 Found 184 hits with Last Name = 'jaeckel' and Initial = 'p'
Found 184 hits with Last Name = 'jaeckel' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072072
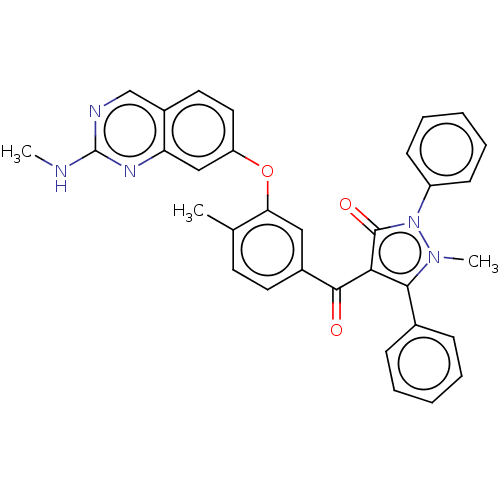
(CHEMBL3407849)Show SMILES CNc1ncc2ccc(Oc3cc(ccc3C)C(=O)c3c(-c4ccccc4)n(C)n(-c4ccccc4)c3=O)cc2n1 Show InChI InChI=1S/C33H27N5O3/c1-21-14-15-23(18-28(21)41-26-17-16-24-20-35-33(34-2)36-27(24)19-26)31(39)29-30(22-10-6-4-7-11-22)37(3)38(32(29)40)25-12-8-5-9-13-25/h4-20H,1-3H3,(H,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072075
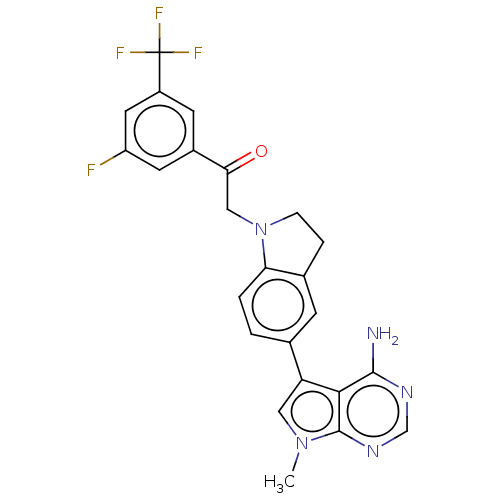
(CHEMBL3407846)Show SMILES Cn1cc(-c2ccc3N(CC(=O)c4cc(F)cc(c4)C(F)(F)F)CCc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C24H19F4N5O/c1-32-10-18(21-22(29)30-12-31-23(21)32)13-2-3-19-14(6-13)4-5-33(19)11-20(34)15-7-16(24(26,27)28)9-17(25)8-15/h2-3,6-10,12H,4-5,11H2,1H3,(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043768

(CHEMBL3355999)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 |r,wU:1.0,wD:4.7,(70.01,-31.83,;70.01,-30.29,;68.68,-29.52,;68.68,-27.99,;70.01,-27.22,;71.35,-27.97,;71.35,-29.52,;70.01,-25.68,;68.67,-24.91,;67.33,-25.69,;65.99,-24.92,;65.99,-23.38,;67.33,-22.61,;68.66,-23.37,;67.33,-21.07,;68.66,-20.3,;68.66,-18.76,;67.31,-17.99,;65.98,-18.76,;66,-20.3,;64.66,-21.07,;63.33,-20.31,;63.32,-18.75,;61.98,-17.99,;60.65,-18.76,;59.32,-17.99,;57.99,-18.76,;57.83,-20.29,;56.32,-20.61,;55.56,-21.94,;54.02,-21.94,;53.24,-20.61,;54.01,-19.27,;55.55,-19.27,;56.58,-18.13,;60.65,-20.31,;59.32,-21.08,;59.32,-22.62,;60.67,-23.39,;61.99,-22.61,;61.99,-21.08,)| Show InChI InChI=1S/C32H30N8O/c33-20-11-13-21(14-12-20)36-31-35-19-17-26(37-31)24-8-5-18-34-30(24)41-29-16-15-25(22-6-1-2-7-23(22)29)38-32-39-27-9-3-4-10-28(27)40-32/h1-10,15-21H,11-14,33H2,(H,35,36,37)(H2,38,39,40)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043704

(CHEMBL3356001)Show SMILES Cc1cc(Nc2nc3ccccc3[nH]2)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:37.42,wD:34.38,(29.58,-30.1,;28.24,-30.87,;26.91,-30.11,;25.58,-30.88,;24.24,-30.11,;22.91,-30.88,;22.75,-32.41,;21.24,-32.73,;20.48,-34.06,;18.95,-34.06,;18.17,-32.73,;18.94,-31.39,;20.47,-31.39,;21.5,-30.25,;25.58,-32.43,;24.25,-33.2,;24.25,-34.74,;25.59,-35.51,;26.92,-34.73,;26.91,-33.2,;28.25,-32.43,;29.59,-33.19,;30.92,-32.42,;30.91,-30.88,;32.23,-30.11,;33.58,-30.88,;33.58,-32.42,;32.25,-33.19,;32.25,-34.73,;30.92,-35.5,;30.92,-37.04,;32.25,-37.81,;33.59,-37.03,;34.93,-37.8,;34.93,-39.34,;33.6,-40.1,;33.6,-41.64,;34.94,-42.41,;34.94,-43.95,;36.27,-41.64,;36.27,-40.09,;33.58,-35.49,)| Show InChI InChI=1S/C33H32N8O/c1-20-19-29(41-33-39-27-10-4-5-11-28(27)40-33)23-7-2-3-8-24(23)30(20)42-31-25(9-6-17-35-31)26-16-18-36-32(38-26)37-22-14-12-21(34)13-15-22/h2-11,16-19,21-22H,12-15,34H2,1H3,(H,36,37,38)(H2,39,40,41)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072038

