

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50090462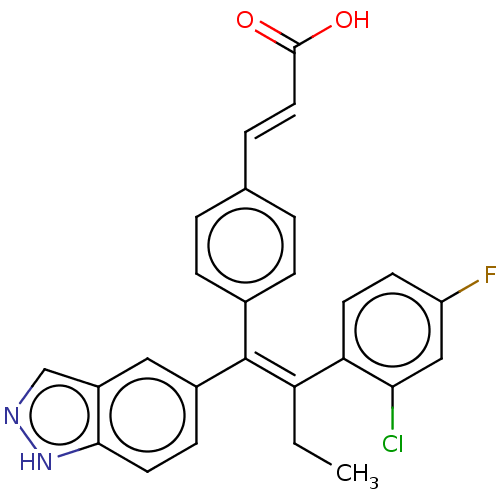 (CHEMBL3581693 | US20240043442, Example GDC-0810) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length recombinant human ESR1 expressed in insect cells by radiometric assay | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233215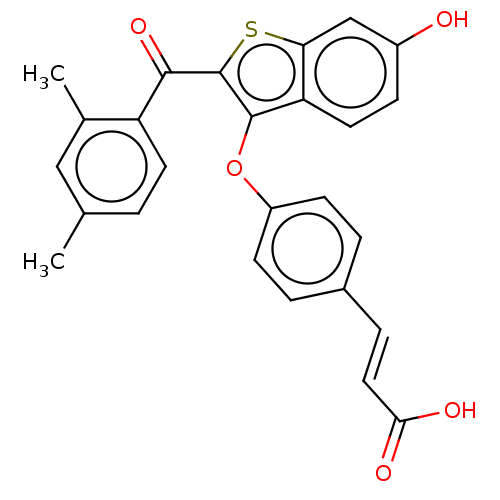 (CHEMBL4070169) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length recombinant human ESR1 expressed in insect cells by radiometric assay | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233216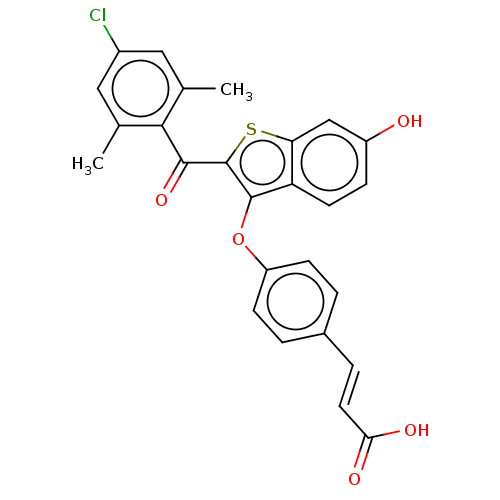 (CHEMBL4081807) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length recombinant human ESR1 expressed in insect cells by radiometric assay | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233203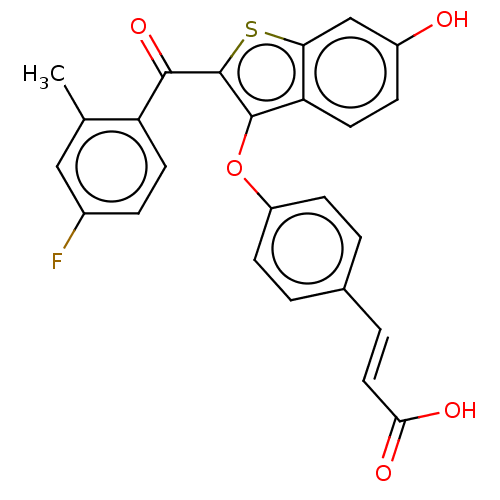 (CHEMBL4098867) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length recombinant human ESR1 expressed in insect cells by radiometric assay | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233205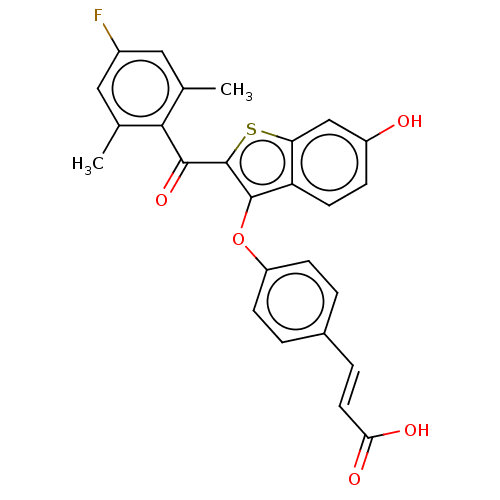 (CHEMBL4059888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length recombinant human ESR1 expressed in insect cells by radiometric assay | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233202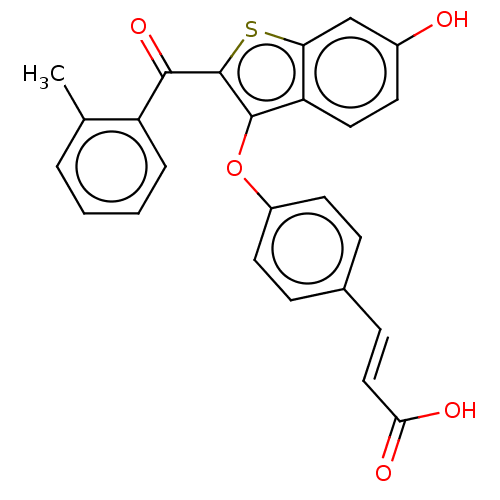 (CHEMBL4092208) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length recombinant human ESR1 expressed in insect cells by radiometric assay | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233204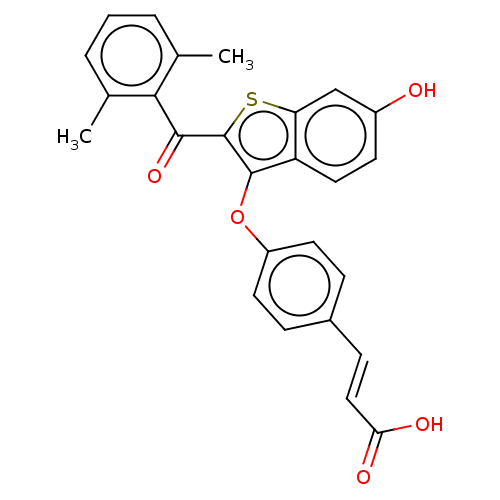 (CHEMBL4094499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length recombinant human ESR1 expressed in insect cells by radiometric assay | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233216 (CHEMBL4081807) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in tamoxifen-sensitive human MCF7:WS8 cells assessed as inhibition of estradiol-induced response after 18 hrs by lucif... | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233205 (CHEMBL4059888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in tamoxifen-sensitive human MCF7:WS8 cells assessed as inhibition of estradiol-induced response after 18 hrs by lucif... | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233204 (CHEMBL4094499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in tamoxifen-sensitive human MCF7:WS8 cells assessed as inhibition of estradiol-induced response after 18 hrs by lucif... | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233215 (CHEMBL4070169) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in tamoxifen-sensitive human MCF7:WS8 cells assessed as inhibition of estradiol-induced response after 18 hrs by lucif... | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233203 (CHEMBL4098867) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in tamoxifen-sensitive human MCF7:WS8 cells assessed as inhibition of estradiol-induced response after 18 hrs by lucif... | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50090462 (CHEMBL3581693 | US20240043442, Example GDC-0810) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in tamoxifen-sensitive human MCF7:WS8 cells assessed as inhibition of estradiol-induced response after 18 hrs by lucif... | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233202 (CHEMBL4092208) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in tamoxifen-sensitive human MCF7:WS8 cells assessed as inhibition of estradiol-induced response after 18 hrs by lucif... | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50232053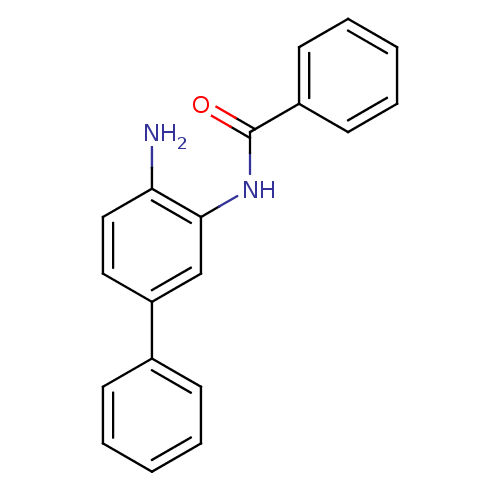 (CHEMBL271741 | N-(4-amino-biphenyl-3-yl)-benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387965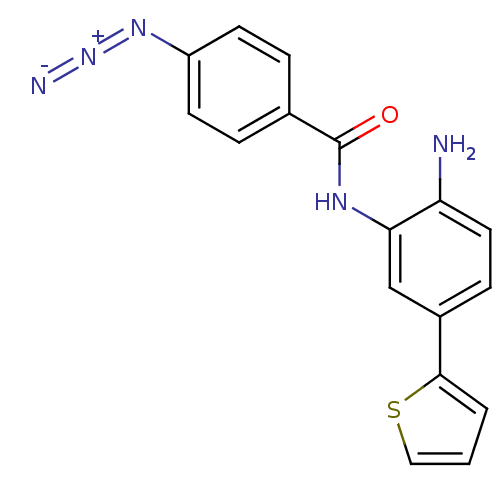 (CHEMBL2057824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387965 (CHEMBL2057824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50232053 (CHEMBL271741 | N-(4-amino-biphenyl-3-yl)-benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387967 (CHEMBL2057823) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387967 (CHEMBL2057823) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387973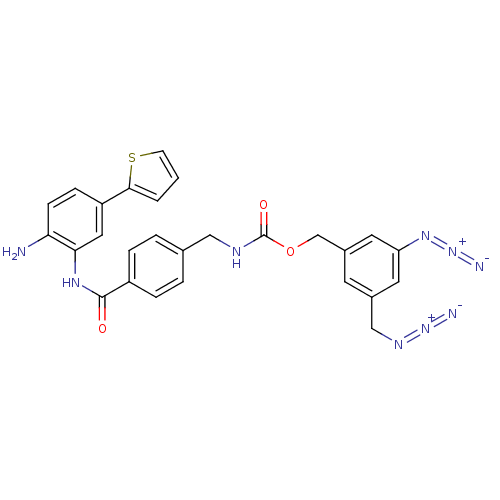 (CHEMBL2057822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387966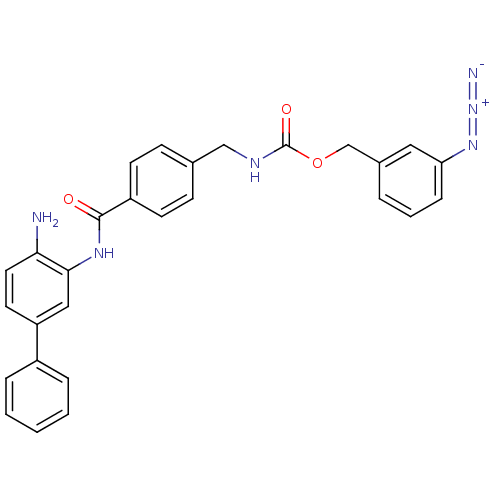 (CHEMBL2057820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50232053 (CHEMBL271741 | N-(4-amino-biphenyl-3-yl)-benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387964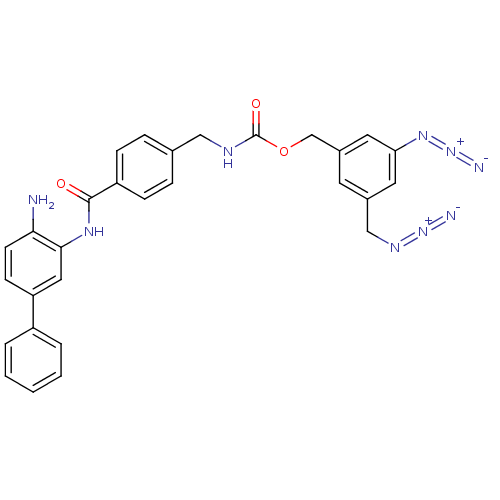 (CHEMBL2057821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50232053 (CHEMBL271741 | N-(4-amino-biphenyl-3-yl)-benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387965 (CHEMBL2057824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50229191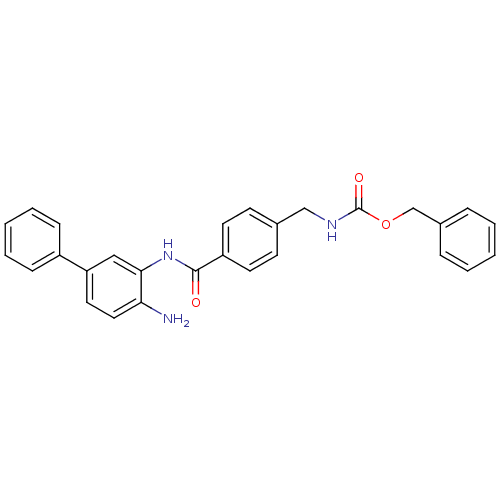 (CHEMBL252409 | US9096559, 15 | [4-(4-amino-bipheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387971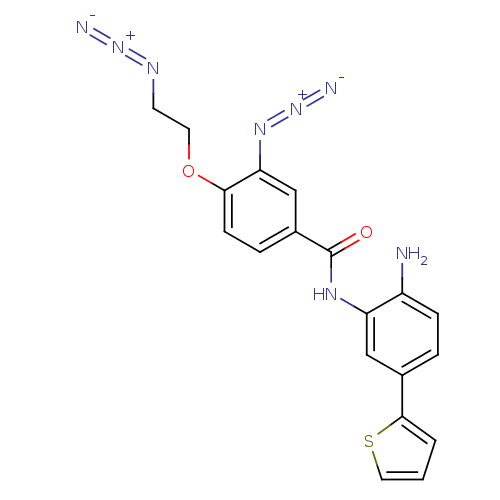 (CHEMBL2057828) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387966 (CHEMBL2057820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387970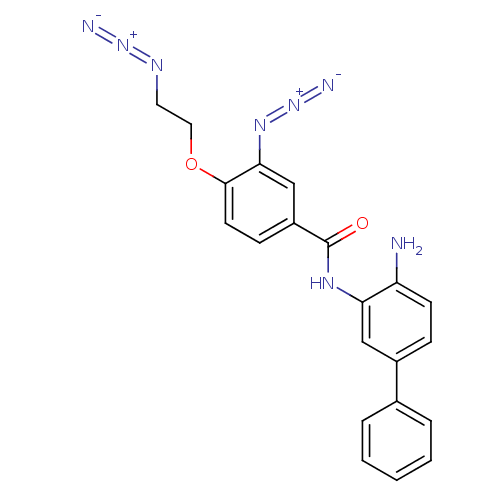 (CHEMBL2057827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387964 (CHEMBL2057821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387973 (CHEMBL2057822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387966 (CHEMBL2057820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387967 (CHEMBL2057823) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387964 (CHEMBL2057821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387965 (CHEMBL2057824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50232053 (CHEMBL271741 | N-(4-amino-biphenyl-3-yl)-benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387973 (CHEMBL2057822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387968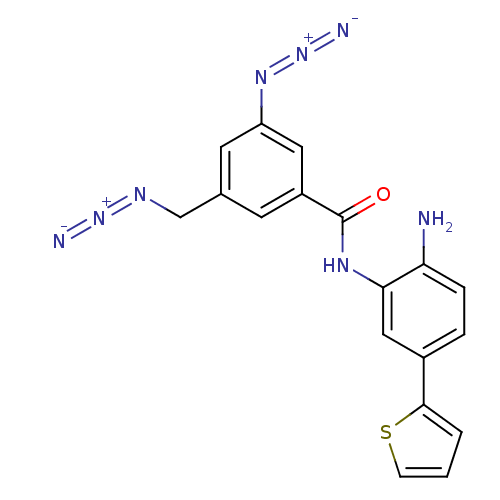 (CHEMBL2057825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229191 (CHEMBL252409 | US9096559, 15 | [4-(4-amino-bipheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387967 (CHEMBL2057823) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387968 (CHEMBL2057825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 24 hrs measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387964 (CHEMBL2057821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387970 (CHEMBL2057827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 3 hrs measured after 35 mins by spectroflu... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50387973 (CHEMBL2057822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50387965 (CHEMBL2057824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using Boc-L-Lys (Ac)-AMC as substrate preincubated with compound for 5 mins measured after 35 mins by spectrofl... | Bioorg Med Chem Lett 22: 5025-30 (2012) Article DOI: 10.1016/j.bmcl.2012.06.017 BindingDB Entry DOI: 10.7270/Q2F76DNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |