 Found 176 hits with Last Name = 'jung' and Initial = 'ys'
Found 176 hits with Last Name = 'jung' and Initial = 'ys' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119798
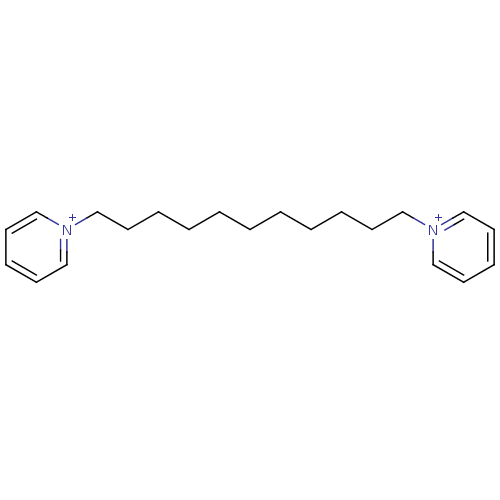
(1,11-bis(pyridinium)-undecane dibromide | 1,11-di(...)Show InChI InChI=1S/C21H32N2/c1(2-4-6-10-16-22-18-12-8-13-19-22)3-5-7-11-17-23-20-14-9-15-21-23/h8-9,12-15,18-21H,1-7,10-11,16-17H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis for enzyme-inhibitor complex |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119798

(1,11-bis(pyridinium)-undecane dibromide | 1,11-di(...)Show InChI InChI=1S/C21H32N2/c1(2-4-6-10-16-22-18-12-8-13-19-22)3-5-7-11-17-23-20-14-9-15-21-23/h8-9,12-15,18-21H,1-7,10-11,16-17H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334300
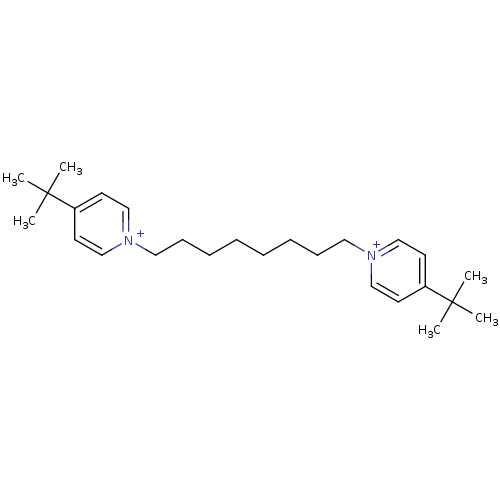
(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50313093
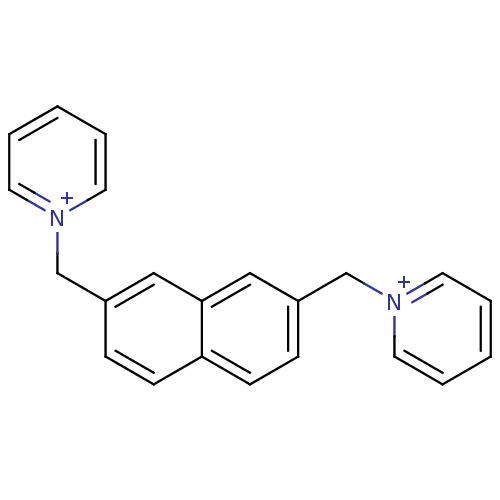
(1,1'-bis(pyridinium)-naphtyl-3,6-dimethylene dibro...)Show InChI InChI=1S/C22H20N2/c1-3-11-23(12-4-1)17-19-7-9-21-10-8-20(16-22(21)15-19)18-24-13-5-2-6-14-24/h1-16H,17-18H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis for enzyme-inhibitor complex |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50313093

(1,1'-bis(pyridinium)-naphtyl-3,6-dimethylene dibro...)Show InChI InChI=1S/C22H20N2/c1-3-11-23(12-4-1)17-19-7-9-21-10-8-20(16-22(21)15-19)18-24-13-5-2-6-14-24/h1-16H,17-18H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334300

