

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50259983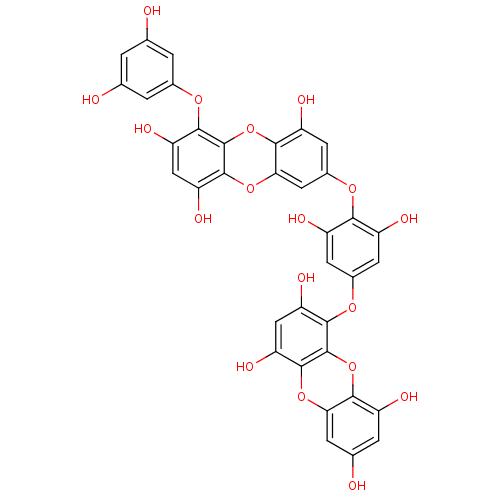 (CHEMBL508791 | US10106521, Compound Dieckol | diec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50259982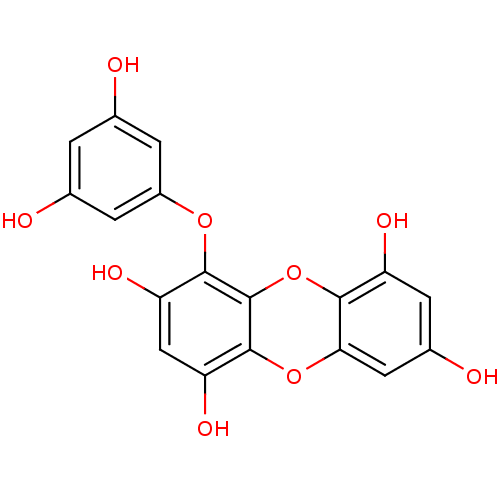 (CHEMBL471187 | US10106521, Compound Eckol | eckol) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM53072 ((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50391431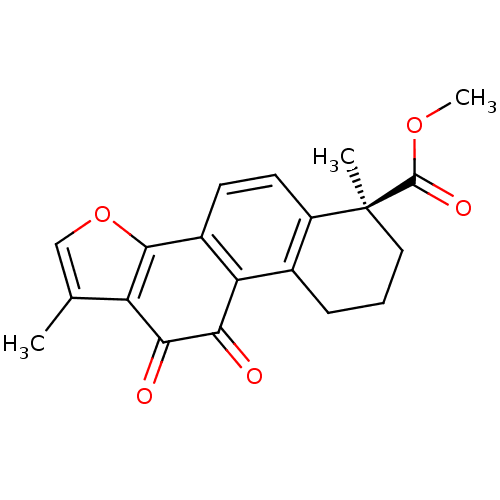 (CHEMBL2146517 | acs.jmedchem.1c00409_ST.502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50276756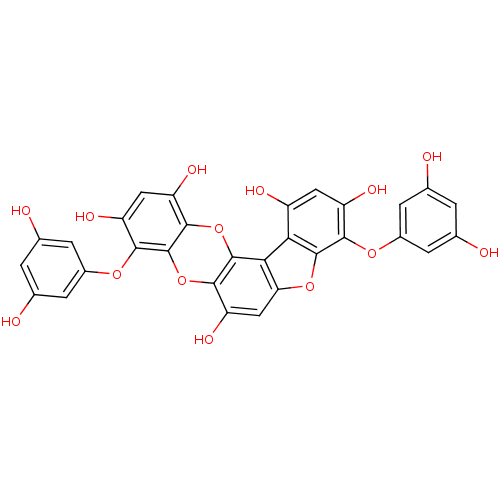 (4,9-bis(3,5-dihydroxyphenoxy)benzofuro[3,2-a]diben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50423877 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM83922 (1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM51317 (1,6-dimethylnaphtho[1,2-g][1]benzofuran-10,11-dion...