

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50279824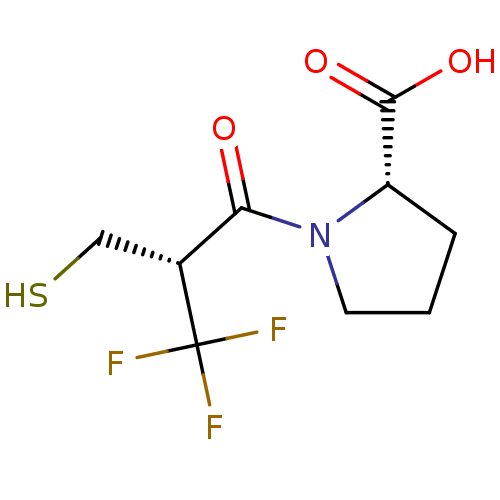 ((S)-1-((R)-3,3,3-Trifluoro-2-mercaptomethyl-propio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50280346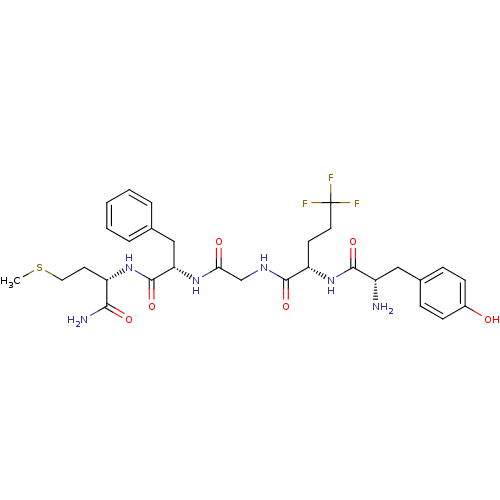 ((S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against enkephalin mu receptor using [3H]-PL-017 as ligand in rat cerebrum in vitro | Bioorg Med Chem Lett 2: 219-222 (1992) Article DOI: 10.1016/S0960-894X(01)81068-4 BindingDB Entry DOI: 10.7270/Q2D50MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50019056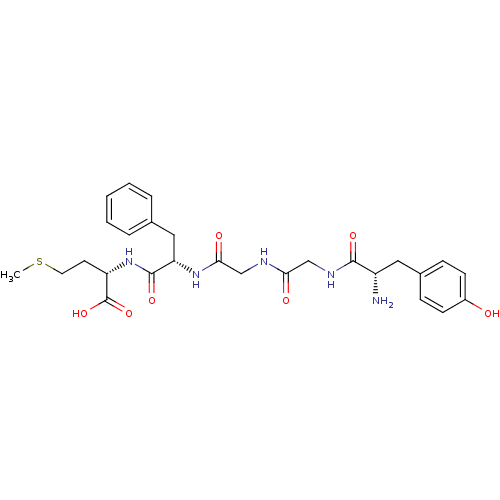 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against enkephalin delta receptor using [3H]-DPDPE as ligand in rat cerebrum in vitro | Bioorg Med Chem Lett 2: 219-222 (1992) Article DOI: 10.1016/S0960-894X(01)81068-4 BindingDB Entry DOI: 10.7270/Q2D50MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50019056 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against enkephalin mu receptor using [3H]-PL-017 as ligand in rat cerebrum in vitro | Bioorg Med Chem Lett 2: 219-222 (1992) Article DOI: 10.1016/S0960-894X(01)81068-4 BindingDB Entry DOI: 10.7270/Q2D50MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50280346 ((S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against enkephalin delta receptor using [3H]-DPDPE as ligand in rat cerebrum in vitro | Bioorg Med Chem Lett 2: 219-222 (1992) Article DOI: 10.1016/S0960-894X(01)81068-4 BindingDB Entry DOI: 10.7270/Q2D50MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base. (reported from ref. 1b) | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50280346 ((S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against enkephalin kappa receptor using [3H]-U-69,593 as ligand in guinea pig cerebrum in vitro | Bioorg Med Chem Lett 2: 219-222 (1992) Article DOI: 10.1016/S0960-894X(01)81068-4 BindingDB Entry DOI: 10.7270/Q2D50MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50279823 ((S)-1-((S)-3,3,3-Trifluoro-2-mercaptomethyl-propio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50019056 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against enkephalin kappa receptor using [3H]-U-69,593 as ligand in guinea pig cerebrum in vitro | Bioorg Med Chem Lett 2: 219-222 (1992) Article DOI: 10.1016/S0960-894X(01)81068-4 BindingDB Entry DOI: 10.7270/Q2D50MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||