(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8958
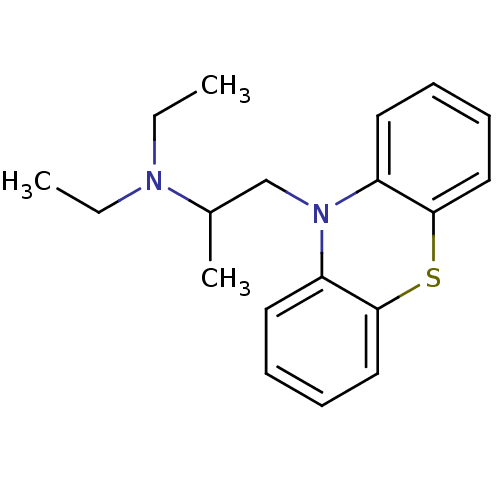
(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8958

(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.21E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334300

(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50304127

(1,12-Bis-(4-tert-butylpyridinium)dodecane dibromid...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C30H50N2/c1-29(2,3)27-17-23-31(24-18-27)21-15-13-11-9-7-8-10-12-14-16-22-32-25-19-28(20-26-32)30(4,5)6/h17-20,23-26H,7-16,21-22H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334301

(1,10-bis(4-tert.butylpyridinium)-dec-1,10-diyl dib...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C28H46N2/c1-27(2,3)25-15-21-29(22-16-25)19-13-11-9-7-8-10-12-14-20-30-23-17-26(18-24-30)28(4,5)6/h15-18,21-24H,7-14,19-20H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334300

(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334302

(1,7-bis(4-tert.butylpyridinium)-hept-1,7-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C25H40N2/c1-24(2,3)22-12-18-26(19-13-22)16-10-8-7-9-11-17-27-20-14-23(15-21-27)25(4,5)6/h12-15,18-21H,7-11,16-17H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50304127

(1,12-Bis-(4-tert-butylpyridinium)dodecane dibromid...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C30H50N2/c1-29(2,3)27-17-23-31(24-18-27)21-15-13-11-9-7-8-10-12-14-16-22-32-25-19-28(20-26-32)30(4,5)6/h17-20,23-26H,7-16,21-22H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins using spectrophotometer by Ellman's method |
Bioorg Med Chem Lett 21: 6563-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.042
BindingDB Entry DOI: 10.7270/Q2HD7W2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334304

(1,11-bis(4-tert.butylpyridinium)-undec-1,11-diyl d...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C29H48N2/c1-28(2,3)26-16-22-30(23-17-26)20-14-12-10-8-7-9-11-13-15-21-31-24-18-27(19-25-31)29(4,5)6/h16-19,22-25H,7-15,20-21H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334305
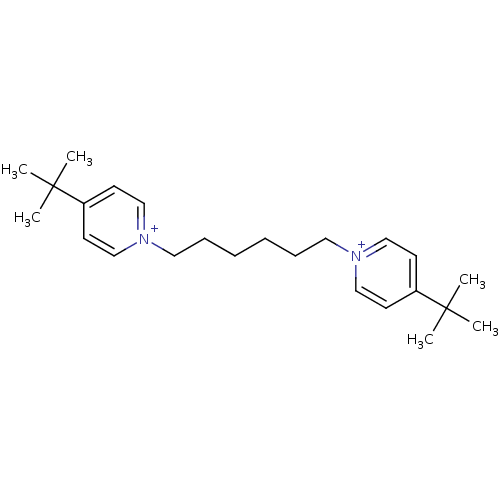
(1,6-bis(4-tert.butylpyridinium)-hex-1,6-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C24H38N2/c1-23(2,3)21-11-17-25(18-12-21)15-9-7-8-10-16-26-19-13-22(14-20-26)24(4,5)6/h11-14,17-20H,7-10,15-16H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334306

(1,9-bis(4-tert.butylpyridinium)-non-1,9-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H44N2/c1-26(2,3)24-14-20-28(21-15-24)18-12-10-8-7-9-11-13-19-29-22-16-25(17-23-29)27(4,5)6/h14-17,20-23H,7-13,18-19H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50327936
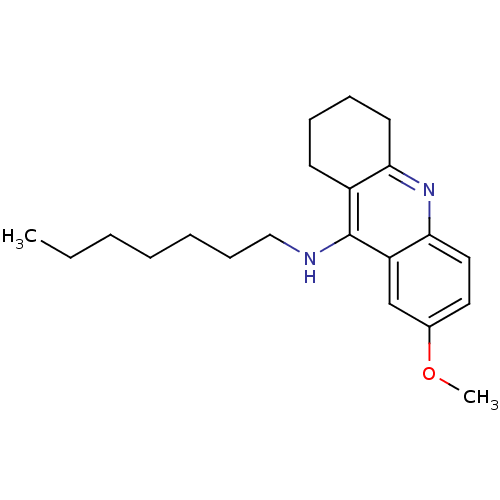
(CHEMBL1257767 | CHEMBL1618106 | N-n-heptyl-7-metho...)Show InChI InChI=1S/C21H30N2O/c1-3-4-5-6-9-14-22-21-17-10-7-8-11-19(17)23-20-13-12-16(24-2)15-18(20)21/h12-13,15H,3-11,14H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins using spectrophotometer by Ellman's method |
Bioorg Med Chem Lett 21: 6563-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.042
BindingDB Entry DOI: 10.7270/Q2HD7W2Q |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334301

(1,10-bis(4-tert.butylpyridinium)-dec-1,10-diyl dib...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C28H46N2/c1-27(2,3)25-15-21-29(22-16-25)19-13-11-9-7-8-10-12-14-20-30-23-17-26(18-24-30)28(4,5)6/h15-18,21-24H,7-14,19-20H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50327935
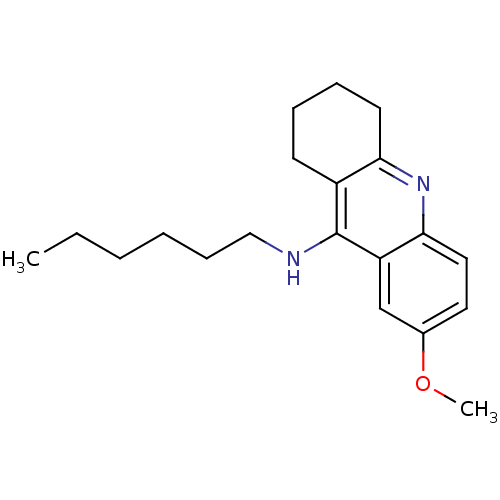
(CHEMBL1257885 | CHEMBL1618217 | N-n-hexyl-7-methox...)Show InChI InChI=1S/C20H28N2O/c1-3-4-5-8-13-21-20-16-9-6-7-10-18(16)22-19-12-11-15(23-2)14-17(19)20/h11-12,14H,3-10,13H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins using spectrophotometer by Ellman's method |
Bioorg Med Chem Lett 21: 6563-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.042
BindingDB Entry DOI: 10.7270/Q2HD7W2Q |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334304

(1,11-bis(4-tert.butylpyridinium)-undec-1,11-diyl d...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C29H48N2/c1-28(2,3)26-16-22-30(23-17-26)20-14-12-10-8-7-9-11-13-15-21-31-24-18-27(19-25-31)29(4,5)6/h16-19,22-25H,7-15,20-21H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334306

(1,9-bis(4-tert.butylpyridinium)-non-1,9-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H44N2/c1-26(2,3)24-14-20-28(21-15-24)18-12-10-8-7-9-11-13-19-29-22-16-25(17-23-29)27(4,5)6/h14-17,20-23H,7-13,18-19H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50313093

