

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of 5-HT2A receptor | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of SERT | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of SERT | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of 5-HT2A receptor | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253594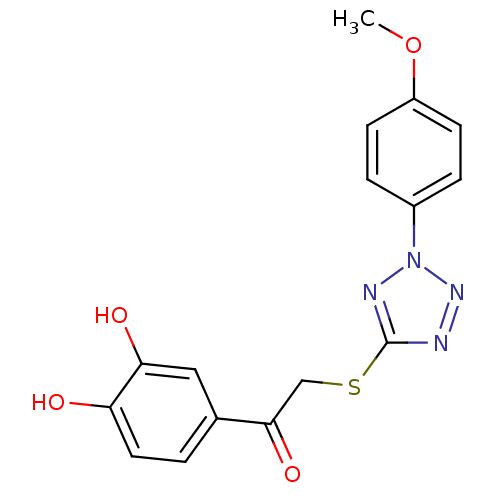 (1-(3,4-dihydroxyphenyl)-2-[2-(4-methoxyphenyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253593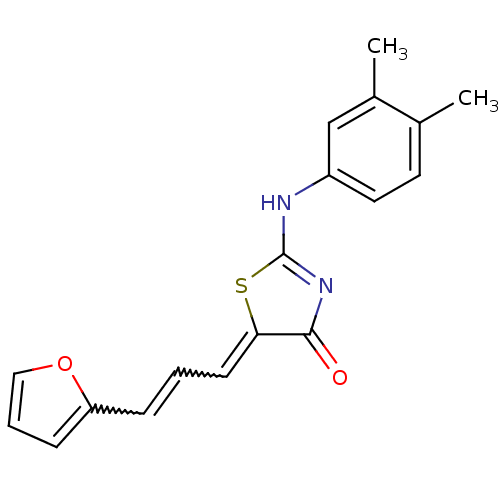 (2-(3,4-Dimethylphenylamino)-5-(3-furan-2-ylallylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50237948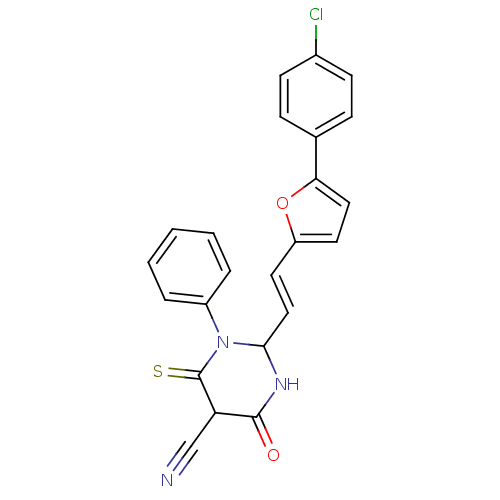 ((E)-2-(2-(5-(4-chlorophenyl)furan-2-yl)vinyl)-6-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253622 (2-[4-(4-chlorophenyl)-5-p-tolyl-4H-[1,2,4]triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Ability to displace [3H]-oxotremorine from Muscarinic acetylcholine receptor M1 expressed in CHO cells. | Bioorg Med Chem Lett 11: 2855-7 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313272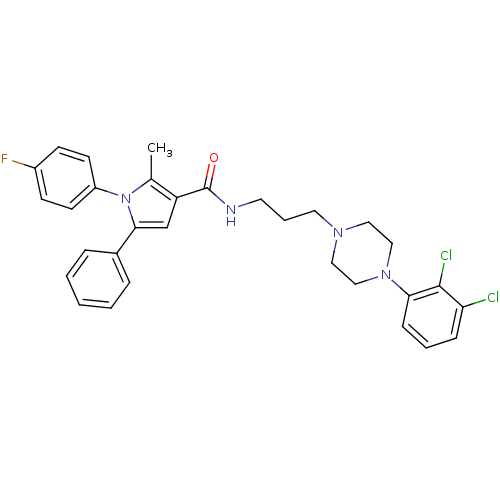 (CHEMBL1088314 | N-(3-(4-(2,3-dichlorophenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313264 (CHEMBL1087773 | N-(3-(4-(2,3-dimethylphenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50081724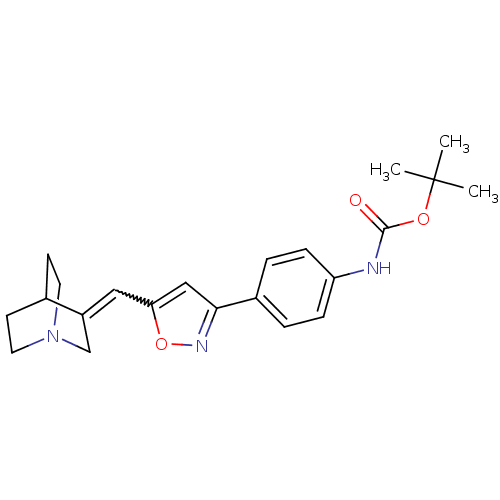 ((4-{5-[1-Aza-bicyclo[2.2.2]oct-(3Z)-ylidenemethyl]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description