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50129952 (2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis | Bioorg Med Chem 18: 7940-7 (2010) Article DOI: 10.1016/j.bmc.2010.09.035 BindingDB Entry DOI: 10.7270/Q2MG7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352810 (CHEMBL1711961) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50276826 ((1-(3',5'-dihydroxyphenoxy)-7-(2'',4'',6-trihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352812 (CHEMBL1823414) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM76798 ((E)-1-[2,4-dihydroxy-3-[(2E)-6-hydroxy-3,7-dimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50009219 (2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenant...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50391429 (CHEMBL215254 | acs.jmedchem.1c00409_ST.620) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50491981 (2-PHLOROECKOL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323199 (5,7-dihydroxy-8-(5-(5-hydroxy-7-methoxy-4-oxo-4H-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis | Bioorg Med Chem 18: 7940-7 (2010) Article DOI: 10.1016/j.bmc.2010.09.035 BindingDB Entry DOI: 10.7270/Q2MG7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352808 (CHEMBL1823413) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323206 (CHEMBL208908 | sciadopitisin | sciadopitysin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis | Bioorg Med Chem 18: 7940-7 (2010) Article DOI: 10.1016/j.bmc.2010.09.035 BindingDB Entry DOI: 10.7270/Q2MG7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352809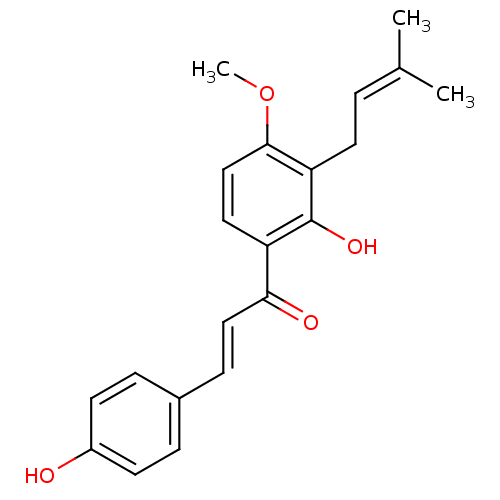 (CHEMBL458094) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50491983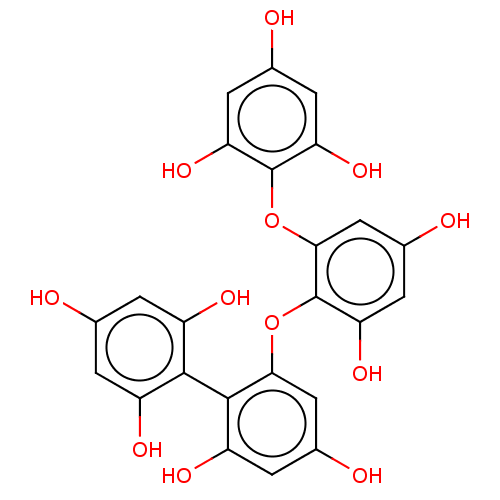 (CHEBI:65918 | Fucodiphloroethol G) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323196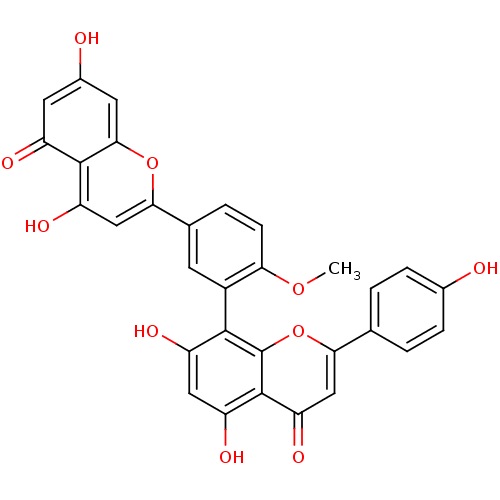 (4'-methylamentoflavone | CHEMBL378188 | bilobetin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis | Bioorg Med Chem 18: 7940-7 (2010) Article DOI: 10.1016/j.bmc.2010.09.035 BindingDB Entry DOI: 