(1,1'-bis(pyridinium)-naphtyl-3,6-dimethylene dibro...)Show InChI InChI=1S/C22H20N2/c1-3-11-23(12-4-1)17-19-7-9-21-10-8-20(16-22(21)15-19)18-24-13-5-2-6-14-24/h1-16H,17-18H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115725

(3-(1-Indol-1-yl-3,4-dioxo-3,4-dihydro-naphthalen-2...)Show SMILES CCOC(=O)CCC1=C(c2ccccc2C(=O)C1=O)n1ccc2ccccc12 |t:7| Show InChI InChI=1S/C23H19NO4/c1-2-28-20(25)12-11-18-21(24-14-13-15-7-3-6-10-19(15)24)16-8-4-5-9-17(16)22(26)23(18)27/h3-10,13-14H,2,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50327936

(CHEMBL1257767 | CHEMBL1618106 | N-n-heptyl-7-metho...)Show InChI InChI=1S/C21H30N2O/c1-3-4-5-6-9-14-22-21-17-10-7-8-11-19(17)23-20-13-12-16(24-2)15-18(20)21/h12-13,15H,3-11,14H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins using spectrophotometer by Ellman's method |
Bioorg Med Chem Lett 21: 6563-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.042
BindingDB Entry DOI: 10.7270/Q2HD7W2Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334307
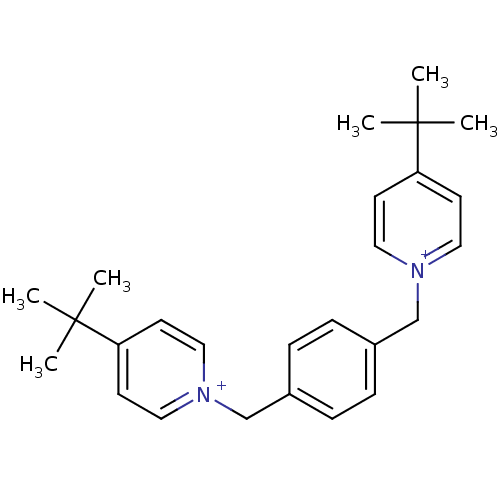
(1,1'-bis(4-tert.butylpyridinium)-1,4-phenyldimethy...)Show SMILES CC(C)(C)c1cc[n+](Cc2ccc(C[n+]3ccc(cc3)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C26H34N2/c1-25(2,3)23-11-15-27(16-12-23)19-21-7-9-22(10-8-21)20-28-17-13-24(14-18-28)26(4,5)6/h7-18H,19-20H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115722

(4-Cyclohexyl-[1,2]naphthoquinone | CHEMBL58737)Show InChI InChI=1S/C16H16O2/c17-15-10-14(11-6-2-1-3-7-11)12-8-4-5-9-13(12)16(15)18/h4-5,8-11H,1-3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115756

(3-(5,6-Dioxo-8-phenyl-5,6-dihydro-naphthalen-2-yl)...)Show SMILES COC(=O)CCc1ccc2C(=O)C(=O)C=C(c3ccccc3)c2c1 |t:14| Show InChI InChI=1S/C20H16O4/c1-24-19(22)10-8-13-7-9-15-17(11-13)16(12-18(21)20(15)23)14-5-3-2-4-6-14/h2-7,9,11-12H,8,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119779
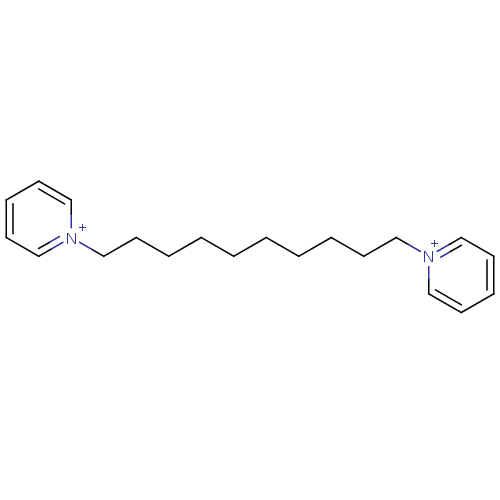
(1,1'-(decane-1,10-diyl)dipyridinium iodide | 1,10-...)Show InChI InChI=1S/C20H30N2/c1(3-5-9-15-21-17-11-7-12-18-21)2-4-6-10-16-22-19-13-8-14-20-22/h7-8,11-14,17-20H,1-6,9-10,15-16H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115733
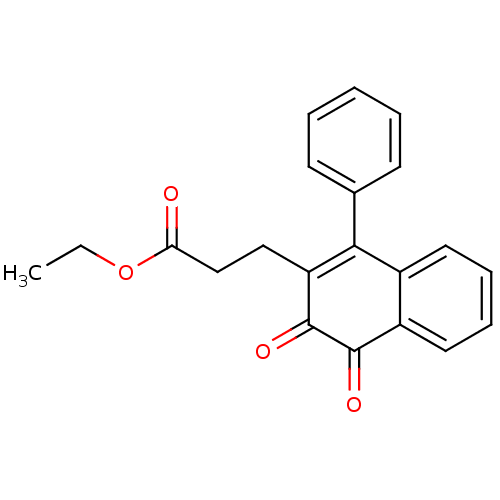
(3-(3,4-Dioxo-1-phenyl-3,4-dihydro-naphthalen-2-yl)...)Show SMILES CCOC(=O)CCC1=C(c2ccccc2)c2ccccc2C(=O)C1=O |c:7| Show InChI InChI=1S/C21H18O4/c1-2-25-18(22)13-12-17-19(14-8-4-3-5-9-14)15-10-6-7-11-16(15)20(23)21(17)24/h3-11H,2,12-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115766
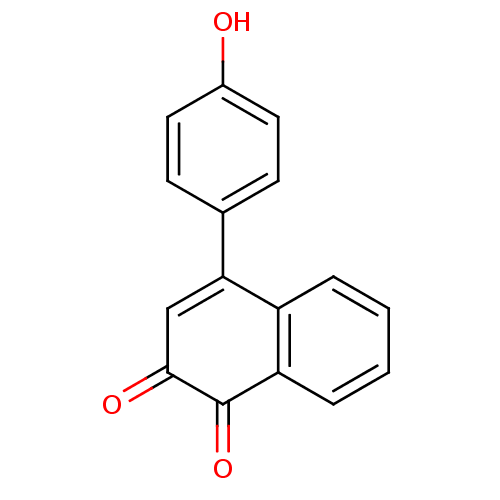
(4-(4-Hydroxy-phenyl)-[1,2]naphthoquinone | CHEMBL5...)Show InChI InChI=1S/C16H10O3/c17-11-7-5-10(6-8-11)14-9-15(18)16(19)13-4-2-1-3-12(13)14/h1-9,17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115760

(4-(2,5-Difluoro-phenyl)-[1,2]naphthoquinone | CHEM...)Show SMILES Fc1ccc(F)c(c1)C1=CC(=O)C(=O)c2ccccc12 |t:9| Show InChI InChI=1S/C16H8F2O2/c17-9-5-6-14(18)13(7-9)12-8-15(19)16(20)11-4-2-1-3-10(11)12/h1-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins using spectrophotometer by Ellman's method |
Bioorg Med Chem Lett 21: 6563-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.042
BindingDB Entry DOI: 10.7270/Q2HD7W2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334305

(1,6-bis(4-tert.butylpyridinium)-hex-1,6-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C24H38N2/c1-23(2,3)21-11-17-25(18-12-21)15-9-7-8-10-16-26-19-13-22(14-20-26)24(4,5)6/h11-14,17-20H,7-10,15-16H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115721

(3-(3,4-Dioxo-1-phenyl-3,4-dihydro-naphthalen-2-yl)...)Show SMILES CCN(CC)C(=O)CCC1=C(c2ccccc2)c2ccccc2C(=O)C1=O |c:9| Show InChI InChI=1S/C23H23NO3/c1-3-24(4-2)20(25)15-14-19-21(16-10-6-5-7-11-16)17-12-8-9-13-18(17)22(26)23(19)27/h5-13H,3-4,14-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115740
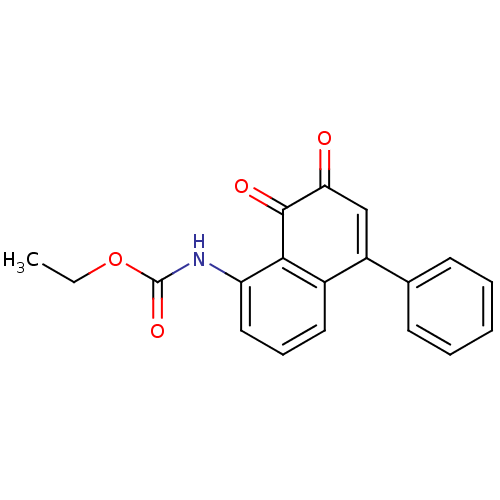
((7,8-Dioxo-5-phenyl-7,8-dihydro-naphthalen-1-yl)-c...)Show SMILES CCOC(=O)Nc1cccc2C(=CC(=O)C(=O)c12)c1ccccc1 |c:11| Show InChI InChI=1S/C19H15NO4/c1-2-24-19(23)20-15-10-6-9-13-14(12-7-4-3-5-8-12)11-16(21)18(22)17(13)15/h3-11H,2H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115739

(CHEMBL56948 | [4-(1-Indol-1-yl-3,4-dioxo-3,4-dihyd...)Show SMILES CC(C)(C)OC(=O)COc1ccc(CC2=C(c3ccccc3C(=O)C2=O)n2ccc3ccccc23)cc1 |t:14| Show InChI InChI=1S/C31H27NO5/c1-31(2,3)37-27(33)19-36-22-14-12-20(13-15-22)18-25-28(32-17-16-21-8-4-7-11-26(21)32)23-9-5-6-10-24(23)29(34)30(25)35/h4-17H,18-19H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115746

(3-[7-(2-Methoxycarbonyl-ethyl)-3,4-dioxo-1-phenyl-...)Show SMILES COC(=O)CCC1=C(c2ccccc2)c2cc(CCC(=O)OC)ccc2C(=O)C1=O |c:6| Show InChI InChI=1S/C24H22O6/c1-29-20(25)12-9-15-8-10-17-19(14-15)22(16-6-4-3-5-7-16)18(24(28)23(17)27)11-13-21(26)30-2/h3-8,10,14H,9,11-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) |
Bioorg Med Chem Lett 12: 1941-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QZ2999 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334308

(1,1'-bis(4-tert.butylpyridinium)-1,3-phenyldimethy...)Show SMILES CC(C)(C)c1cc[n+](Cc2cccc(C[n+]3ccc(cc3)C(C)(C)C)c2)cc1 Show InChI InChI=1S/C26H34N2/c1-25(2,3)23-10-14-27(15-11-23)19-21-8-7-9-22(18-21)20-28-16-12-24(13-17-28)26(4,5)6/h7-18H,19-20H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334302

(1,7-bis(4-tert.butylpyridinium)-hept-1,7-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C25H40N2/c1-24(2,3)22-12-18-26(19-13-22)16-10-8-7-9-11-17-27-20-14-23(15-21-27)25(4,5)6/h12-15,18-21H,7-11,16-17H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119798

(1,11-bis(pyridinium)-undecane dibromide | 1,11-di(...)Show InChI InChI=1S/C21H32N2/c1(2-4-6-10-16-22-18-12-8-13-19-22)3-5-7-11-17-23-20-14-9-15-21-23/h8-9,12-15,18-21H,1-7,10-11,16-17H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data