(CHEMBL3407863)Show SMILES Cc1nc(N)c2c(cn(C)c2n1)-c1ccc2N(CC(=O)c3cc(F)cc(c3)C(F)(F)F)CCc2c1 Show InChI InChI=1S/C25H21F4N5O/c1-13-31-23(30)22-19(11-33(2)24(22)32-13)14-3-4-20-15(7-14)5-6-34(20)12-21(35)16-8-17(25(27,28)29)10-18(26)9-16/h3-4,7-11H,5-6,12H2,1-2H3,(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072070
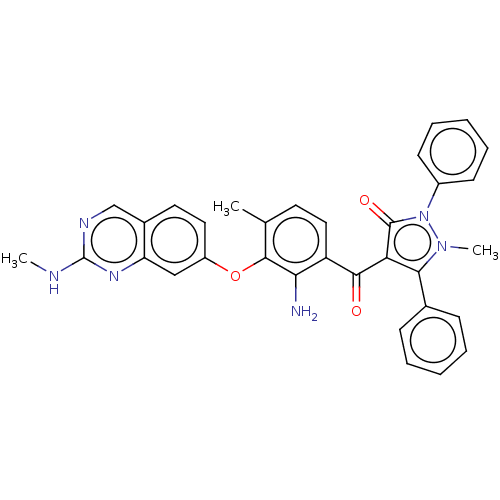
(CHEMBL3407504)Show SMILES CNc1ncc2ccc(Oc3c(C)ccc(C(=O)c4c(-c5ccccc5)n(C)n(-c5ccccc5)c4=O)c3N)cc2n1 Show InChI InChI=1S/C33H28N6O3/c1-20-14-17-25(28(34)31(20)42-24-16-15-22-19-36-33(35-2)37-26(22)18-24)30(40)27-29(21-10-6-4-7-11-21)38(3)39(32(27)41)23-12-8-5-9-13-23/h4-19H,34H2,1-3H3,(H,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50071957
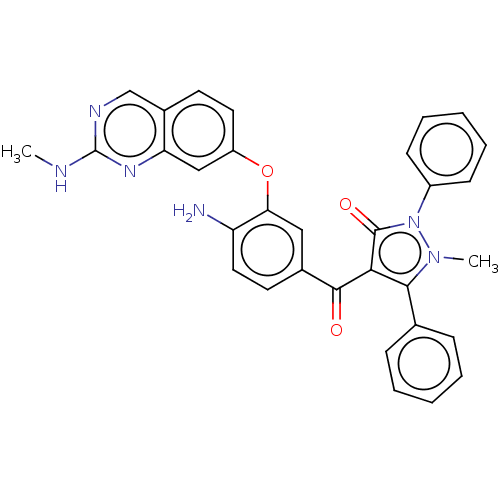
(CHEMBL3407850)Show SMILES CNc1ncc2ccc(Oc3cc(ccc3N)C(=O)c3c(-c4ccccc4)n(C)n(-c4ccccc4)c3=O)cc2n1 Show InChI InChI=1S/C32H26N6O3/c1-34-32-35-19-22-13-15-24(18-26(22)36-32)41-27-17-21(14-16-25(27)33)30(39)28-29(20-9-5-3-6-10-20)37(2)38(31(28)40)23-11-7-4-8-12-23/h3-19H,33H2,1-2H3,(H,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072073
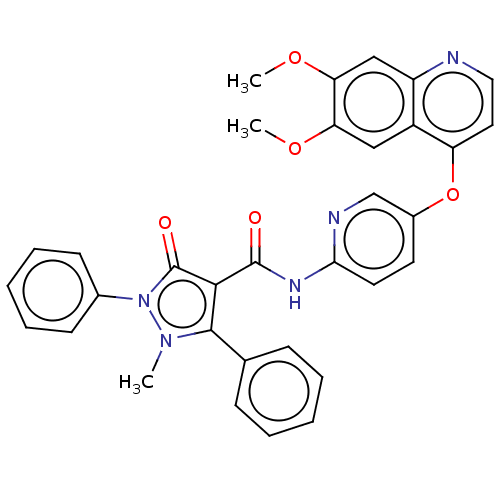
(CHEMBL3407848)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)c4c(-c5ccccc5)n(C)n(-c5ccccc5)c4=O)nc3)c2cc1OC Show InChI InChI=1S/C33H27N5O5/c1-37-31(21-10-6-4-7-11-21)30(33(40)38(37)22-12-8-5-9-13-22)32(39)36-29-15-14-23(20-35-29)43-26-16-17-34-25-19-28(42-3)27(41-2)18-24(25)26/h4-20H,1-3H3,(H,35,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072027
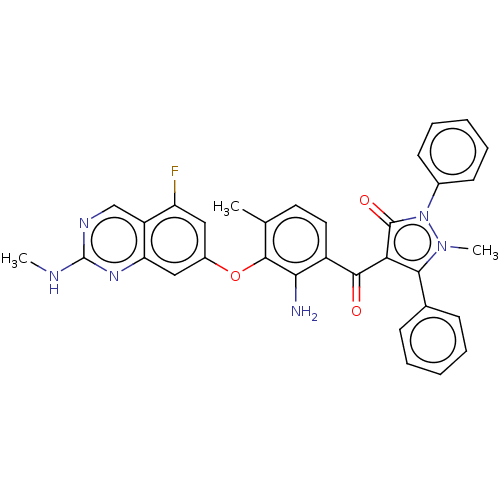
(CHEMBL3407852)Show SMILES CNc1ncc2c(F)cc(Oc3c(C)ccc(C(=O)c4c(-c5ccccc5)n(C)n(-c5ccccc5)c4=O)c3N)cc2n1 Show InChI InChI=1S/C33H27FN6O3/c1-19-14-15-23(28(35)31(19)43-22-16-25(34)24-18-37-33(36-2)38-26(24)17-22)30(41)27-29(20-10-6-4-7-11-20)39(3)40(32(27)42)21-12-8-5-9-13-21/h4-18H,35H2,1-3H3,(H,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072030
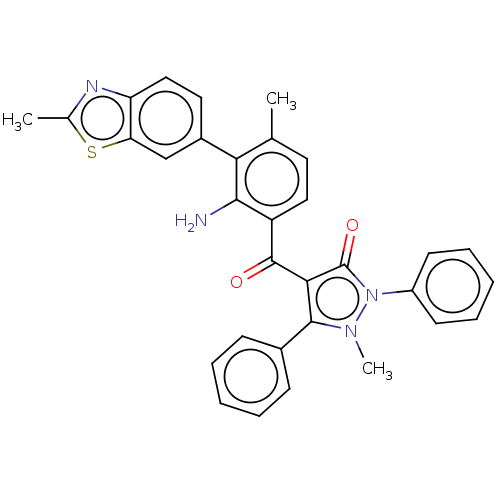
(CHEMBL3407856)Show SMILES Cc1nc2ccc(cc2s1)-c1c(C)ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c1N Show InChI InChI=1S/C32H26N4O2S/c1-19-14-16-24(29(33)27(19)22-15-17-25-26(18-22)39-20(2)34-25)31(37)28-30(21-10-6-4-7-11-21)35(3)36(32(28)38)23-12-8-5-9-13-23/h4-18H,33H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072026

(CHEMBL3407851)Show SMILES CNc1ncc2ccc(Oc3cc(ccc3Cl)C(=O)c3c(-c4ccccc4)n(C)n(-c4ccccc4)c3=O)cc2n1 Show InChI InChI=1S/C32H24ClN5O3/c1-34-32-35-19-22-13-15-24(18-26(22)36-32)41-27-17-21(14-16-25(27)33)30(39)28-29(20-9-5-3-6-10-20)37(2)38(31(28)40)23-11-7-4-8-12-23/h3-19H,1-2H3,(H,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072029
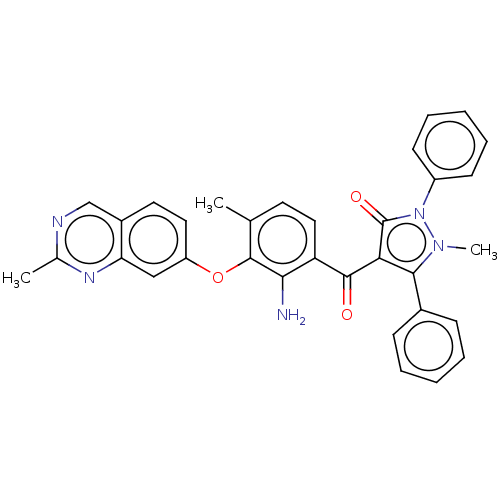
(CHEMBL3407854)Show SMILES Cc1ncc2ccc(Oc3c(C)ccc(C(=O)c4c(-c5ccccc5)n(C)n(-c5ccccc5)c4=O)c3N)cc2n1 Show InChI InChI=1S/C33H27N5O3/c1-20-14-17-26(29(34)32(20)41-25-16-15-23-19-35-21(2)36-27(23)18-25)31(39)28-30(22-10-6-4-7-11-22)37(3)38(33(28)40)24-12-8-5-9-13-24/h4-19H,34H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50072072