The compound was tested for its binding affinity against M1 human recombinant muscarinic receptor in CHO cells. | Bioorg Med Chem Lett 9: 2795-800 (1999) BindingDB Entry DOI: 10.7270/Q26H4GM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50081729 (4-{5-[1-Aza-bicyclo[2.2.2]oct-(3Z)-ylidenemethyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description The compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. | Bioorg Med Chem Lett 9: 2795-800 (1999) BindingDB Entry DOI: 10.7270/Q26H4GM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313280 (CHEMBL1080712 | N-(4-(4-(2,3-dimethylphenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313250 (CHEMBL1080726 | N-(3-(4-(3-chlorophenyl)piperazin-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313259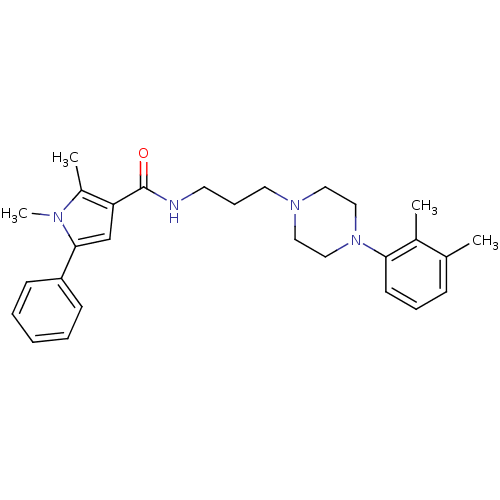 (CHEMBL1081644 | N-(3-(4-(2,3-Dimethylphenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50081733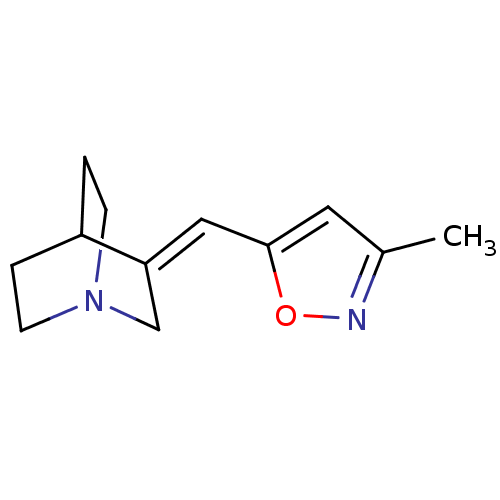 (3-(3-Methyl-isoxazol-5-ylmethylene)-1-aza-bicyclo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. | Bioorg Med Chem Lett 9: 2795-800 (1999) BindingDB Entry DOI: 10.7270/Q26H4GM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50313248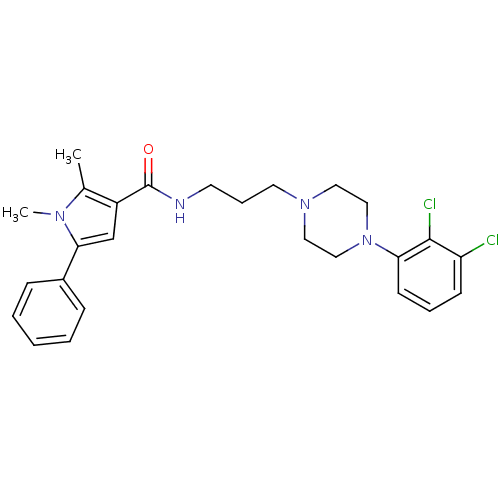 (CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313265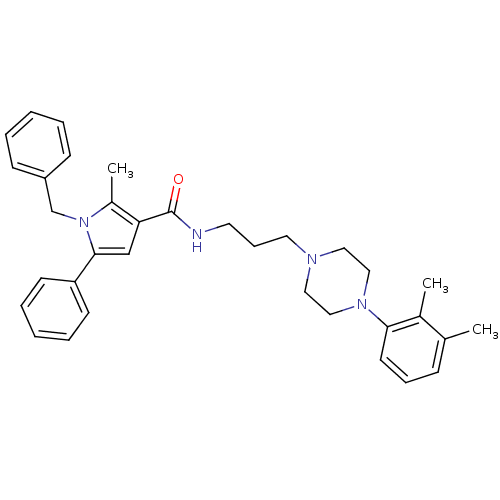 (1-benzyl-N-(3-(4-(2,3-dimethylphenyl)piperazin-1-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. | Bioorg