

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470392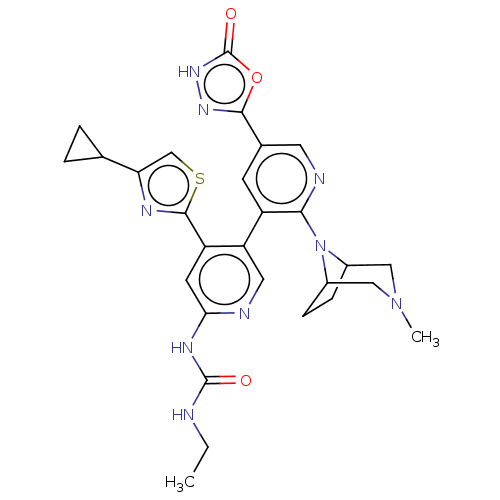 (CHEMBL4283208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470399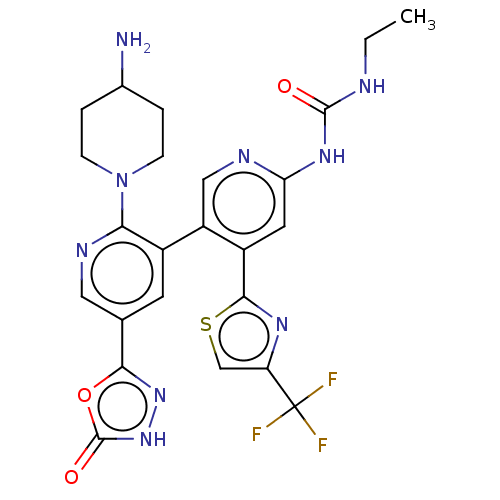 (CHEMBL4286573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286099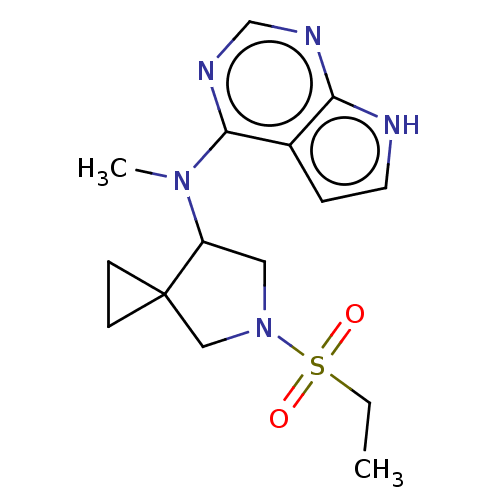 (N-(5-(ethylsulfonyl)-5-azaspiro[2.4]heptan-7-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470397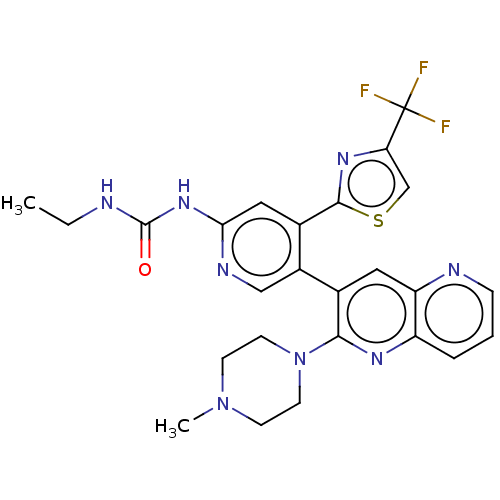 (CHEMBL4294536) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470406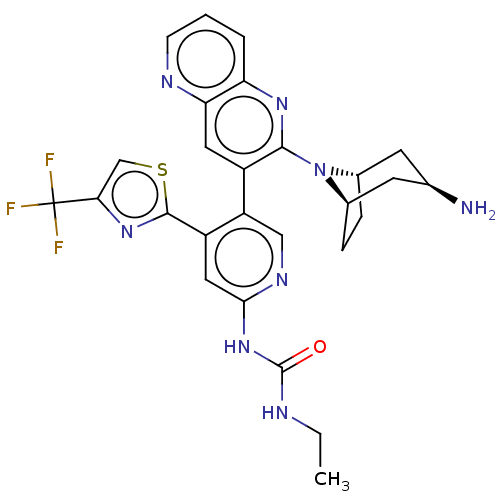 (CHEMBL4295081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470392 (CHEMBL4283208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470393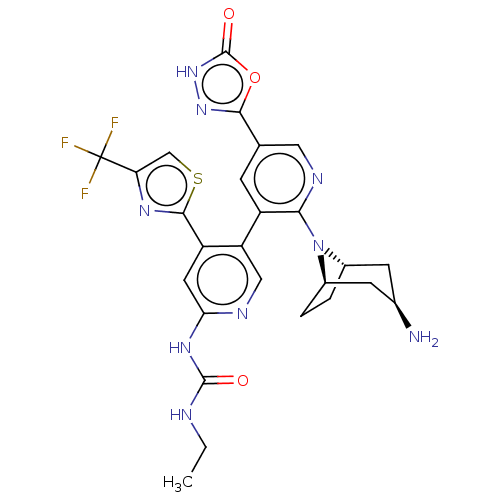 (CHEMBL4291061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470403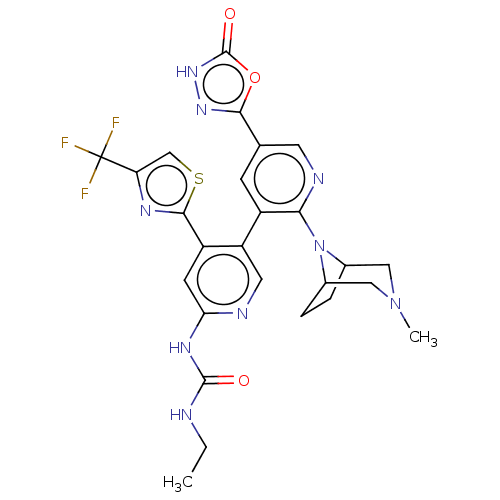 (CHEMBL4294467) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470400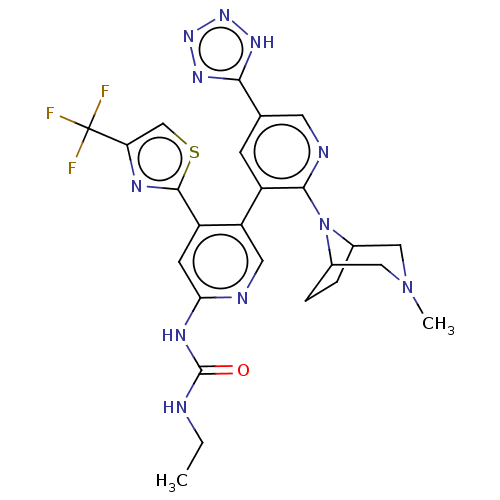 (CHEMBL4289998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470407 (CHEMBL4286625) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470401 (CHEMBL4290062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286098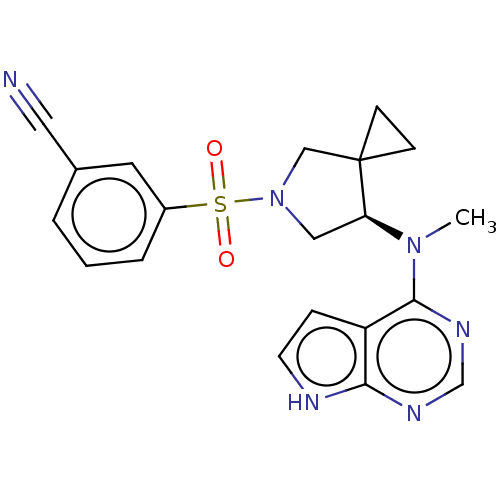 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50006565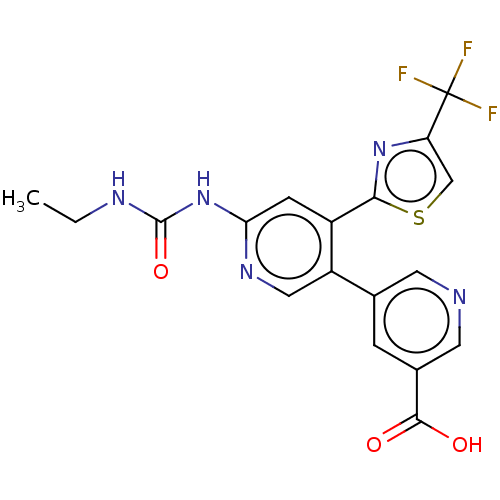 (CHEMBL3235085) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470394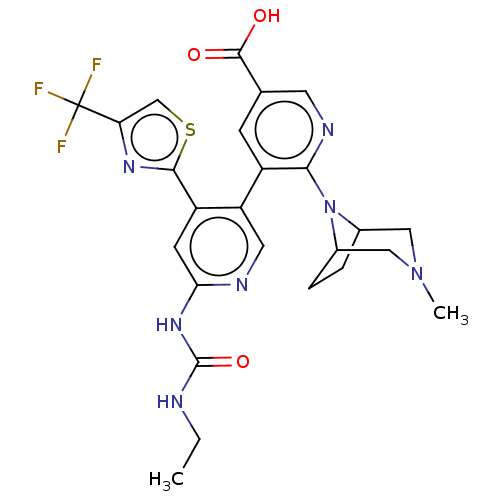 (CHEMBL4281775) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470396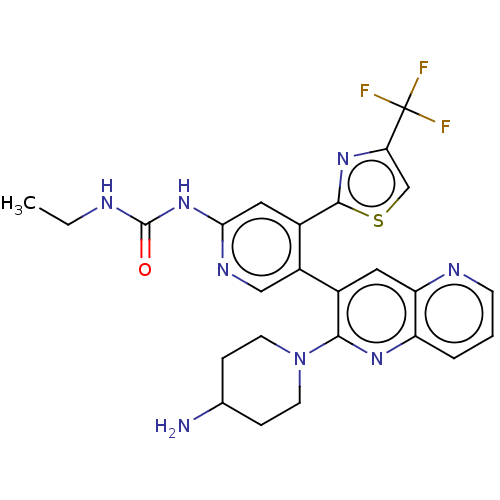 (CHEMBL4293447) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470403 (CHEMBL4294467) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470404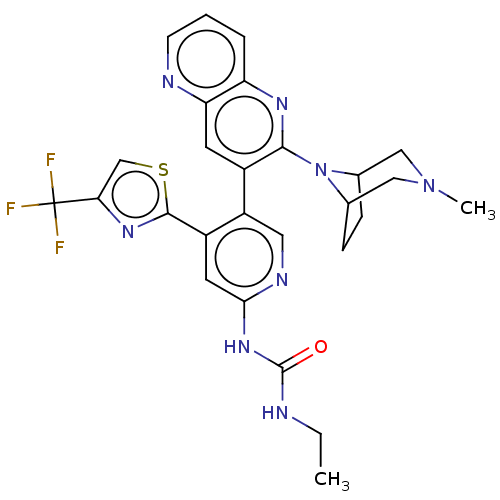 (CHEMBL4279382) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470395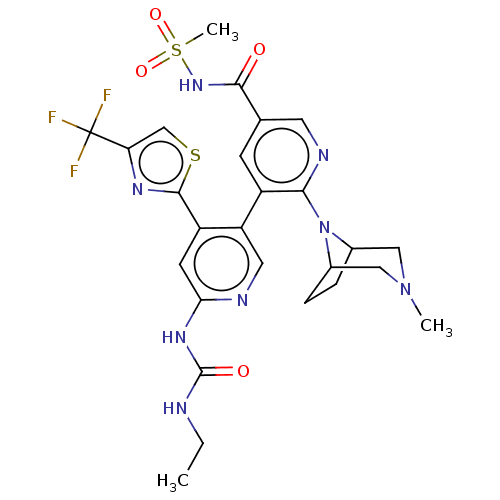 (CHEMBL4279313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470394 (CHEMBL4281775) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470402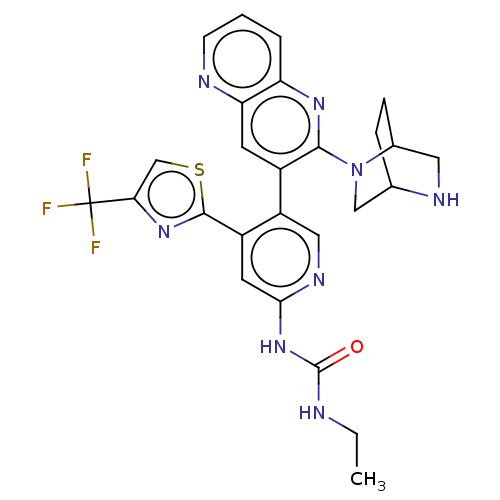 (CHEMBL4278317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286095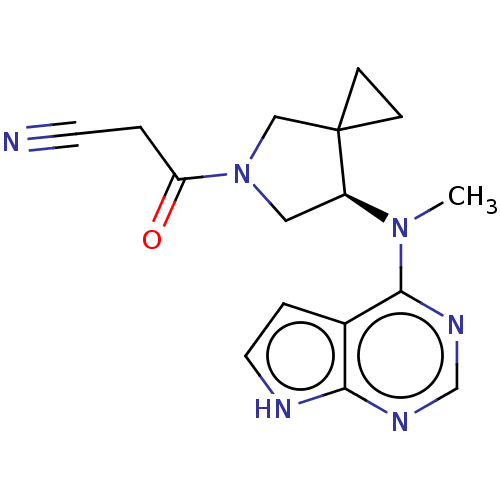 ((R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470395 (CHEMBL4279313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470405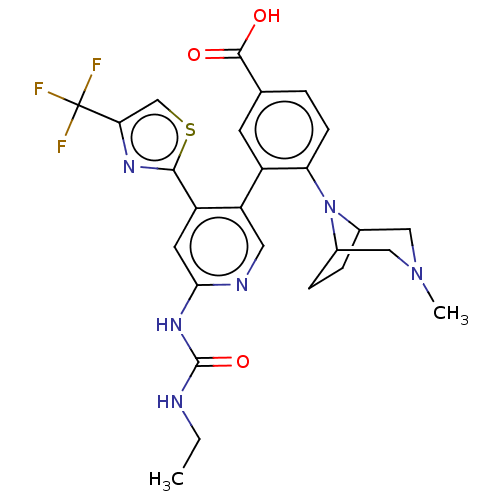 (CHEMBL4289650) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470400 (CHEMBL4289998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470405 (CHEMBL4289650) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470393 (CHEMBL4291061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470404 (CHEMBL4279382) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470398 (CHEMBL4286232) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470402 (CHEMBL4278317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470399 (CHEMBL4286573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470406 (CHEMBL4295081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286098 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.7 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470398 (CHEMBL4286232) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286096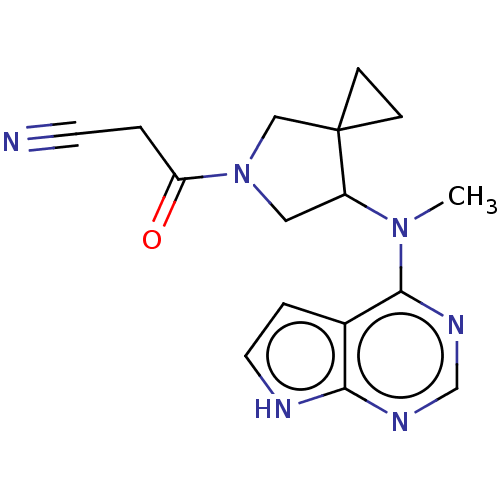 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29.3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286096 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40.4 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470396 (CHEMBL4293447) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286098 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 58.6 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM286098 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286096 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286099 (N-(5-(ethylsulfonyl)-5-azaspiro[2.4]heptan-7-yl)-N...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470401 (CHEMBL4290062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286095 ((R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470397 (CHEMBL4294536) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286095 ((R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286099 (N-(5-(ethylsulfonyl)-5-azaspiro[2.4]heptan-7-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM286096 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 694 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50006565 (CHEMBL3235085) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286097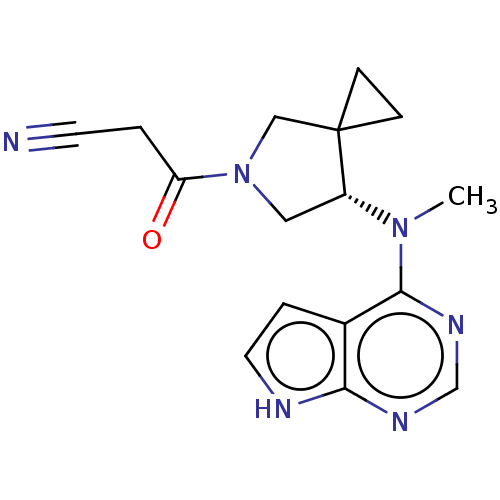 ((S)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 787 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286097 ((S)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286097 ((S)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |