

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423394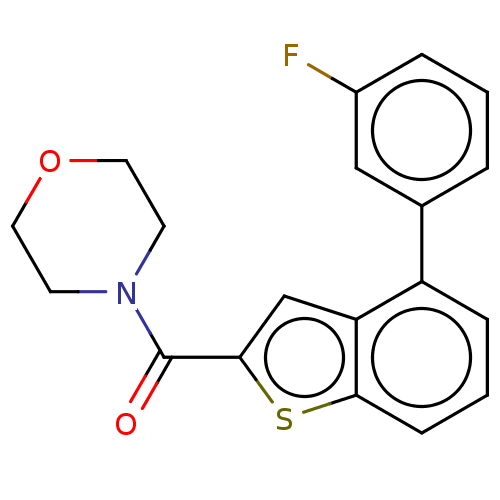 (US10501473, Example 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423407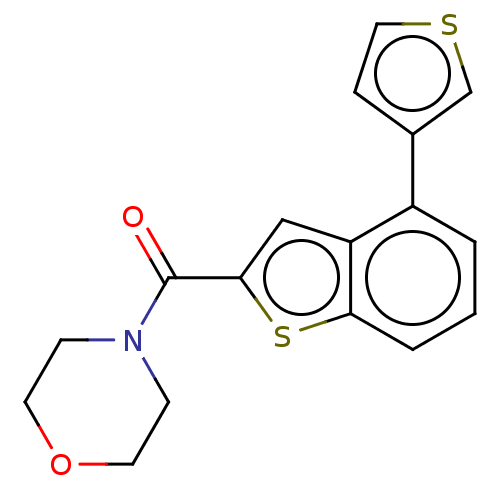 (US10501473, Example 76) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 61.2 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423404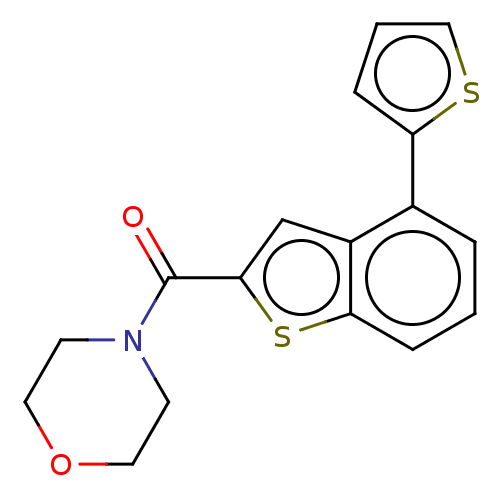 (US10501473, Example 74) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65.6 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423405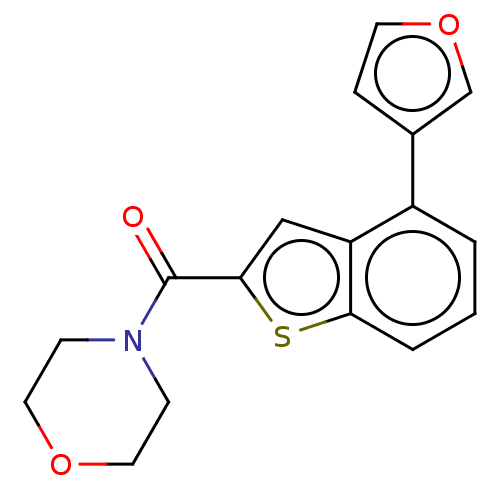 (US10501473, Example 75) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 73.4 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM36371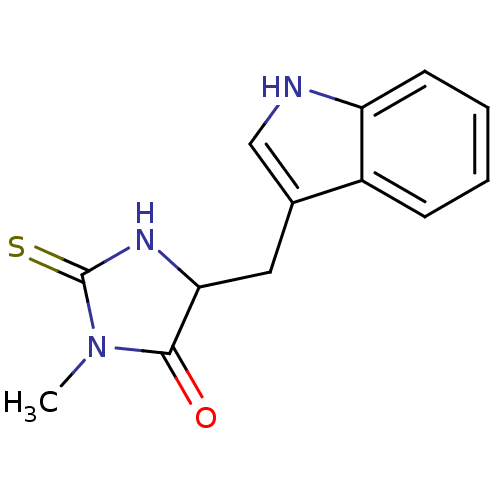 (5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4-Imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 95.5 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423379 (US10501473, Example 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423400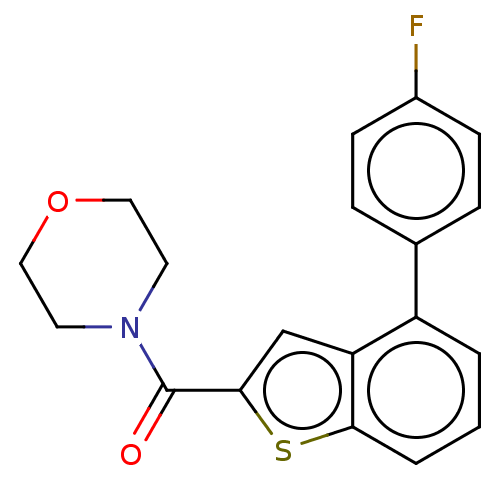 (US10501473, Example 39) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423401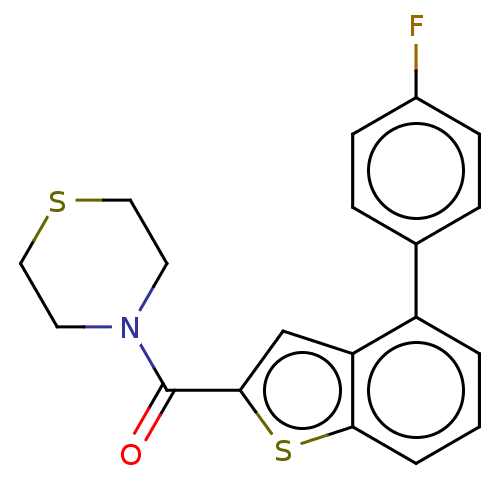 (US10501473, Example 51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423403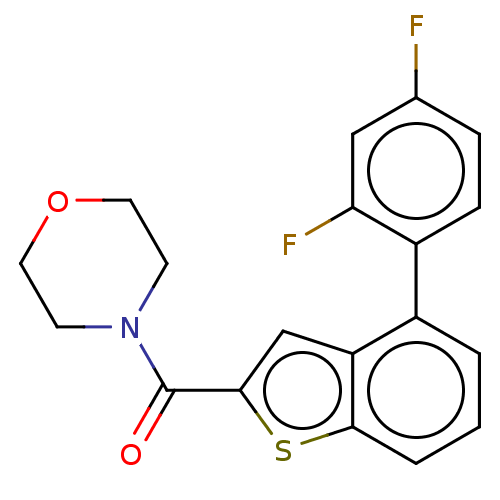 (US10501473, Example 63) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423389 (US10501473, Example 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356166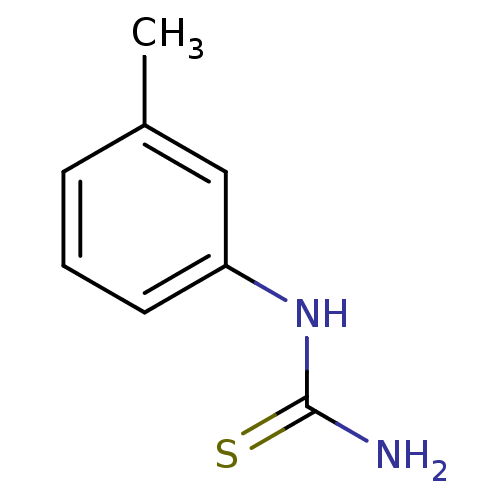 (CHEMBL1088657) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356164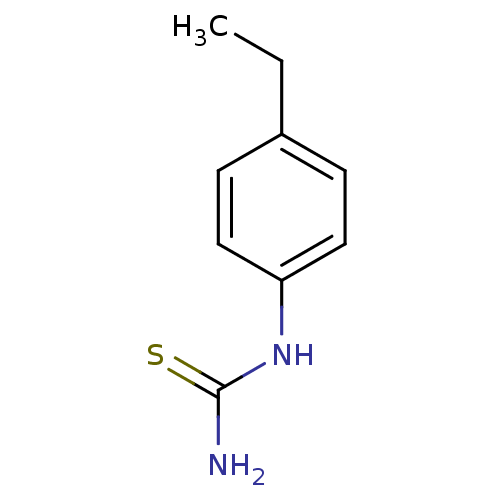 (CHEMBL1088361) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356167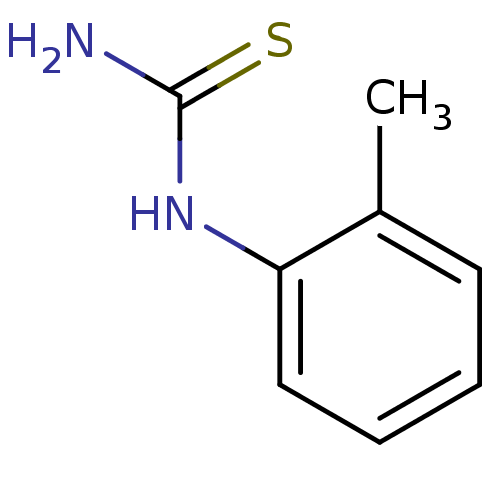 (CHEMBL1088658) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423376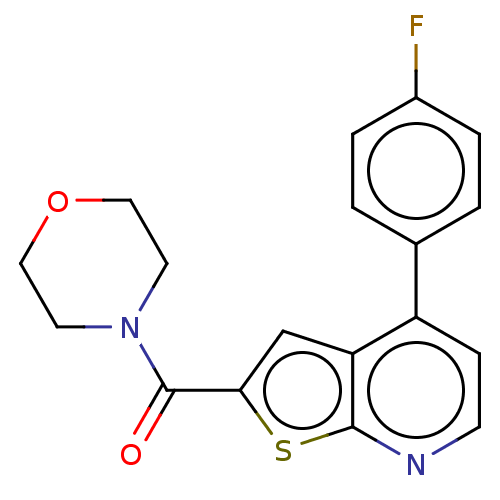 (US10501473, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356163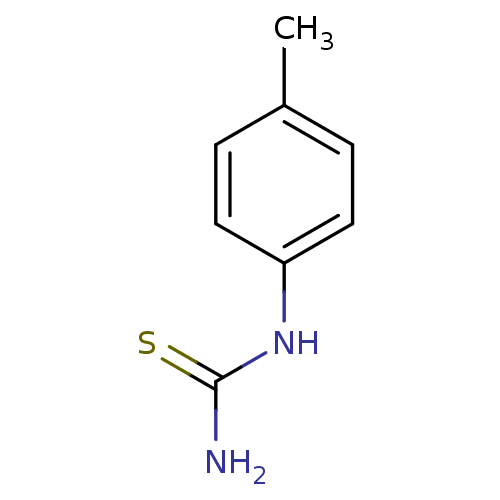 (CHEMBL1088360) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50240041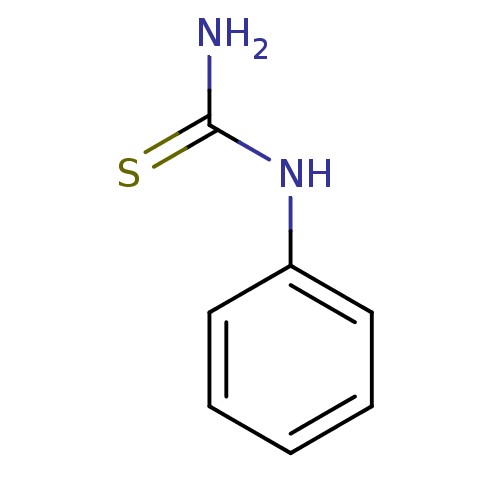 (1-phenyl-2-thiourea | 1-phenylthiourea | CHEMBL263...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356165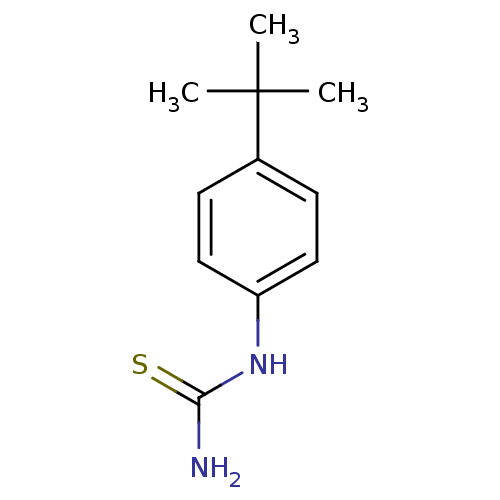 (CHEMBL1088498) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM423402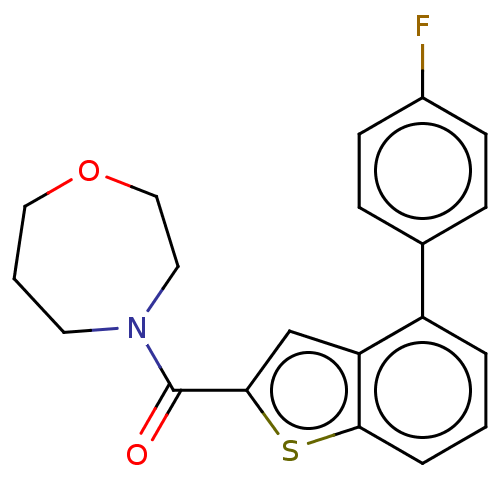 (US10501473, Example 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INDUSTRY & ACADEMIC COOPERATION IN CHUNGNAM NATIONAL UNIVERSITY (IAC) US Patent | Assay Description RIPK1 protein was over-expressed in HEK293 cell line, to which lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.4 mM phenylm... | US Patent US10501473 (2019) BindingDB Entry DOI: 10.7270/Q2TH8Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391556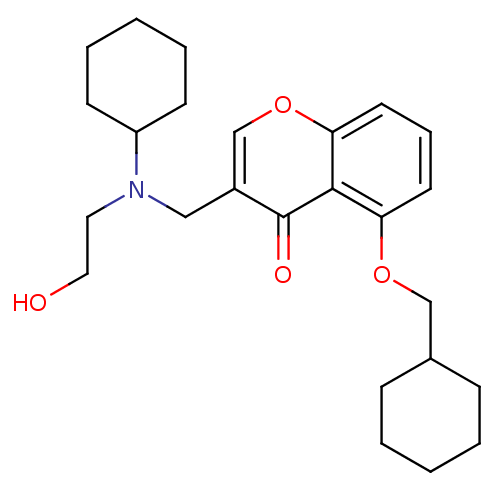 (CHEMBL2147495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391546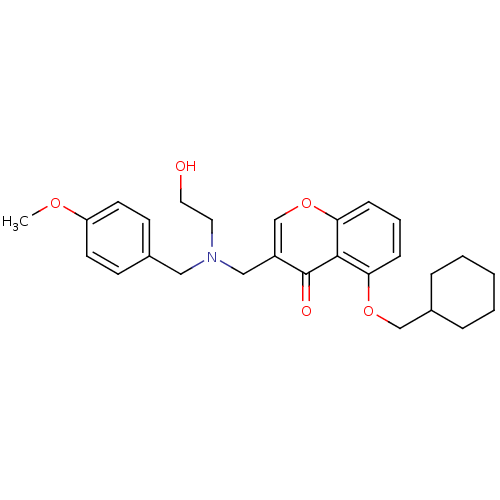 (CHEMBL2146491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391547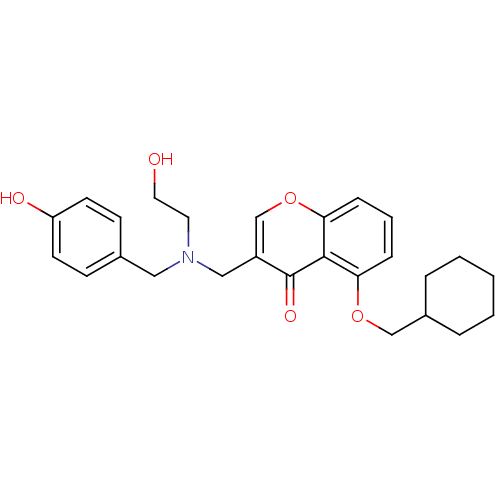 (CHEMBL2147499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391554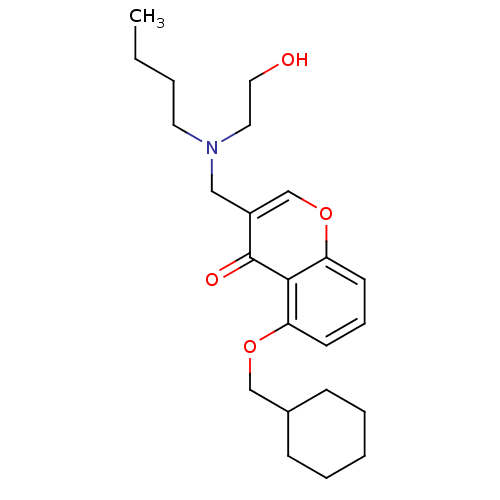 (CHEMBL2147492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391548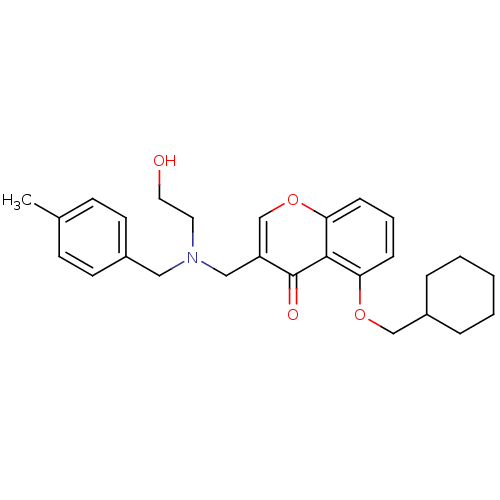 (CHEMBL2147498) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391553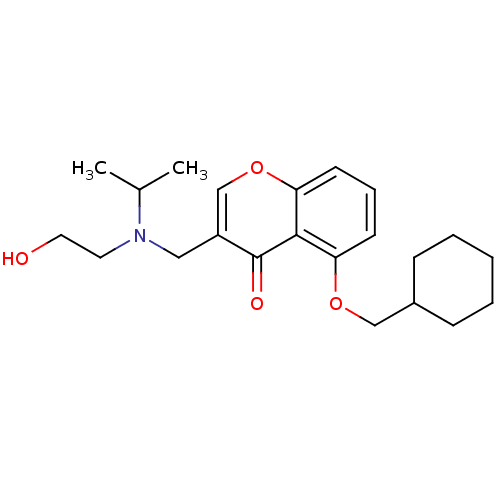 (CHEMBL2147493) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391552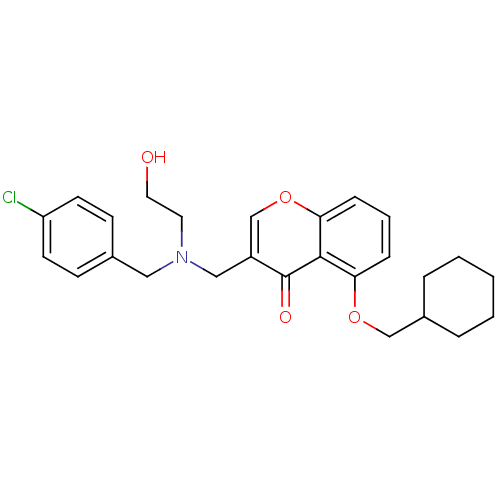 (CHEMBL2147500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50242276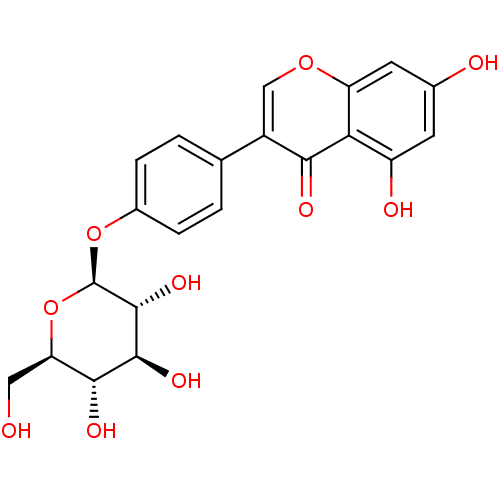 (CHEMBL486626 | Sophoricoside) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391550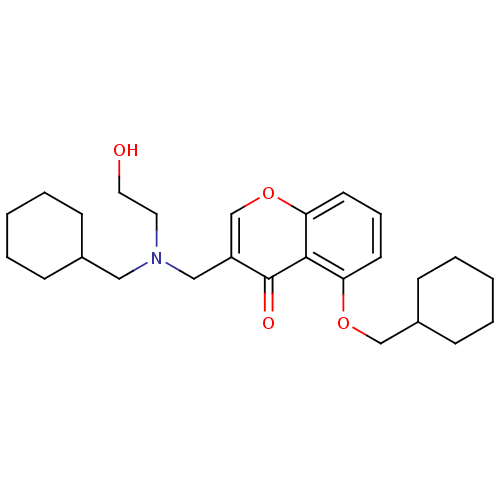 (CHEMBL2147496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391551 (CHEMBL2147501) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391555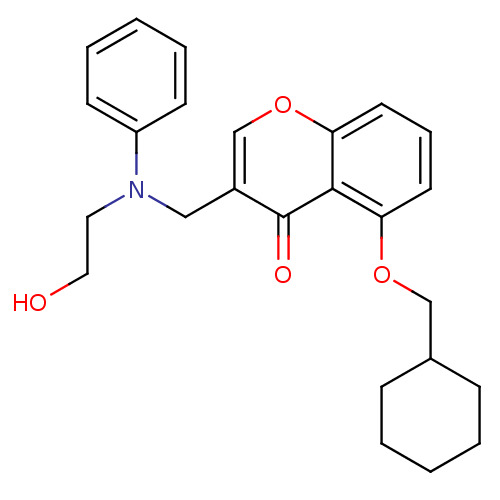 (CHEMBL2147494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391544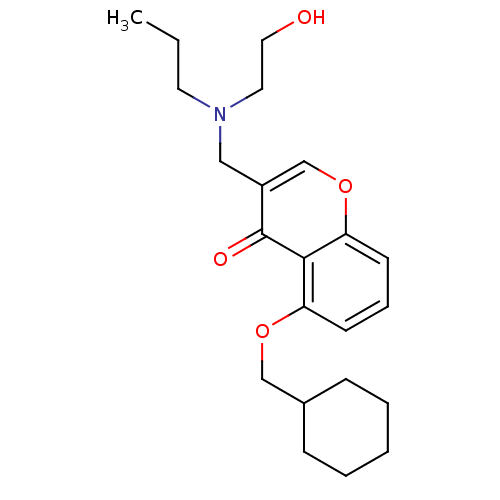 (CHEMBL2147491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391549 (CHEMBL2147497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391545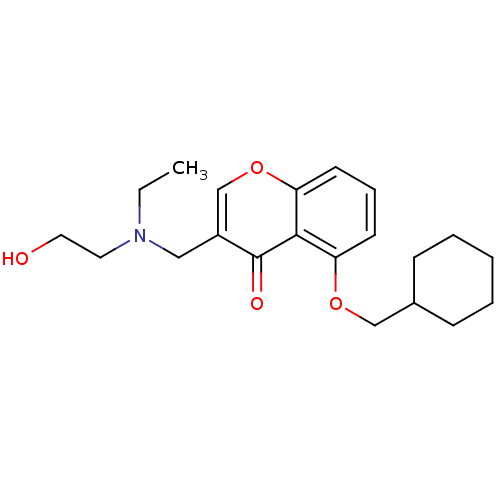 (CHEMBL2147490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356170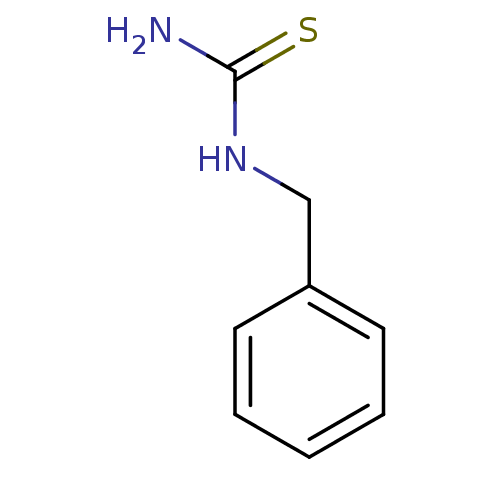 (CHEMBL1087841) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356168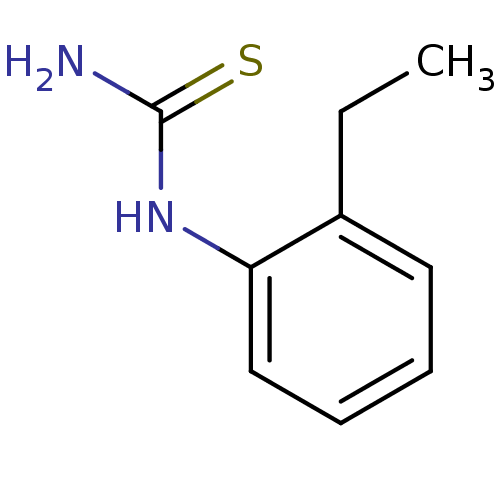 (CHEMBL1087472) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50354850 (BUDESONIDE | US10869929, Compound Budesonide | US1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-5 (Mus musculus) | BDBM50391557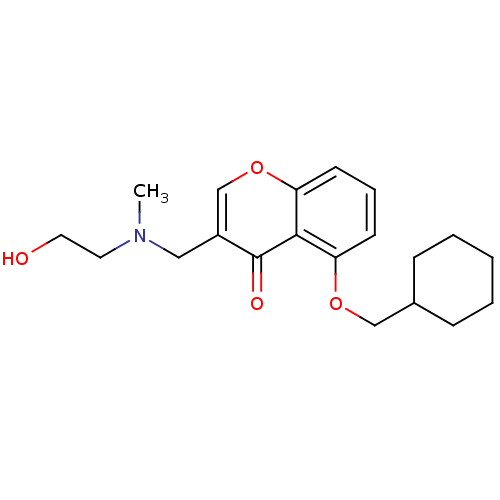 (CHEMBL2147489) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of IL-5 in mouse Y16 cells after 48 hrs by WST1 assay | Bioorg Med Chem 20: 5757-62 (2012) Article