10.7270/Q2MG7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352811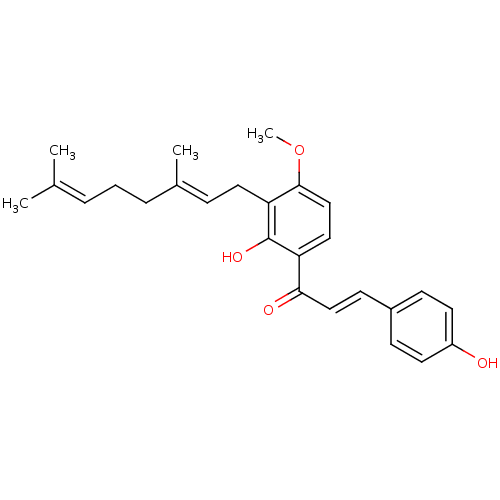 (CHEMBL1722838) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431696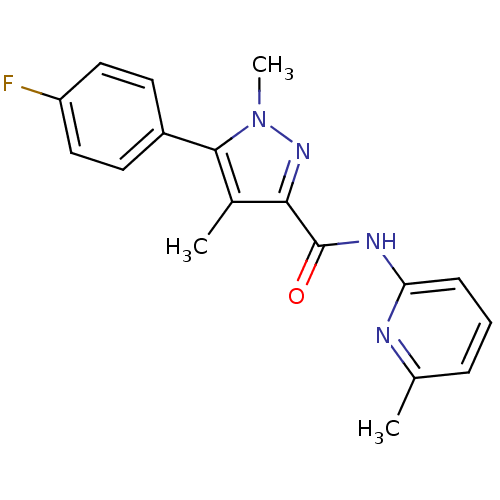 (CHEMBL2349534) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431690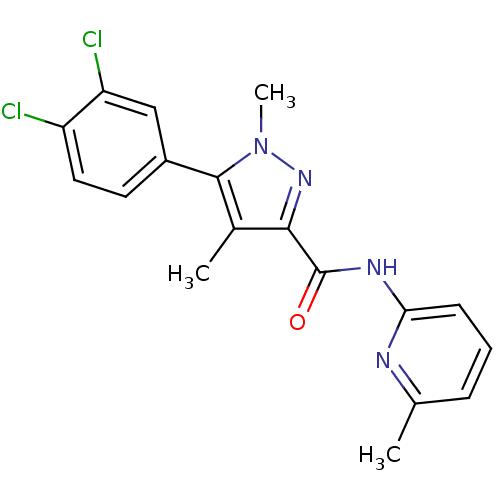 (CHEMBL2349540) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613898 (CHEMBL5283753) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613915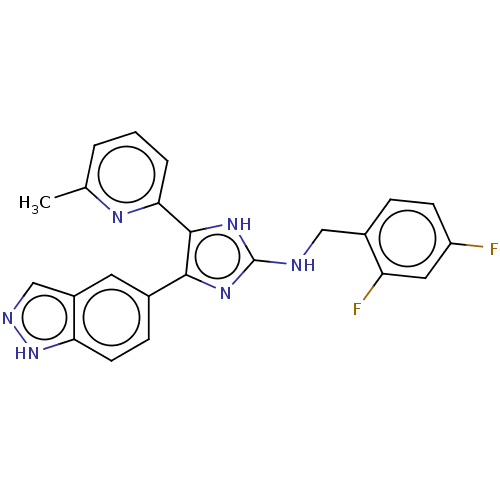 (CHEMBL5268868) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50609365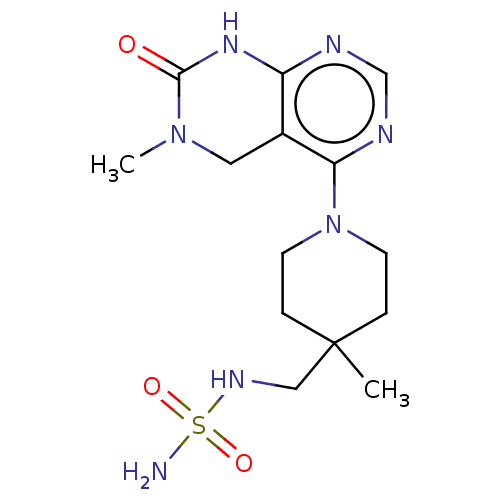 (CHEMBL5282123) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431692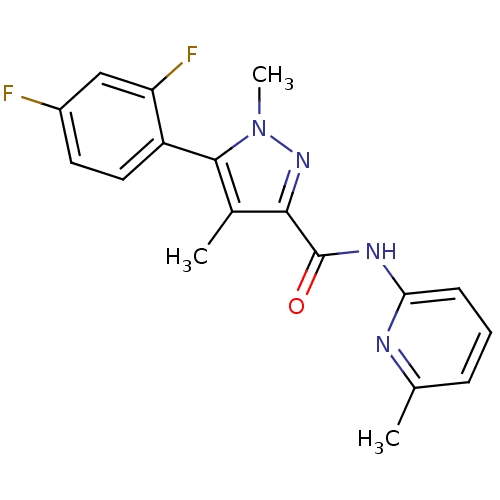 (CHEMBL2349538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431695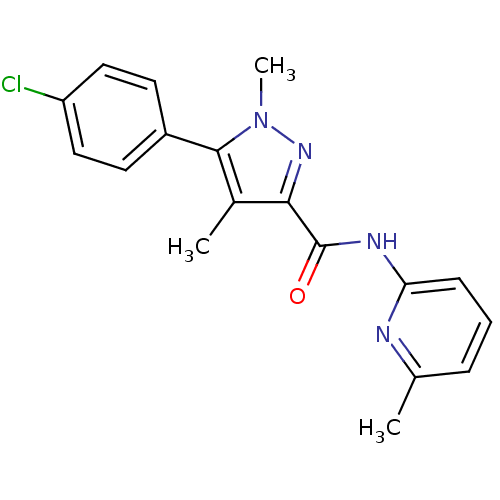 (CHEMBL2349535) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431693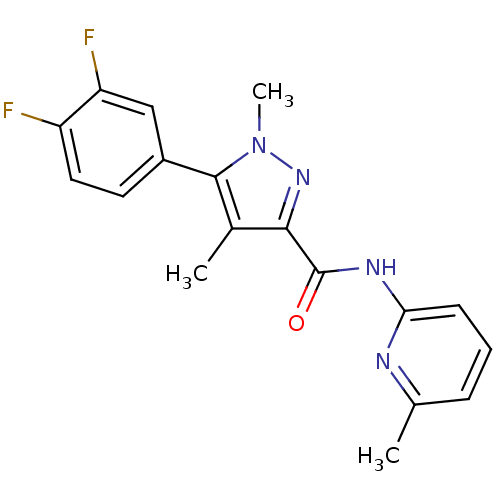 (CHEMBL2349537) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431697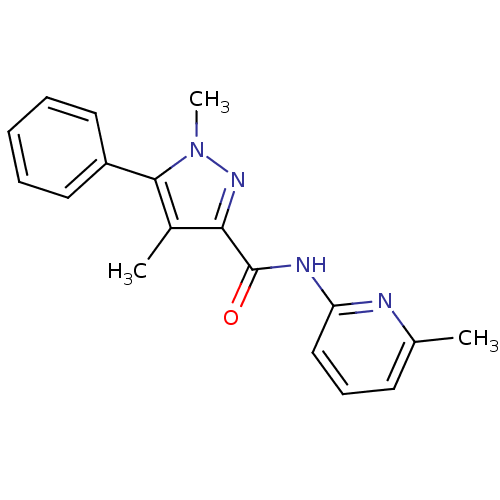 (CHEMBL2349533) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613920 (CHEMBL5273412) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431694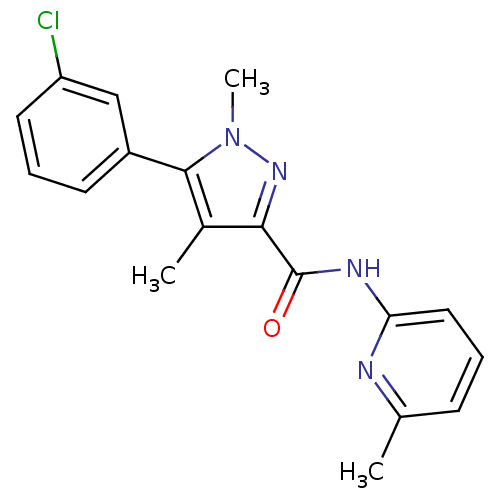 (CHEMBL2349536) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613917 (CHEMBL5271258) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613909 (CHEMBL5279947) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613898 (CHEMBL5283753) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613902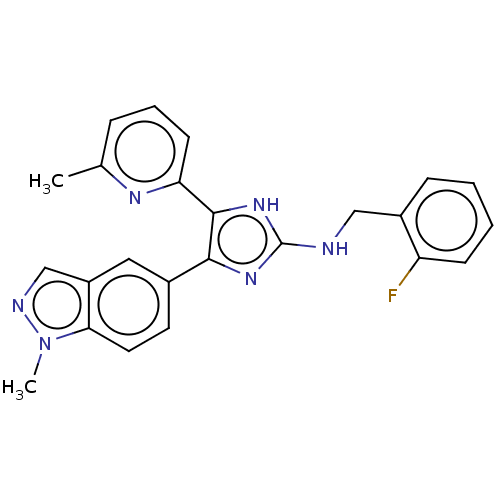 (CHEMBL5282597) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613914 (CHEMBL5274746) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431691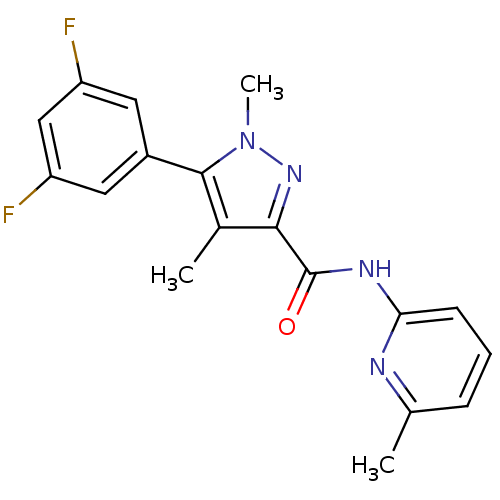 (CHEMBL2349539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431689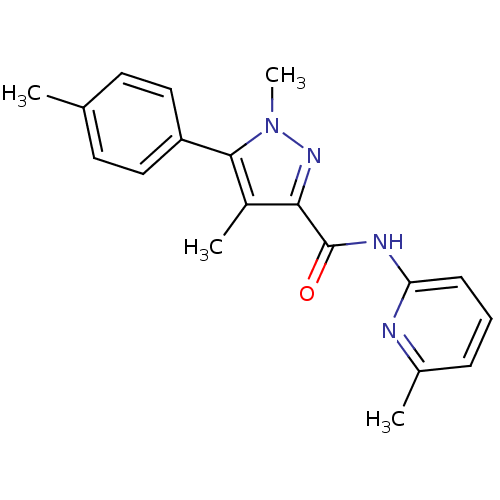 (CHEMBL2349541) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613905 (CHEMBL5289293) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613916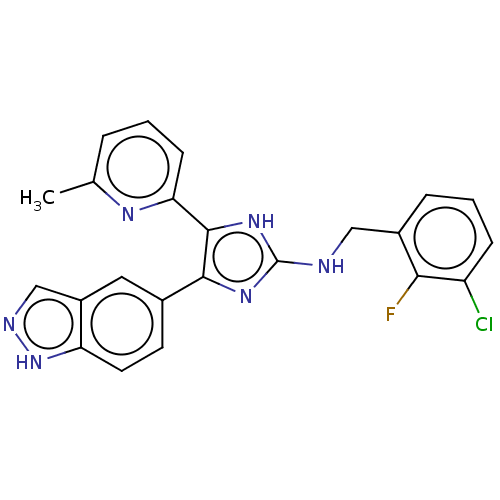 (CHEMBL5278659) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431717 (CHEMBL2346726) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613897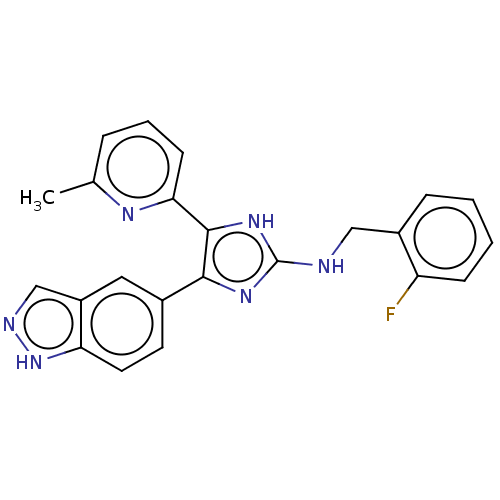 (CHEMBL5275540) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431722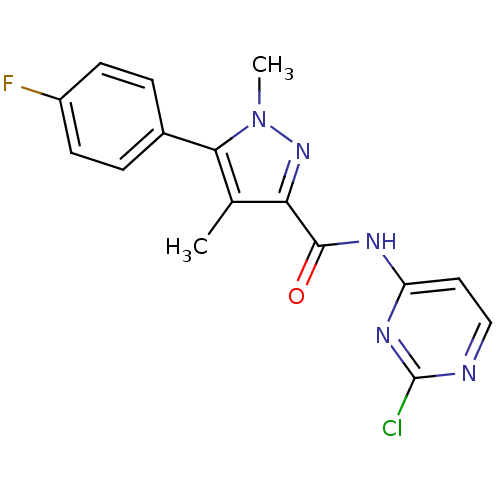 (CHEMBL2346721) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613900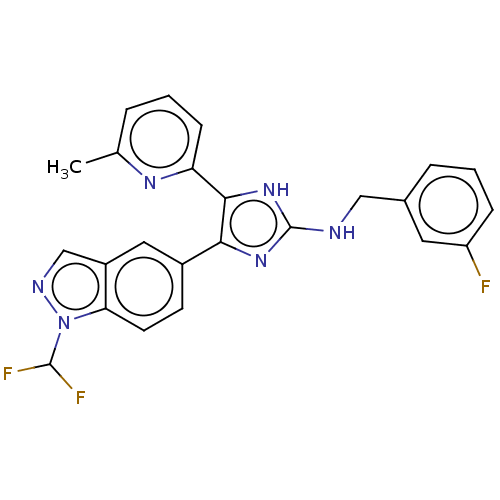 (CHEMBL5290805) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613919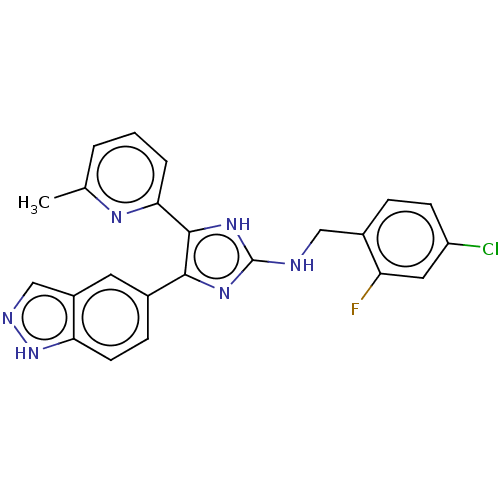 (CHEMBL5275976) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50613903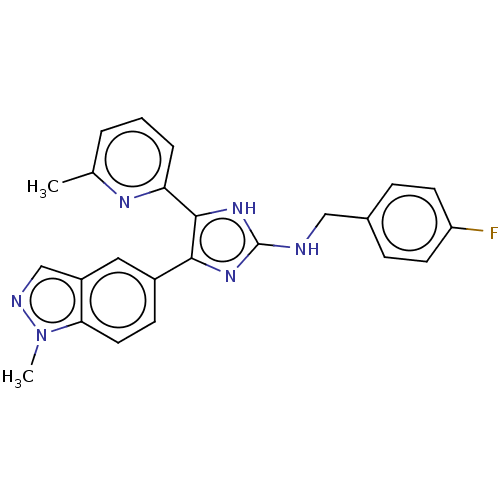 (CHEMBL5276258) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015639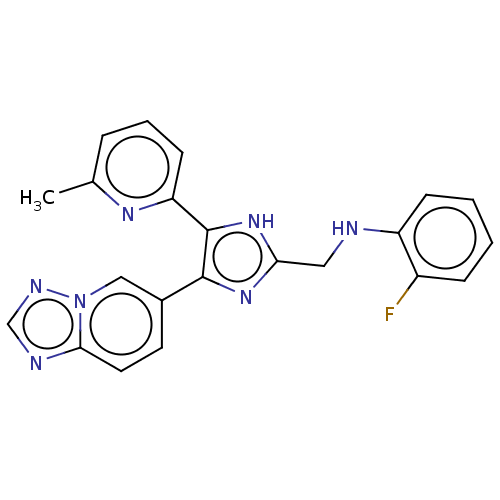 (CHEMBL3260567 | USRE47141, Example 2) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 419 total ) | Next | Last >> |