(CHEMBL3407849)Show SMILES CNc1ncc2ccc(Oc3cc(ccc3C)C(=O)c3c(-c4ccccc4)n(C)n(-c4ccccc4)c3=O)cc2n1 Show InChI InChI=1S/C33H27N5O3/c1-21-14-15-23(18-28(21)41-26-17-16-24-20-35-33(34-2)36-27(24)19-26)31(39)29-30(22-10-6-4-7-11-22)37(3)38(32(29)40)25-12-8-5-9-13-25/h4-20H,1-3H3,(H,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His-tagged GCN2 expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043705
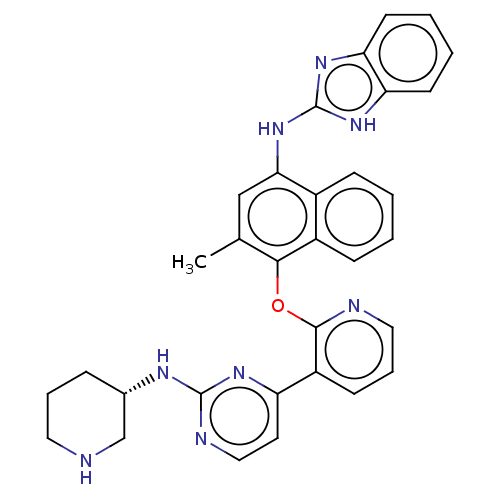
(CHEMBL3356002)Show SMILES Cc1cc(Nc2nc3ccccc3[nH]2)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CCCNC2)n1 |r| Show InChI InChI=1S/C32H30N8O/c1-20-18-28(40-32-38-26-12-4-5-13-27(26)39-32)22-9-2-3-10-23(22)29(20)41-30-24(11-7-16-34-30)25-14-17-35-31(37-25)36-21-8-6-15-33-19-21/h2-5,7,9-14,16-18,21,33H,6,8,15,19H2,1H3,(H,35,36,37)(H2,38,39,40)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043771
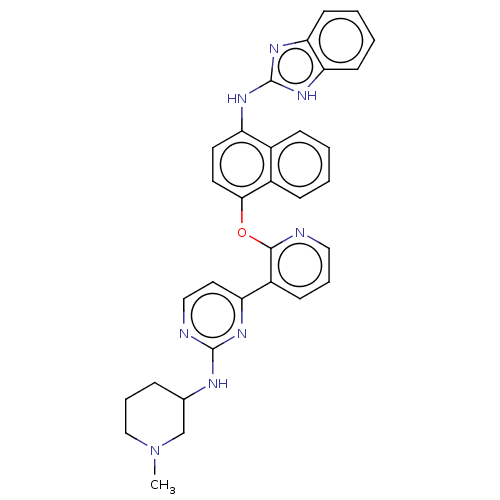
(CHEMBL3355996)Show SMILES CN1CCCC(C1)Nc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 Show InChI InChI=1S/C32H30N8O/c1-40-19-7-8-21(20-40)35-31-34-18-16-26(36-31)24-11-6-17-33-30(24)41-29-15-14-25(22-9-2-3-10-23(22)29)37-32-38-27-12-4-5-13-28(27)39-32/h2-6,9-18,21H,7-8,19-20H2,1H3,(H,34,35,36)(H2,37,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072075

(CHEMBL3407846)Show SMILES Cn1cc(-c2ccc3N(CC(=O)c4cc(F)cc(c4)C(F)(F)F)CCc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C24H19F4N5O/c1-32-10-18(21-22(29)30-12-31-23(21)32)13-2-3-19-14(6-13)4-5-33(19)11-20(34)15-7-16(24(26,27)28)9-17(25)8-15/h2-3,6-10,12H,4-5,11H2,1H3,(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of doxycycline-inducible T-REx-PERK-FLAG (unknown origin) autophosphorylation tranfected in human HT1080 cells after 1 hr by sandwich ELIS... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072033
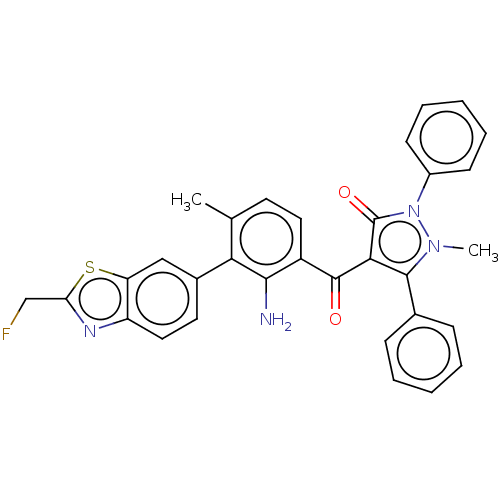
(CHEMBL3407859)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(CF)sc2c1 Show InChI InChI=1S/C32H25FN4O2S/c1-19-13-15-23(29(34)27(19)21-14-16-24-25(17-21)40-26(18-33)35-24)31(38)28-30(20-9-5-3-6-10-20)36(2)37(32(28)39)22-11-7-4-8-12-22/h3-17H,18,34H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072036

(CHEMBL3407862)Show SMILES Cc1ccc2cc(ccc2n1)-c1c(C)ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c1N Show InChI InChI=1S/C34H28N4O2/c1-21-14-18-27(31(35)29(21)25-17-19-28-24(20-25)16-15-22(2)36-28)33(39)30-32(23-10-6-4-7-11-23)37(3)38(34(30)40)26-12-8-5-9-13-26/h4-20H,35H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072028
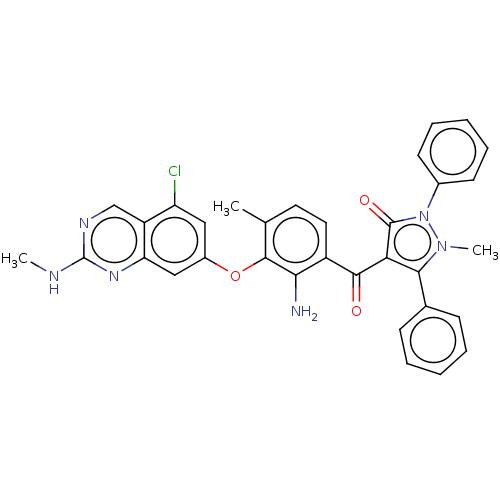
(CHEMBL3407853)Show SMILES CNc1ncc2c(Cl)cc(Oc3c(C)ccc(C(=O)c4c(-c5ccccc5)n(C)n(-c5ccccc5)c4=O)c3N)cc2n1 Show InChI InChI=1S/C33H27ClN6O3/c1-19-14-15-23(28(35)31(19)43-22-16-25(34)24-18-37-33(36-2)38-26(24)17-22)30(41)27-29(20-10-6-4-7-11-20)39(3)40(32(27)42)21-12-8-5-9-13-21/h4-18H,35H2,1-3H3,(H,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043704

(CHEMBL3356001)Show SMILES Cc1cc(Nc2nc3ccccc3[nH]2)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:37.42,wD:34.38,(29.58,-30.1,;28.24,-30.87,;26.91,-30.11,;25.58,-30.88,;24.24,-30.11,;22.91,-30.88,;22.75,-32.41,;21.24,-32.73,;20.48,-34.06,;18.95,-34.06,;18.17,-32.73,;18.94,-31.39,;20.47,-31.39,;21.5,-30.25,;25.58,-32.43,;24.25,-33.2,;24.25,-34.74,;25.59,-35.51,;26.92,-34.73,;26.91,-33.2,;28.25,-32.43,;29.59,-33.19,;30.92,-32.42,;30.91,-30.88,;32.23,-30.11,;33.58,-30.88,;33.58,-32.42,;32.25,-33.19,;32.25,-34.73,;30.92,-35.5,;30.92,-37.04,;32.25,-37.81,;33.59,-37.03,;34.93,-37.8,;34.93,-39.34,;33.6,-40.1,;33.6,-41.64,;34.94,-42.41,;34.94,-43.95,;36.27,-41.64,;36.27,-40.09,;33.58,-35.49,)| Show InChI InChI=1S/C33H32N8O/c1-20-19-29(41-33-39-27-10-4-5-11-28(27)40-33)23-7-2-3-8-24(23)30(20)42-31-25(9-6-17-35-31)26-16-18-36-32(38-26)37-22-14-12-21(34)13-15-22/h2-11,16-19,21-22H,12-15,34H2,1H3,(H,36,37,38)(H2,39,40,41)/t21-,22- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043706
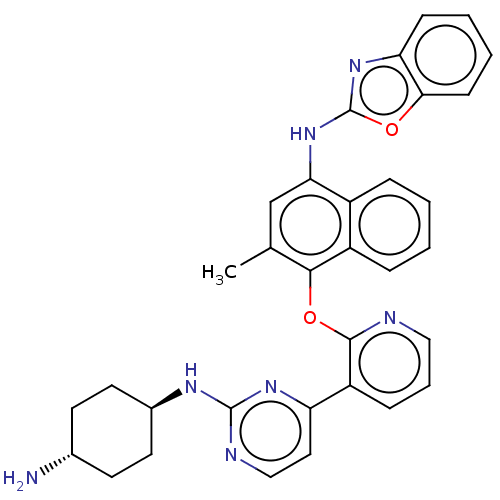
(CHEMBL3356003)Show SMILES Cc1cc(Nc2nc3ccccc3o2)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:37.42,wD:34.38,(11.12,-44.15,;9.78,-44.92,;8.45,-44.16,;7.12,-44.93,;5.78,-44.16,;4.45,-44.93,;4.29,-46.46,;2.78,-46.78,;2.01,-48.11,;.48,-48.11,;-.3,-46.78,;.48,-45.44,;2.01,-45.45,;3.04,-44.3,;7.12,-46.47,;5.78,-47.24,;5.79,-48.79,;7.13,-49.55,;8.46,-48.78,;8.45,-47.25,;9.79,-46.47,;11.13,-47.24,;12.46,-46.47,;12.45,-44.93,;13.77,-44.16,;15.12,-44.92,;15.12,-46.47,;13.79,-47.24,;13.79,-48.78,;12.46,-49.55,;12.46,-51.09,;13.79,-51.86,;15.13,-51.08,;16.47,-51.84,;17.8,-51.07,;19.12,-51.84,;20.45,-51.07,;20.45,-49.53,;21.79,-48.76,;19.12,-48.76,;17.78,-49.53,;15.12,-49.54,)| Show InChI InChI=1S/C33H31N7O2/c1-20-19-28(40-33-39-27-10-4-5-11-29(27)41-33)23-7-2-3-8-24(23)30(20)42-31-25(9-6-17-35-31)26-16-18-36-32(38-26)37-22-14-12-21(34)13-15-22/h2-11,16-19,21-22H,12-15,34H2,1H3,(H,39,40)(H,36,37,38)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043703
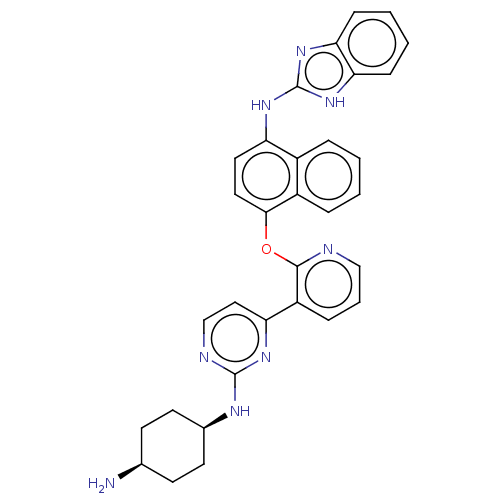
(CHEMBL3356000)Show SMILES N[C@H]1CC[C@H](CC1)Nc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 |r,wU:4.7,1.0,(18.1,-43.96,;18.1,-42.42,;16.76,-41.64,;16.76,-40.11,;18.1,-39.34,;19.44,-40.1,;19.43,-41.64,;18.09,-37.8,;16.75,-37.03,;15.41,-37.81,;14.08,-37.04,;14.08,-35.5,;15.42,-34.73,;16.75,-35.5,;15.41,-33.19,;16.74,-32.42,;16.74,-30.88,;15.4,-30.11,;14.07,-30.89,;14.08,-32.42,;12.75,-33.2,;11.41,-32.43,;11.41,-30.88,;10.07,-30.11,;8.74,-30.88,;7.4,-30.11,;6.07,-30.88,;5.91,-32.41,;4.4,-32.73,;3.64,-34.06,;2.11,-34.07,;1.33,-32.73,;2.1,-31.4,;3.63,-31.4,;4.66,-30.25,;8.74,-32.43,;7.41,-33.2,;7.41,-34.74,;8.75,-35.51,;10.08,-34.73,;10.07,-33.2,)| Show InChI InChI=1S/C32H30N8O/c33-20-11-13-21(14-12-20)36-31-35-19-17-26(37-31)24-8-5-18-34-30(24)41-29-16-15-25(22-6-1-2-7-23(22)29)38-32-39-27-9-3-4-10-28(27)40-32/h1-10,15-21H,11-14,33H2,(H,35,36,37)(H2,38,39,40)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50072074

(CHEMBL3407847)Show SMILES CNc1ncc2cc(ccc2n1)-c1c(C)ccc(C(=O)Nc2ccc(OC)c(c2)C(F)(F)F)c1N Show InChI InChI=1S/C25H22F3N5O2/c1-13-4-7-17(23(34)32-16-6-9-20(35-3)18(11-16)25(26,27)28)22(29)21(13)14-5-8-19-15(10-14)12-31-24(30-2)33-19/h4-12H,29H2,1-3H3,(H,32,34)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant BRAF V600E kinase domain mutant (unknown origin) |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50072072

(CHEMBL3407849)Show SMILES CNc1ncc2ccc(Oc3cc(ccc3C)C(=O)c3c(-c4ccccc4)n(C)n(-c4ccccc4)c3=O)cc2n1 Show InChI InChI=1S/C33H27N5O3/c1-21-14-15-23(18-28(21)41-26-17-16-24-20-35-33(34-2)36-27(24)19-26)31(39)29-30(22-10-6-4-7-11-22)37(3)38(32(29)40)25-12-8-5-9-13-25/h4-20H,1-3H3,(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant BRAF V600E kinase domain mutant (unknown origin) |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072031
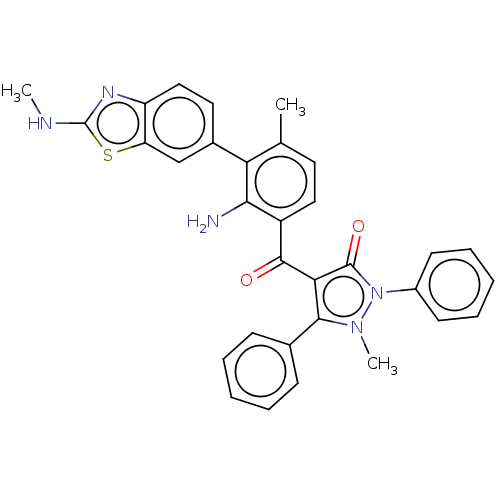
(CHEMBL3407857)Show SMILES CNc1nc2ccc(cc2s1)-c1c(C)ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c1N Show InChI InChI=1S/C32H27N5O2S/c1-19-14-16-23(28(33)26(19)21-15-17-24-25(18-21)40-32(34-2)35-24)30(38)27-29(20-10-6-4-7-11-20)36(3)37(31(27)39)22-12-8-5-9-13-22/h4-18H,33H2,1-3H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043769
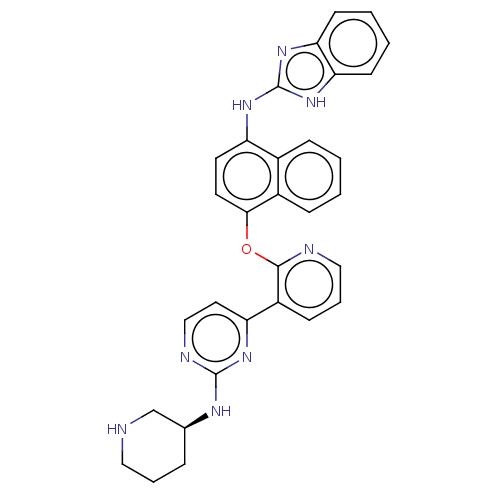
(CHEMBL3355998)Show SMILES C1CNC[C@H](C1)Nc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 |r| Show InChI InChI=1S/C31H28N8O/c1-2-9-22-21(8-1)24(37-31-38-26-11-3-4-12-27(26)39-31)13-14-28(22)40-29-23(10-6-17-33-29)25-15-18-34-30(36-25)35-20-7-5-16-32-19-20/h1-4,6,8-15,17-18,20,32H,5,7,16,19H2,(H,34,35,36)(H2,37,38,39)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50072026

(CHEMBL3407851)Show SMILES CNc1ncc2ccc(Oc3cc(ccc3Cl)C(=O)c3c(-c4ccccc4)n(C)n(-c4ccccc4)c3=O)cc2n1 Show InChI InChI=1S/C32H24ClN5O3/c1-34-32-35-19-22-13-15-24(18-26(22)36-32)41-27-17-21(14-16-25(27)33)30(39)28-29(20-9-5-3-6-10-20)37(2)38(31(28)40)23-11-7-4-8-12-23/h3-19H,1-2H3,(H,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His-tagged GCN2 expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043768

(CHEMBL3355999)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 |r,wU:1.0,wD:4.7,(70.01,-31.83,;70.01,-30.29,;68.68,-29.52,;68.68,-27.99,;70.01,-27.22,;71.35,-27.97,;71.35,-29.52,;70.01,-25.68,;68.67,-24.91,;67.33,-25.69,;65.99,-24.92,;65.99,-23.38,;67.33,-22.61,;68.66,-23.37,;67.33,-21.07,;68.66,-20.3,;68.66,-18.76,;67.31,-17.99,;65.98,-18.76,;66,-20.3,;64.66,-21.07,;63.33,-20.31,;63.32,-18.75,;61.98,-17.99,;60.65,-18.76,;59.32,-17.99,;57.99,-18.76,;57.83,-20.29,;56.32,-20.61,;55.56,-21.94,;54.02,-21.94,;53.24,-20.61,;54.01,-19.27,;55.55,-19.27,;56.58,-18.13,;60.65,-20.31,;59.32,-21.08,;59.32,-22.62,;60.67,-23.39,;61.99,-22.61,;61.99,-21.08,)| Show InChI InChI=1S/C32H30N8O/c33-20-11-13-21(14-12-20)36-31-35-19-17-26(37-31)24-8-5-18-34-30(24)41-29-16-15-25(22-6-1-2-7-23(22)29)38-32-39-27-9-3-4-10-28(27)40-32/h1-10,15-21H,11-14,33H2,(H,35,36,37)(H2,38,39,40)/t20-,21- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072032
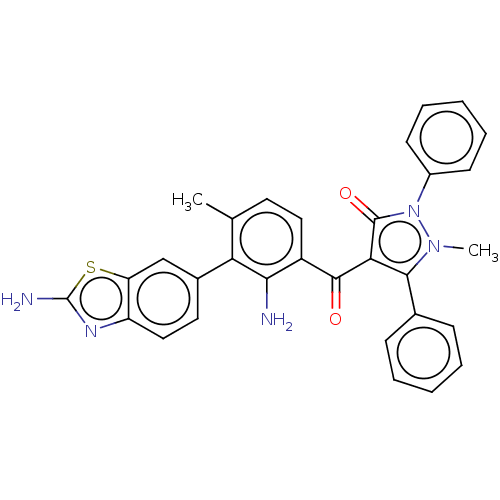
(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072034

(CHEMBL3407860)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(sc2c1)C(F)F Show InChI InChI=1S/C32H24F2N4O2S/c1-18-13-15-22(27(35)25(18)20-14-16-23-24(17-20)41-31(36-23)30(33)34)29(39)26-28(19-9-5-3-6-10-19)37(2)38(32(26)40)21-11-7-4-8-12-21/h3-17,30H,35H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50072074

(CHEMBL3407847)Show SMILES CNc1ncc2cc(ccc2n1)-c1c(C)ccc(C(=O)Nc2ccc(OC)c(c2)C(F)(F)F)c1N Show InChI InChI=1S/C25H22F3N5O2/c1-13-4-7-17(23(34)32-16-6-9-20(35-3)18(11-16)25(26,27)28)22(29)21(13)14-5-8-19-15(10-14)12-31-24(30-2)33-19/h4-12H,29H2,1-3H3,(H,32,34)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) transfected in human A375 cells assessed as inhibition of ERK phosphorylation by Western blot analys... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043772
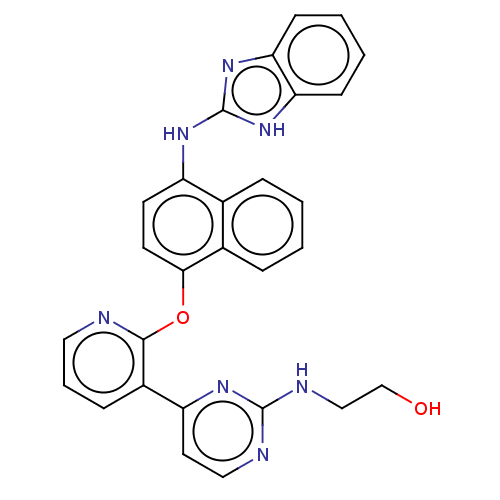
(CHEMBL3355995)Show SMILES OCCNc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 Show InChI InChI=1S/C28H23N7O2/c36-17-16-31-27-30-15-13-22(32-27)20-8-5-14-29-26(20)37-25-12-11-21(18-6-1-2-7-19(18)25)33-28-34-23-9-3-4-10-24(23)35-28/h1-15,36H,16-17H2,(H,30,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072070

(CHEMBL3407504)Show SMILES CNc1ncc2ccc(Oc3c(C)ccc(C(=O)c4c(-c5ccccc5)n(C)n(-c5ccccc5)c4=O)c3N)cc2n1 Show InChI InChI=1S/C33H28N6O3/c1-20-14-17-25(28(34)31(20)42-24-16-15-22-19-36-33(35-2)37-26(22)18-24)30(40)27-29(21-10-6-4-7-11-21)38(3)39(32(27)41)23-12-8-5-9-13-23/h4-19H,34H2,1-3H3,(H,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of doxycycline-inducible T-REx-PERK-FLAG (unknown origin) autophosphorylation tranfected in human HT1080 cells after 1 hr by sandwich ELIS... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50071957

(CHEMBL3407850)Show SMILES CNc1ncc2ccc(Oc3cc(ccc3N)C(=O)c3c(-c4ccccc4)n(C)n(-c4ccccc4)c3=O)cc2n1 Show InChI InChI=1S/C32H26N6O3/c1-34-32-35-19-22-13-15-24(18-26(22)36-32)41-27-17-21(14-16-25(27)33)30(39)28-29(20-9-5-3-6-10-20)37(2)38(31(28)40)23-11-7-4-8-12-23/h3-19H,33H2,1-2H3,(H,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His-tagged GCN2 expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072038

(CHEMBL3407863)Show SMILES Cc1nc(N)c2c(cn(C)c2n1)-c1ccc2N(CC(=O)c3cc(F)cc(c3)C(F)(F)F)CCc2c1 Show InChI InChI=1S/C25H21F4N5O/c1-13-31-23(30)22-19(11-33(2)24(22)32-13)14-3-4-20-15(7-14)5-6-34(20)12-21(35)16-8-17(25(27,28)29)10-18(26)9-16/h3-4,7-11H,5-6,12H2,1-2H3,(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of doxycycline-inducible T-REx-PERK-FLAG (unknown origin) autophosphorylation tranfected in human HT1080 cells after 1 hr by sandwich ELIS... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043709
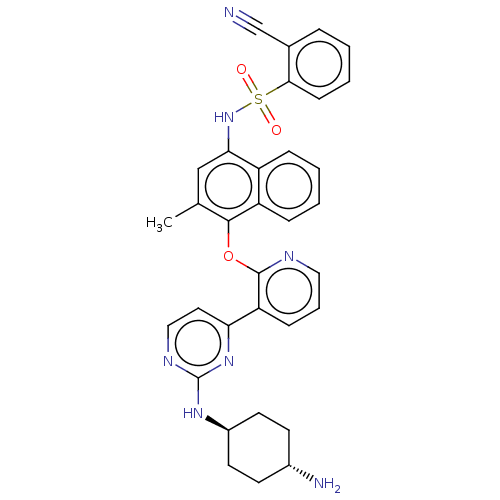
(CHEMBL3356006)Show SMILES Cc1cc(NS(=O)(=O)c2ccccc2C#N)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:39.43,wD:36.39,(11.6,-3.63,;10.27,-4.4,;8.93,-3.64,;7.6,-4.41,;6.27,-3.64,;4.93,-4.41,;5.69,-5.74,;4.16,-5.73,;3.6,-3.64,;3.61,-2.1,;2.28,-1.33,;.94,-2.1,;.95,-3.65,;2.28,-4.41,;2.29,-5.95,;2.29,-7.49,;7.6,-5.95,;6.27,-6.72,;6.27,-8.26,;7.62,-9.03,;8.94,-8.26,;8.94,-6.72,;10.27,-5.95,;11.61,-6.72,;12.94,-5.95,;12.93,-4.41,;14.25,-3.64,;15.6,-4.41,;15.6,-5.95,;14.27,-6.72,;14.27,-8.25,;12.94,-9.02,;12.94,-10.56,;14.27,-11.33,;15.61,-10.56,;16.95,-11.32,;18.28,-10.55,;19.6,-11.32,;20.93,-10.55,;20.93,-9.01,;22.27,-8.24,;19.6,-8.24,;18.26,-9.01,;15.6,-9.02,)| Show InChI InChI=1S/C33H31N7O3S/c1-21-19-29(40-44(41,42)30-11-5-2-7-22(30)20-34)25-8-3-4-9-26(25)31(21)43-32-27(10-6-17-36-32)28-16-18-37-33(39-28)38-24-14-12-23(35)13-15-24/h2-11,16-19,23-24,40H,12-15,35H2,1H3,(H,37,38,39)/t23-,24- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043766
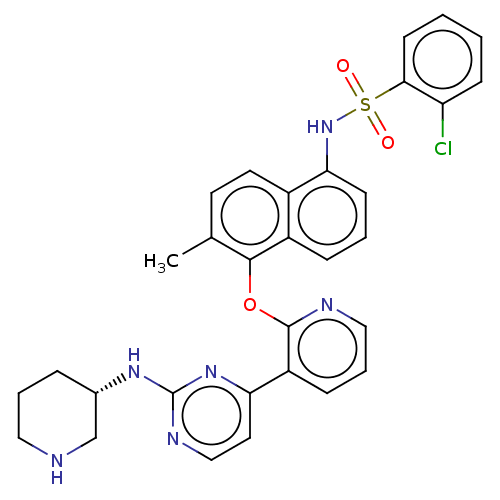
(CHEMBL3356009)Show SMILES Cc1ccc2c(NS(=O)(=O)c3ccccc3Cl)cccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CCCNC2)n1 |r| Show InChI InChI=1S/C31H29ClN6O3S/c1-20-13-14-22-23(8-4-11-27(22)38-42(39,40)28-12-3-2-10-25(28)32)29(20)41-30-24(9-6-17-34-30)26-15-18-35-31(37-26)36-21-7-5-16-33-19-21/h2-4,6,8-15,17-18,21,33,38H,5,7,16,19H2,1H3,(H,35,36,37)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50072026

(CHEMBL3407851)Show SMILES CNc1ncc2ccc(Oc3cc(ccc3Cl)C(=O)c3c(-c4ccccc4)n(C)n(-c4ccccc4)c3=O)cc2n1 Show InChI InChI=1S/C32H24ClN5O3/c1-34-32-35-19-22-13-15-24(18-26(22)36-32)41-27-17-21(14-16-25(27)33)30(39)28-29(20-9-5-3-6-10-20)37(2)38(31(28)40)23-11-7-4-8-12-23/h3-19H,1-2H3,(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant BRAF V600E kinase domain mutant (unknown origin) |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043770
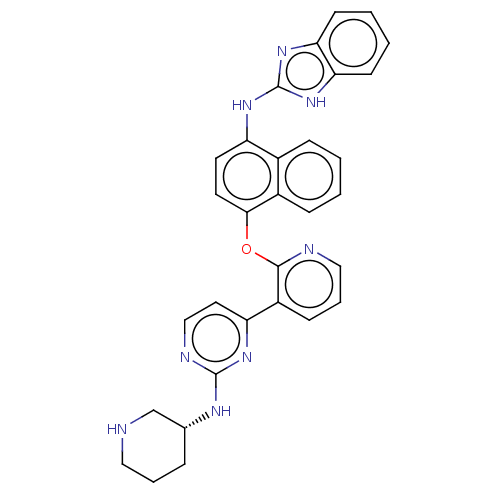
(CHEMBL3355997)Show SMILES C1CNC[C@@H](C1)Nc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 |r| Show InChI InChI=1S/C31H28N8O/c1-2-9-22-21(8-1)24(37-31-38-26-11-3-4-12-27(26)39-31)13-14-28(22)40-29-23(10-6-17-33-29)25-15-18-34-30(36-25)35-20-7-5-16-32-19-20/h1-4,6,8-15,17-18,20,32H,5,7,16,19H2,(H,34,35,36)(H2,37,38,39)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043706

(CHEMBL3356003)Show SMILES Cc1cc(Nc2nc3ccccc3o2)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:37.42,wD:34.38,(11.12,-44.15,;9.78,-44.92,;8.45,-44.16,;7.12,-44.93,;5.78,-44.16,;4.45,-44.93,;4.29,-46.46,;2.78,-46.78,;2.01,-48.11,;.48,-48.11,;-.3,-46.78,;.48,-45.44,;2.01,-45.45,;3.04,-44.3,;7.12,-46.47,;5.78,-47.24,;5.79,-48.79,;7.13,-49.55,;8.46,-48.78,;8.45,-47.25,;9.79,-46.47,;11.13,-47.24,;12.46,-46.47,;12.45,-44.93,;13.77,-44.16,;15.12,-44.92,;15.12,-46.47,;13.79,-47.24,;13.79,-48.78,;12.46,-49.55,;12.46,-51.09,;13.79,-51.86,;15.13,-51.08,;16.47,-51.84,;17.8,-51.07,;19.12,-51.84,;20.45,-51.07,;20.45,-49.53,;21.79,-48.76,;19.12,-48.76,;17.78,-49.53,;15.12,-49.54,)| Show InChI InChI=1S/C33H31N7O2/c1-20-19-28(40-33-39-27-10-4-5-11-29(27)41-33)23-7-2-3-8-24(23)30(20)42-31-25(9-6-17-35-31)26-16-18-36-32(38-26)37-22-14-12-21(34)13-15-22/h2-11,16-19,21-22H,12-15,34H2,1H3,(H,39,40)(H,36,37,38)/t21-,22- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043710
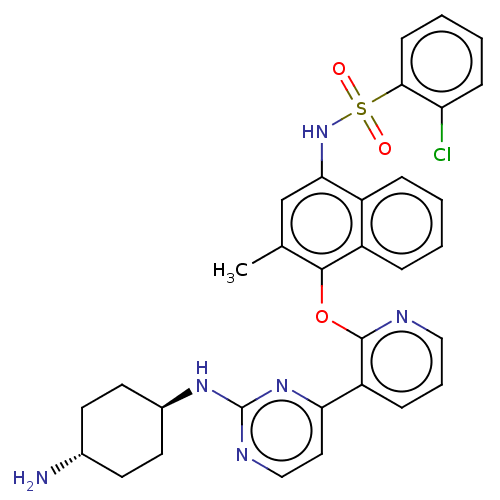
(CHEMBL3356007)Show SMILES Cc1cc(NS(=O)(=O)c2ccccc2Cl)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:38.42,wD:35.38,(34.99,-3.81,;33.66,-4.59,;32.33,-3.83,;31,-4.6,;29.66,-3.83,;28.33,-4.6,;29.09,-5.93,;27.56,-5.92,;27,-3.83,;27,-2.29,;25.67,-1.52,;24.34,-2.29,;24.34,-3.84,;25.67,-4.6,;25.68,-6.14,;31,-6.14,;29.67,-6.91,;29.67,-8.45,;31.01,-9.22,;32.34,-8.44,;32.33,-6.91,;33.67,-6.14,;35,-6.91,;36.34,-6.14,;36.32,-4.6,;37.65,-3.82,;38.99,-4.59,;39,-6.13,;37.66,-6.9,;37.67,-8.44,;36.33,-9.21,;36.33,-10.75,;37.67,-11.52,;39,-10.74,;40.34,-11.51,;41.67,-10.73,;43,-11.5,;44.32,-10.74,;44.33,-9.2,;45.66,-8.43,;42.99,-8.43,;41.65,-9.2,;39,-9.21,)| Show InChI InChI=1S/C32H31ClN6O3S/c1-20-19-28(39-43(40,41)29-11-5-4-10-26(29)33)23-7-2-3-8-24(23)30(20)42-31-25(9-6-17-35-31)27-16-18-36-32(38-27)37-22-14-12-21(34)13-15-22/h2-11,16-19,21-22,39H,12-15,34H2,1H3,(H,36,37,38)/t21-,22- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043776
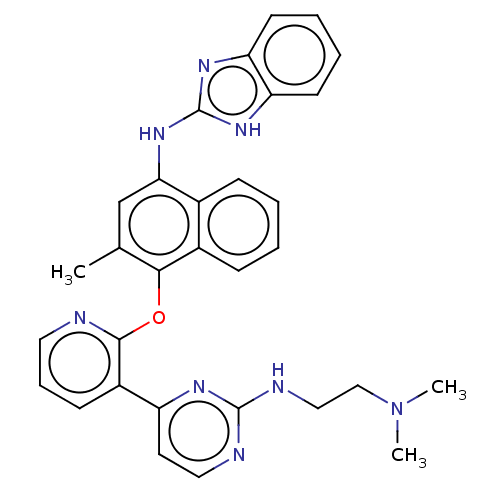
(CHEMBL3355993)Show SMILES CN(C)CCNc1nccc(n1)-c1cccnc1Oc1c(C)cc(Nc2nc3ccccc3[nH]2)c2ccccc12 Show InChI InChI=1S/C31H30N8O/c1-20-19-27(38-31-36-25-12-6-7-13-26(25)37-31)21-9-4-5-10-22(21)28(20)40-29-23(11-8-15-32-29)24-14-16-33-30(35-24)34-17-18-39(2)3/h4-16,19H,17-18H2,1-3H3,(H,33,34,35)(H2,36,37,38) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072070

(CHEMBL3407504)Show SMILES CNc1ncc2ccc(Oc3c(C)ccc(C(=O)c4c(-c5ccccc5)n(C)n(-c5ccccc5)c4=O)c3N)cc2n1 Show InChI InChI=1S/C33H28N6O3/c1-20-14-17-25(28(34)31(20)42-24-16-15-22-19-36-33(35-2)37-26(22)18-24)30(40)27-29(21-10-6-4-7-11-21)38(3)39(32(27)41)23-12-8-5-9-13-23/h4-19H,34H2,1-3H3,(H,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GCN2 phosphorylation in human U2OS cells preincubated for 1 hr followed by drug wash out and incubated for 2 hrs by MSD assay |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072075

(CHEMBL3407846)Show SMILES Cn1cc(-c2ccc3N(CC(=O)c4cc(F)cc(c4)C(F)(F)F)CCc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C24H19F4N5O/c1-32-10-18(21-22(29)30-12-31-23(21)32)13-2-3-19-14(6-13)4-5-33(19)11-20(34)15-7-16(24(26,27)28)9-17(25)8-15/h2-3,6-10,12H,4-5,11H2,1H3,(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PERK in human HT1080 cells assessed as inhibition of thapsigargin-induced CHoP mRNA expression preincubated for 1 hr followed by thapsi... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043705

(CHEMBL3356002)Show SMILES Cc1cc(Nc2nc3ccccc3[nH]2)c2ccccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CCCNC2)n1 |r| Show InChI InChI=1S/C32H30N8O/c1-20-18-28(40-32-38-26-12-4-5-13-27(26)39-32)22-9-2-3-10-23(22)29(20)41-30-24(11-7-16-34-30)25-14-17-35-31(37-25)36-21-8-6-15-33-19-21/h2-5,7,9-14,16-18,21,33H,6,8,15,19H2,1H3,(H,35,36,37)(H2,38,39,40)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072030

(CHEMBL3407856)Show SMILES Cc1nc2ccc(cc2s1)-c1c(C)ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c1N Show InChI InChI=1S/C32H26N4O2S/c1-19-14-16-24(29(33)27(19)22-15-17-25-26(18-22)39-20(2)34-25)31(37)28-30(21-10-6-4-7-11-21)35(3)36(32(28)38)23-12-8-5-9-13-23/h4-18H,33H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of doxycycline-inducible T-REx-PERK-FLAG (unknown origin) autophosphorylation tranfected in human HT1080 cells after 1 hr by sandwich ELIS... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043726
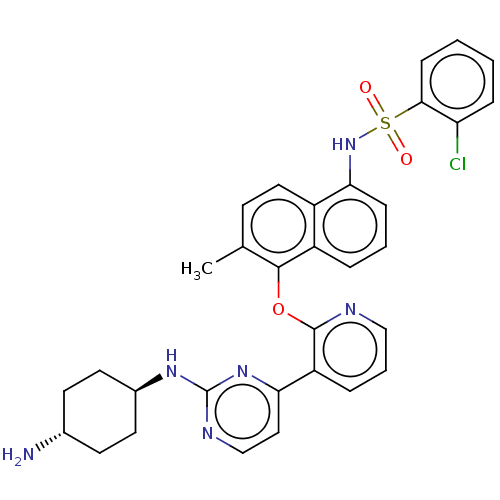
(CHEMBL3356008)Show SMILES Cc1ccc2c(NS(=O)(=O)c3ccccc3Cl)cccc2c1Oc1ncccc1-c1ccnc(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:38.42,wD:35.38,(58.89,-4.22,;57.56,-5,;56.22,-4.23,;54.89,-5.01,;54.89,-6.55,;53.56,-7.32,;52.22,-6.55,;52.21,-5.01,;52.98,-3.67,;53.75,-5,;50.88,-4.24,;50.88,-2.71,;49.55,-1.95,;48.22,-2.72,;48.23,-4.26,;49.56,-5.02,;49.58,-6.56,;53.56,-8.86,;54.9,-9.63,;56.23,-8.86,;56.22,-7.32,;57.56,-6.55,;58.9,-7.32,;60.23,-6.55,;60.22,-5.01,;61.55,-4.23,;62.89,-5,;62.89,-6.54,;61.56,-7.32,;61.57,-8.85,;60.23,-9.62,;60.23,-11.16,;61.56,-11.94,;62.9,-11.16,;64.24,-11.92,;65.57,-11.15,;66.9,-11.92,;68.23,-11.15,;68.23,-9.61,;69.56,-8.84,;66.89,-8.84,;65.55,-9.61,;62.9,-9.62,)| Show InChI InChI=1S/C32H31ClN6O3S/c1-20-11-16-23-24(6-4-9-28(23)39-43(40,41)29-10-3-2-8-26(29)33)30(20)42-31-25(7-5-18-35-31)27-17-19-36-32(38-27)37-22-14-12-21(34)13-15-22/h2-11,16-19,21-22,39H,12-15,34H2,1H3,(H,36,37,38)/t21-,22- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase/endoribonuclease IRE1
(Homo sapiens (Human)) | BDBM50043771

(CHEMBL3355996)Show SMILES CN1CCCC(C1)Nc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 Show InChI InChI=1S/C32H30N8O/c1-40-19-7-8-21(20-40)35-31-34-18-16-26(36-31)24-11-6-17-33-30(24)41-29-15-14-25(22-9-2-3-10-23(22)29)37-32-38-27-12-4-5-13-28(27)39-32/h2-6,9-18,21H,7-8,19-20H2,1H3,(H,34,35,36)(H2,37,38,39) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IRE1alpha using 5'[6FAM]-GAGUCCGCAGCACUC-[BHQ1]3' substrate by biochemical fluorescence quenching assay |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072027

(CHEMBL3407852)Show SMILES CNc1ncc2c(F)cc(Oc3c(C)ccc(C(=O)c4c(-c5ccccc5)n(C)n(-c5ccccc5)c4=O)c3N)cc2n1 Show InChI InChI=1S/C33H27FN6O3/c1-19-14-15-23(28(35)31(19)43-22-16-25(34)24-18-37-33(36-2)38-26(24)17-22)30(41)27-29(20-10-6-4-7-11-20)39(3)40(32(27)42)21-12-8-5-9-13-21/h4-18H,35H2,1-3H3,(H,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of doxycycline-inducible T-REx-PERK-FLAG (unknown origin) autophosphorylation tranfected in human HT1080 cells after 1 hr by sandwich ELIS... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50043769

(CHEMBL3355998)Show SMILES C1CNC[C@H](C1)Nc1nccc(n1)-c1cccnc1Oc1ccc(Nc2nc3ccccc3[nH]2)c2ccccc12 |r| Show InChI InChI=1S/C31H28N8O/c1-2-9-22-21(8-1)24(37-31-38-26-11-3-4-12-27(26)39-31)13-14-28(22)40-29-23(10-6-17-33-29)25-15-18-34-30(36-25)35-20-7-5-16-32-19-20/h1-4,6,8-15,17-18,20,32H,5,7,16,19H2,(H,34,35,36)(H2,37,38,39)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type JNK3 using biotinylated ATF2 substrate assessed as phosphorylation at thr53 on ATF2 by fluorescent plate reader |
ACS Med Chem Lett 6: 68-72 (2015)
Article DOI: 10.1021/ml500315b
BindingDB Entry DOI: 10.7270/Q2DF6STC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data