Med Chem Lett 9: 2795-800 (1999) BindingDB Entry DOI: 10.7270/Q26H4GM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50313279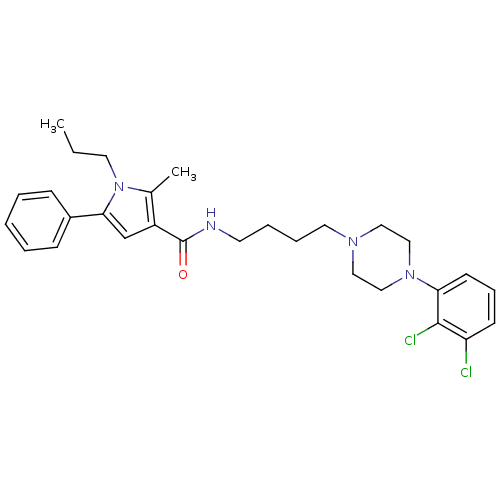 (CHEMBL1080711 | N-(4-(4-(2,3-dichlorophenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313280 (CHEMBL1080712 | N-(4-(4-(2,3-dimethylphenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313255 (CHEMBL1080747 | N-(3-(4-(3-chlorophenyl)piperazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50105712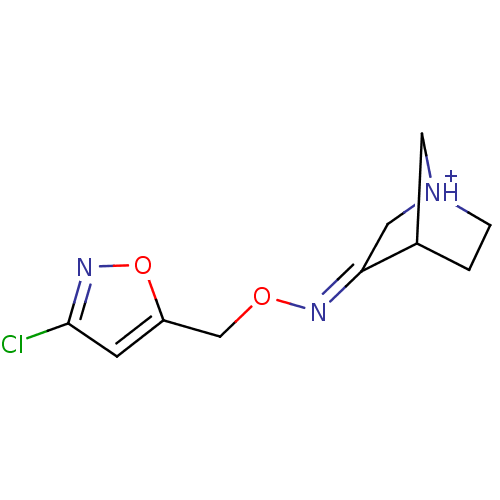 (3-[(Z)-3-Chloro-isoxazol-5-ylmethoxyimino]-1-azoni...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Ability to displace [3H]-oxotremorine from Muscarinic acetylcholine receptor M1 expressed in CHO cells. | Bioorg Med Chem Lett 11: 2855-7 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313251 (CHEMBL1080727 | N-(3-(4-(3-chlorophenyl)piperazin-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313278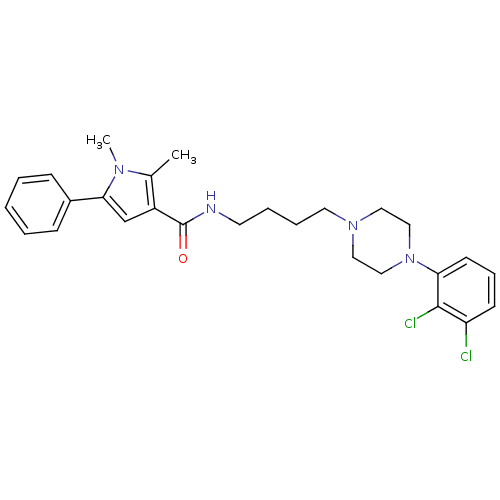 (CHEMBL1081036 | N-(4-(4-(2,3-dichlorophenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50313278 (CHEMBL1081036 | N-(4-(4-(2,3-dichlorophenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313277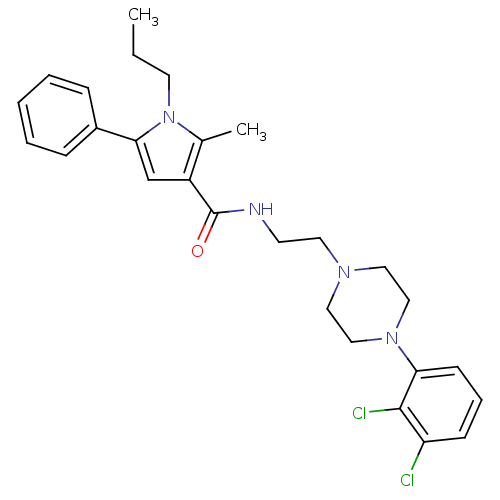 (CHEMBL1081035 | N-(2-(4-(2,3-dichlorophenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313260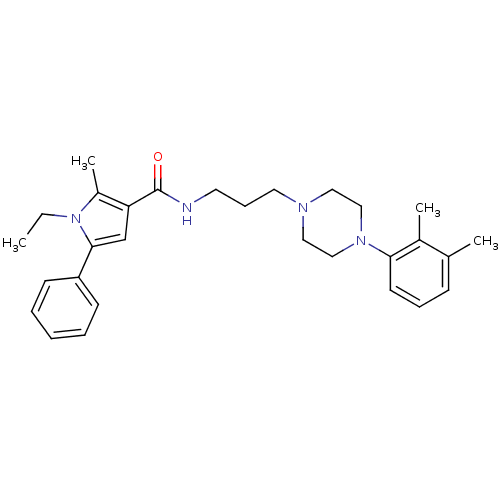 (CHEMBL1086613 | N-(3-(4-(2,3-Dimethylphenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50313275 (CHEMBL1080522 | N-(2-(4-(2,3-dichlorophenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313275 (CHEMBL1080522 | N-(2-(4-(2,3-dichlorophenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50105708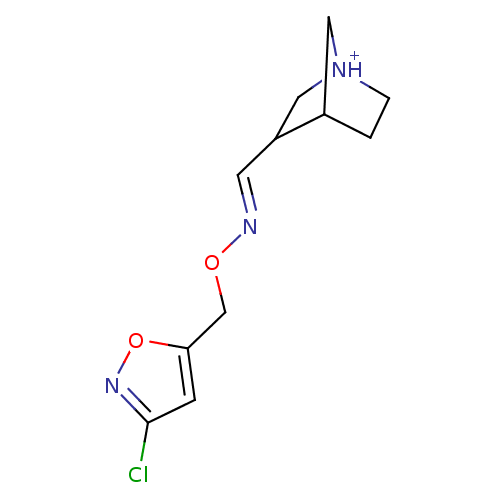 (3-[(3-Chloro-isoxazol-5-ylmethoxyimino)-methyl]-1-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Ability to displace [3H]-N-methylscopolamine from Muscarinic acetylcholine receptor M1 expressed in CHO cells. | Bioorg Med Chem Lett 11: 2855-7 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313248 (CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313264 (CHEMBL1087773 | N-(3-(4-(2,3-dimethylphenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50313248 (CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of 5HT1A receptor | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313259 (CHEMBL1081644 | N-(3-(4-(2,3-Dimethylphenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313276 (CHEMBL1080683 | N-(2-(4-(2,3-Dichlorophenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50105710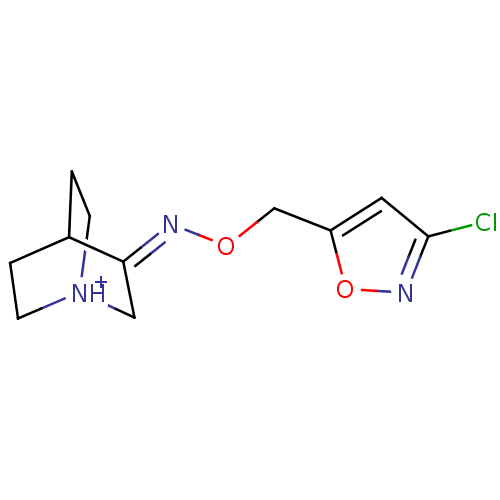 (3-[(Z)-3-Chloro-isoxazol-5-ylmethoxyimino]-1-azoni...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Ability to displace [3H]-oxotremorine from Muscarinic acetylcholine receptor M1 expressed in CHO cells. | Bioorg Med Chem Lett 11: 2855-7 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313281 (CHEMBL1080554 | N-(4-(4-(2,3-dimethylphenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50313281 (CHEMBL1080554 | N-(4-(4-(2,3-dimethylphenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313248 (CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50313280 (CHEMBL1080712 | N-(4-(4-(2,3-dimethylphenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50313268 (CHEMBL1086755 | N-(3-(4-(2,3-dichlorophenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313271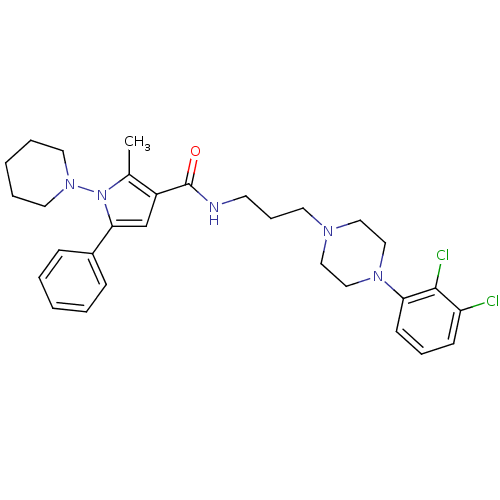 (CHEMBL1088313 | N-(3-(4-(2,3-dichlorophenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50105721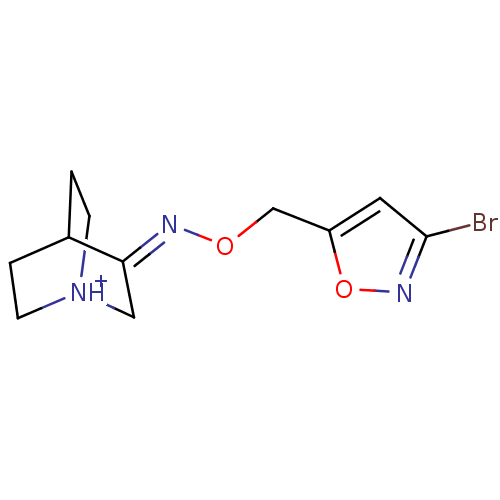 (3-[(Z)-3-Bromo-isoxazol-5-ylmethoxyimino]-1-azonia...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Ability to displace [3H]-oxotremorine from Muscarinic acetylcholine receptor M1 expressed in CHO cells. | Bioorg Med Chem Lett 11: 2855-7 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313260 (CHEMBL1086613 | N-(3-(4-(2,3-Dimethylphenyl)pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313282 (CHEMBL1080555 | N-(4-(4-(2,3-dimethylphenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313279 (CHEMBL1080711 | N-(4-(4-(2,3-dichlorophenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313261 (CHEMBL1088163 | N-(3-(4-(2,3-dimethylphenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313258 (CHEMBL1081468 | N-(3-(4-(2,3-Dimethylphenyl)pipera...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 335 total ) | Next | Last >> |