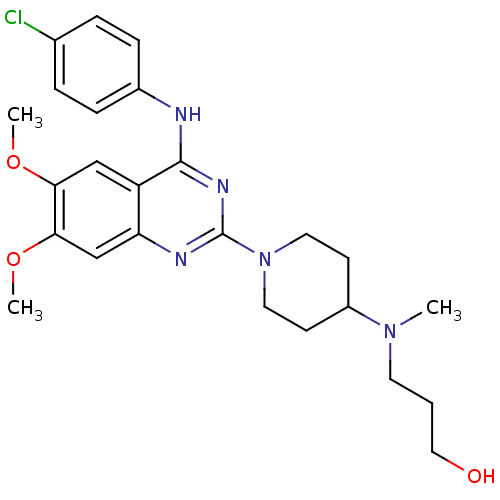 (3-[(1-{4-[(4-Chlorophenyl)amino]-6,7-dimethoxyquin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7968-74 (2008) Article DOI: 10.1016/j.bmc.2008.07.062 BindingDB Entry DOI: 10.7270/Q2QJ7H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50265667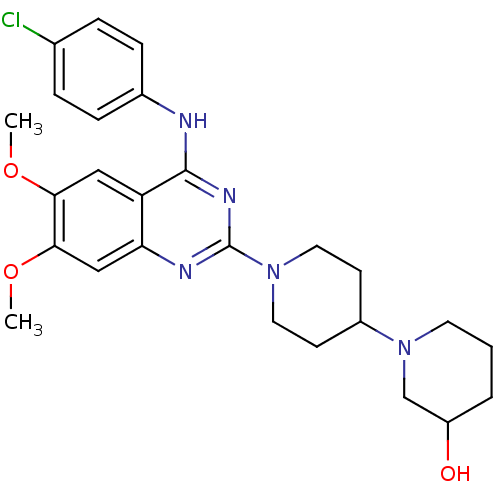 (1'-{4-[(4-Chlorophenyl)amino]-6,7-dimethoxyquinazo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50265670 (CHEMBL526474 | N-(4-Chlorophenyl)-6,7-dimethoxy-2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50265669 ((1'-{4-[(4-Chlorophenyl)amino]-6,7-dimethoxyquinaz...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at mouse CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50265749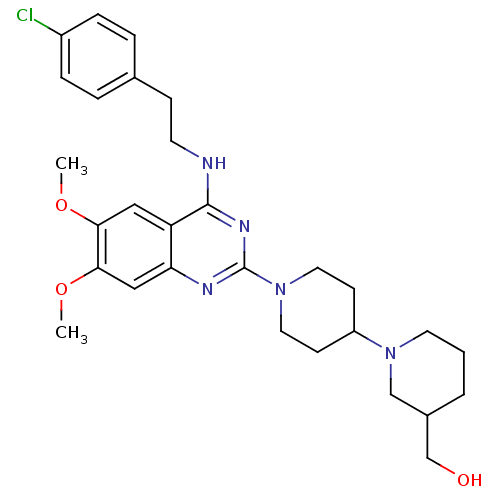 (1'-(4-{[2-(4-Chlorophenyl)ethyl]amino}-6,7-dimetho...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50245072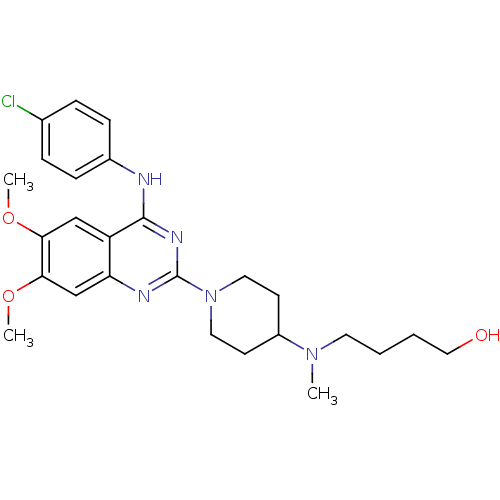 (4-[(1-{4-[(4-Chlorophenyl)amino]-6,7-dimethoxyquin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem 16: 7968-74 (2008) Article DOI: 10.1016/j.bmc.2008.07.062 BindingDB Entry DOI: 10.7270/Q2QJ7H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119 total ) | Next | Last >> |