DOI: 10.1016/j.bmc.2012.08.006 BindingDB Entry DOI: 10.7270/Q2PG1SSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356172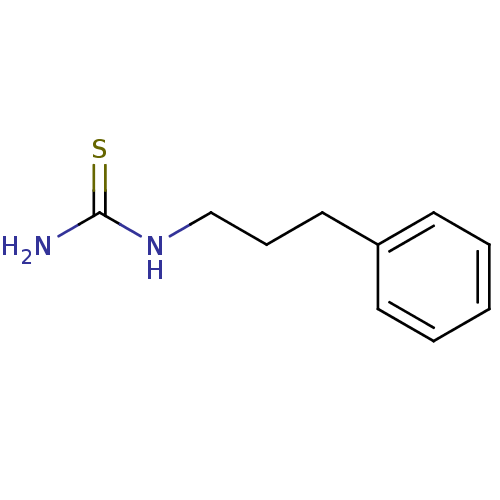 (CHEMBL1087843) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356182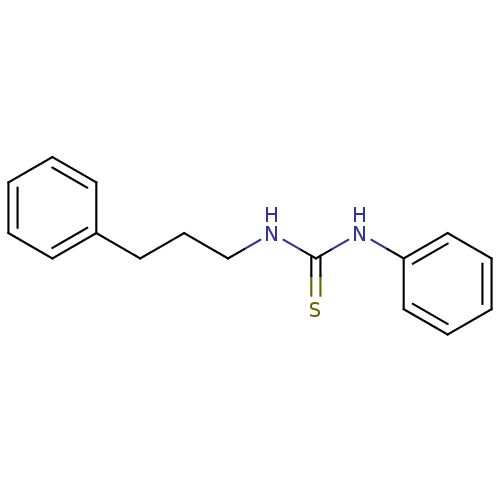 (CHEMBL1521932) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356181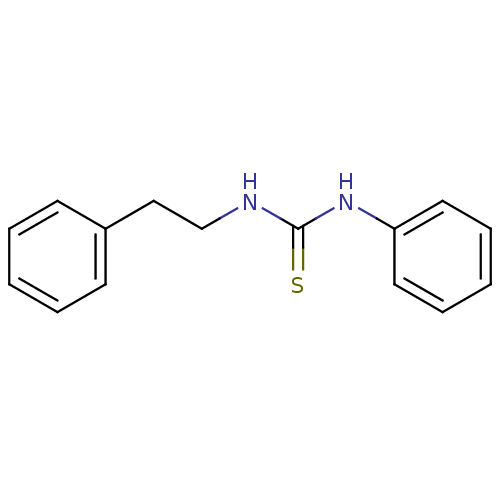 (CHEMBL1497537) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356183 (CHEMBL1910061) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356175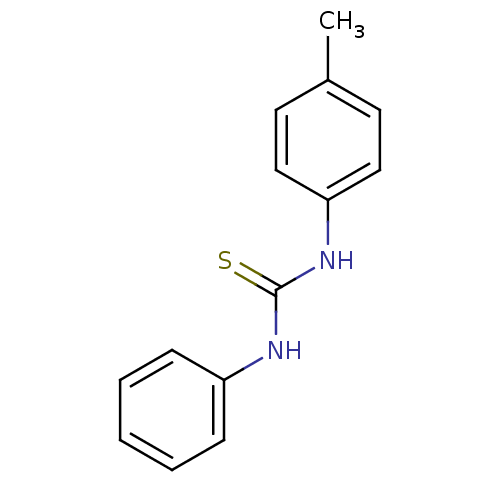 (CHEMBL1910057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356174 (CHEMBL275260) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356173 (CHEMBL1910056) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356176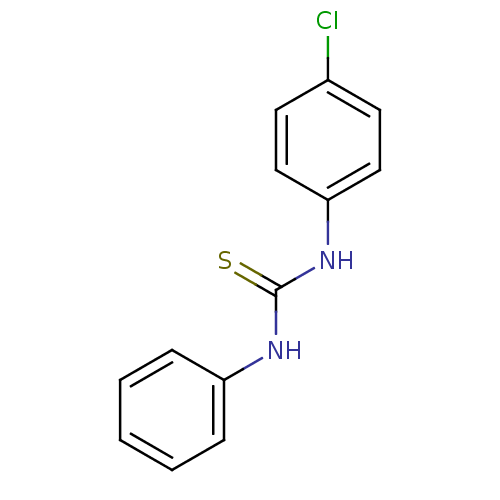 (CHEMBL1910058) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356171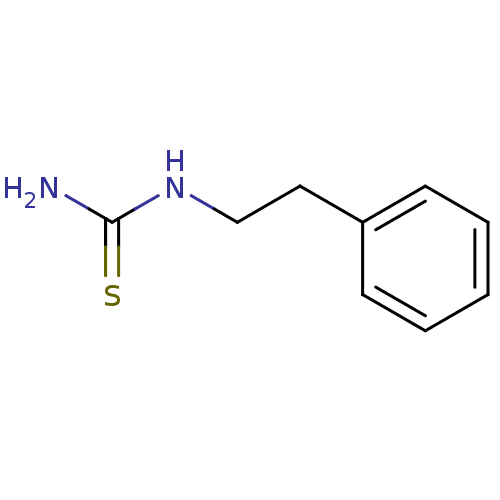 (CHEMBL1087842) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356184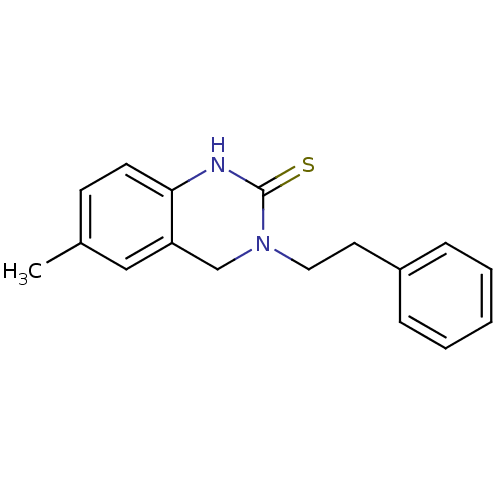 (CHEMBL591208) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356179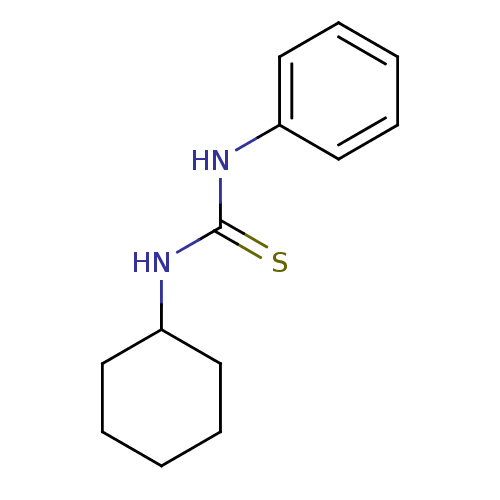 (CHEMBL1910059) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356169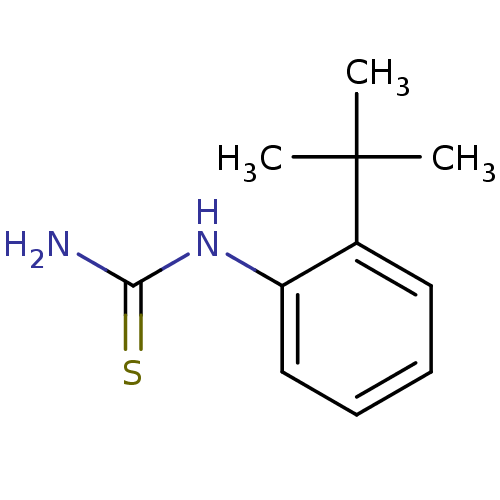 (CHEMBL1094725) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50356185 (CHEMBL1208820) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis | Bioorg Med Chem Lett 21: 6824-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.024 BindingDB Entry DOI: 10.7270/Q2M9093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |