 Found 4706 hits with Last Name = 'kato' and Initial = 'm'
Found 4706 hits with Last Name = 'kato' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586369

(CHEMBL5094265)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586368

(CHEMBL5073848)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50004519

(CHEMBL2181929)Show SMILES CC1(C)Oc2cc(NS(C)(=O)=O)ccc2N(C1=O)c1ccc(F)cc1 Show InChI InChI=1S/C17H17FN2O4S/c1-17(2)16(21)20(13-7-4-11(18)5-8-13)14-9-6-12(10-15(14)24-17)19-25(3,22)23/h4-10,19H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK1
(Homo sapiens (Human)) | BDBM50258546
(CHEMBL4087727)Show SMILES COc1cccc(c1)-c1cccc(c1)-n1cc(CNCC2CCCCC2)c2ccccc12 Show InChI InChI=1S/C29H32N2O/c1-32-27-14-8-12-24(18-27)23-11-7-13-26(17-23)31-21-25(28-15-5-6-16-29(28)31)20-30-19-22-9-3-2-4-10-22/h5-8,11-18,21-22,30H,2-4,9-10,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States.
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant human N-terminal GST-tagged WNK1 (1 to 491 residues) expressed in baculovirus expression system using fluor... |
J Med Chem 60: 7099-7107 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00708
BindingDB Entry DOI: 10.7270/Q29W0HXP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50318300
(CHEMBL1095097 | EPLERENONE | SC-66110)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]23O[C@@H]2C[C@@]2(C)[C@@H](CC[C@@]22CCC(=O)O2)[C@H]13 |r,t:6| Show InChI InChI=1S/C24H30O6/c1-21-7-4-14(25)10-13(21)11-15(20(27)28-3)19-16-5-8-23(9-6-18(26)30-23)22(16,2)12-17-24(19,21)29-17/h10,15-17,19H,4-9,11-12H2,1-3H3/t15-,16+,17-,19+,21+,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86754
(LY 466195 | LY-466195)Show SMILES OC(=O)C1CC(F)(F)CN1C[C@H]1CC[C@H]2CN[C@@H](C[C@H]2C1)C(O)=O |r| Show InChI InChI=1S/C16H24F2N2O4/c17-16(18)5-13(15(23)24)20(8-16)7-9-1-2-10-6-19-12(14(21)22)4-11(10)3-9/h9-13,19H,1-8H2,(H,21,22)(H,23,24)/t9-,10-,11+,12-,13?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586370
(CHEMBL5084197)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCn2cc(COc3cc(OCc4cn(CCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)cc(c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586371
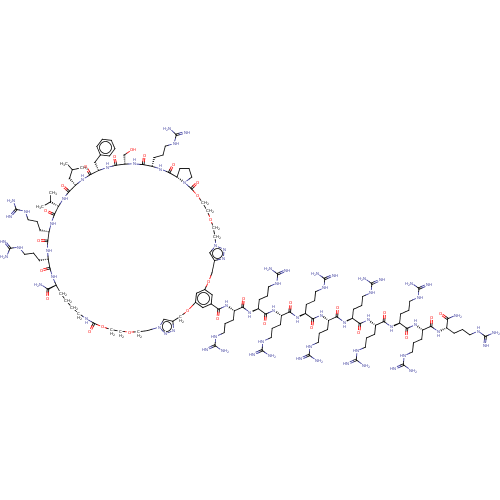
(CHEMBL5089876)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCOCCn2cc(COc3cc(OCc4cn(CCOCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)cc(c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586366

(CHEMBL5093950)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586367

(CHEMBL5089144)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597078

(CHEMBL5181002) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597086
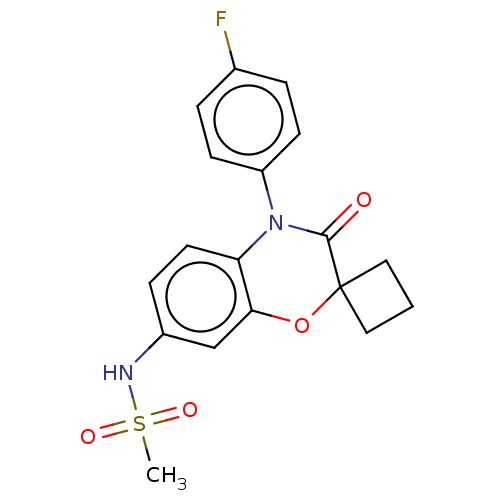
(CHEMBL5187656)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CCC3)Oc2c1)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597084

(CHEMBL5174401)Show SMILES CC1Oc2cc(NS(C)(=O)=O)ccc2N(C1=O)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597087
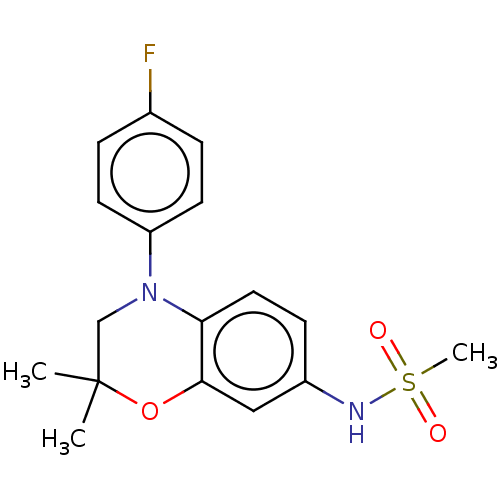
(CHEMBL5181278)Show SMILES CC1(C)CN(c2ccc(F)cc2)c2ccc(NS(C)(=O)=O)cc2O1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586373

(CHEMBL5075544)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1cc(OCc2cn(CCO)nn2)cc(OCc2cn(CCO)nn2)c1)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586365

(CHEMBL5078239)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597084

(CHEMBL5174401)Show SMILES CC1Oc2cc(NS(C)(=O)=O)ccc2N(C1=O)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597083
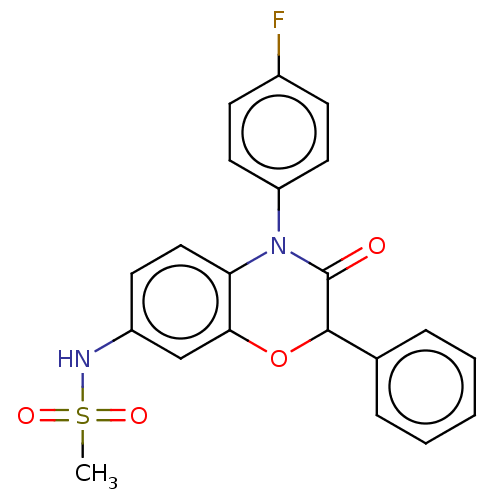
(CHEMBL5171957)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C(Oc2c1)c1ccccc1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 2 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597083

(CHEMBL5171957)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C(Oc2c1)c1ccccc1)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597078

(CHEMBL5181002) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597087

(CHEMBL5181278)Show SMILES CC1(C)CN(c2ccc(F)cc2)c2ccc(NS(C)(=O)=O)cc2O1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Glutamate--cysteine ligase catalytic subunit
(Homo sapiens (Human)) | BDBM50366432
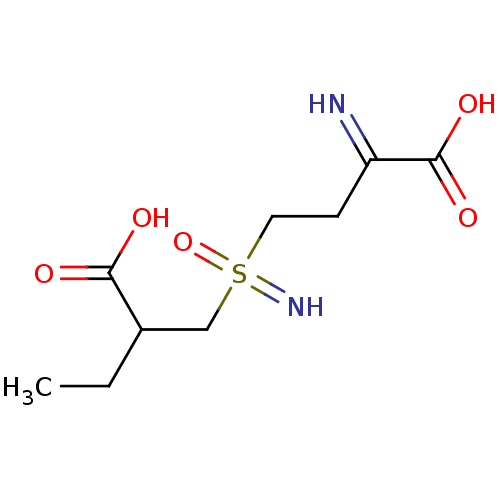
(CHEMBL1627263)Show InChI InChI=1S/C9H18N2O5S/c1-2-6(8(12)13)5-17(11,16)4-3-7(10)9(14)15/h6,10,17H,2-5H2,1H3,(H2,11,16)(H,12,13)(H,14,15) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of Escherichia coli gamma-glutamylcysteine synthetase at a concentration of 5.1 uM |
Bioorg Med Chem Lett 6: 1437-1442 (1996)
Article DOI: 10.1016/S0960-894X(96)00247-8
BindingDB Entry DOI: 10.7270/Q2KH0NV4 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597085
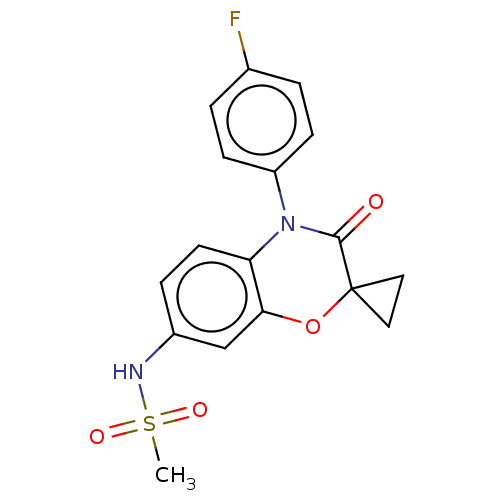
(CHEMBL5183454)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CC3)Oc2c1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597081
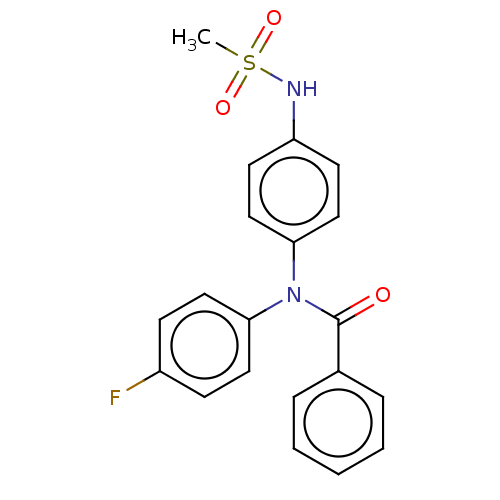
(CHEMBL5184647)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 596 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Glutamate receptor 4
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 4 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597077
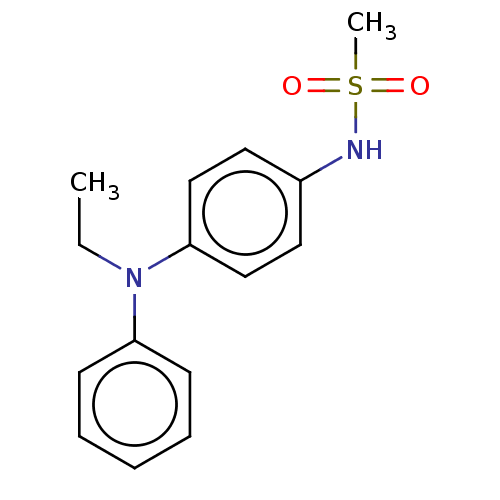
(CHEMBL5172350) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 607 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597081

(CHEMBL5184647)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 611 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597086

(CHEMBL5187656)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CCC3)Oc2c1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586372
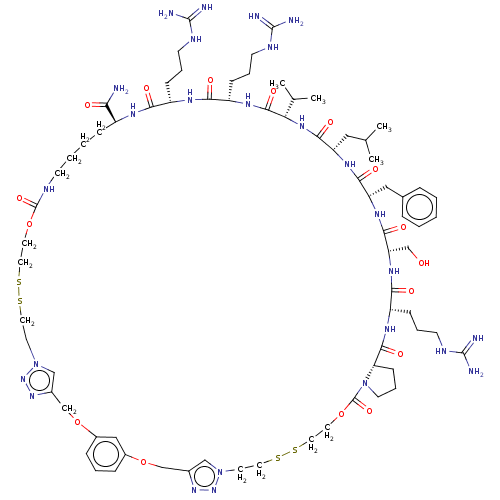
(CHEMBL5085737)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCn2cc(COc3cccc(OCc4cn(CCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)c3)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597076

(CHEMBL5176336) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 735 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597068
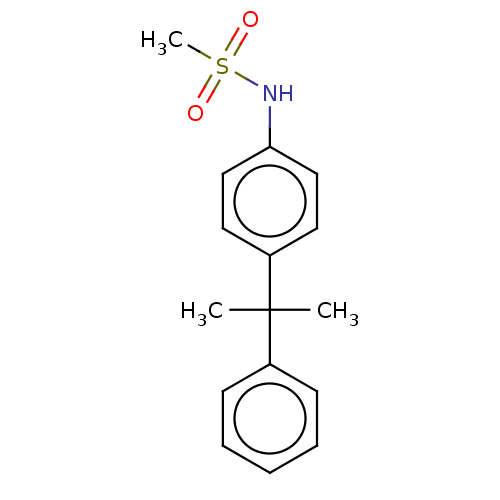
(CHEMBL5201004) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597085

(CHEMBL5183454)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CC3)Oc2c1)c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 808 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86750
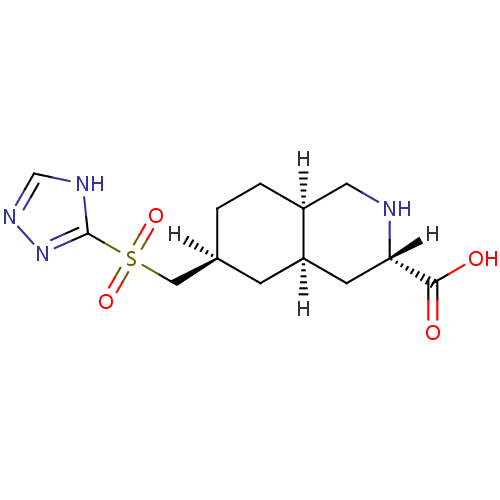
(LY 302679 | LY-302679)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](C[S](=O)(=O)c3nnc[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H20N4O4S/c18-12(19)11-4-10-3-8(1-2-9(10)5-14-11)6-22(20,21)13-15-7-16-17-13/h7-11,14H,1-6H2,(H,18,19)(H,15,16,17)/t8-,9-,10+,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597076

(CHEMBL5176336) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 3 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586364
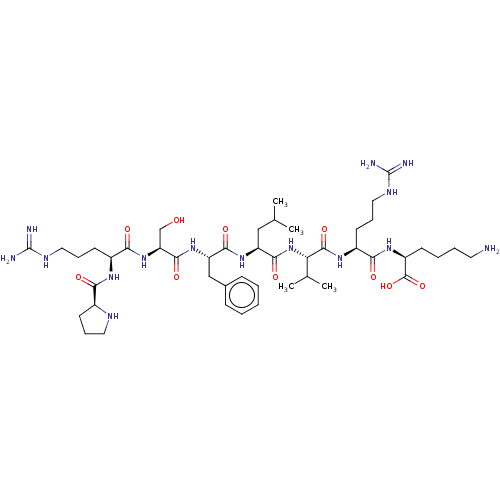
(CHEMBL5084292)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50586363
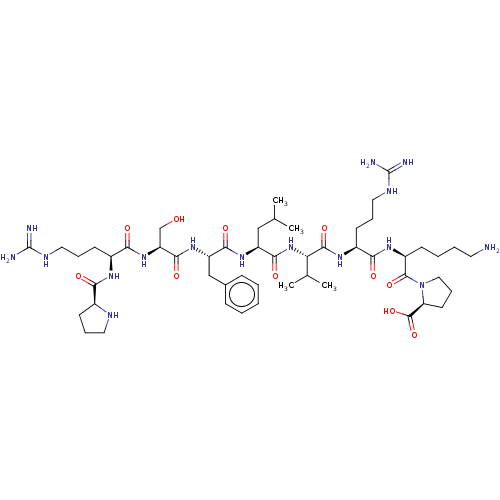
(CHEMBL5079374)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1 using K4me2 peptide as substrate measured after 10 mins by peroxidase-coupled reaction assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597080
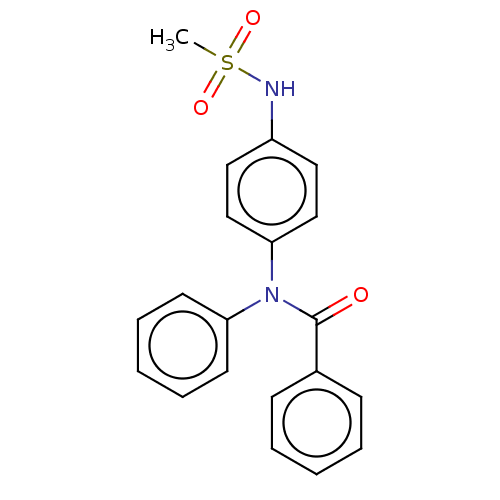
(CHEMBL5207249)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597080

(CHEMBL5207249)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597073
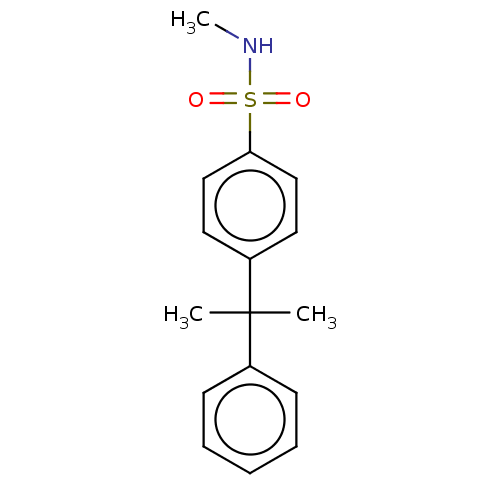
(CHEMBL5182687) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86749

(LY 457691)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Nc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H22N6O2/c24-17(25)15-8-11-7-12(6-5-10(11)9-18-15)19-14-4-2-1-3-13(14)16-20-22-23-21-16/h1-4,10-12,15,18-19H,5-9H2,(H,24,25)(H,20,21,22,23)/t10-,11+,12-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597077

(CHEMBL5172350) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 1 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86755

(LY 458545 | LY-458545)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Oc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H21N5O3/c23-17(24)14-8-11-7-12(6-5-10(11)9-18-14)25-15-4-2-1-3-13(15)16-19-21-22-20-16/h1-4,10-12,14,18H,5-9H2,(H,23,24)(H,19,20,21,22)/t10-,11+,12-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597082

(CHEMBL5204612) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50597070
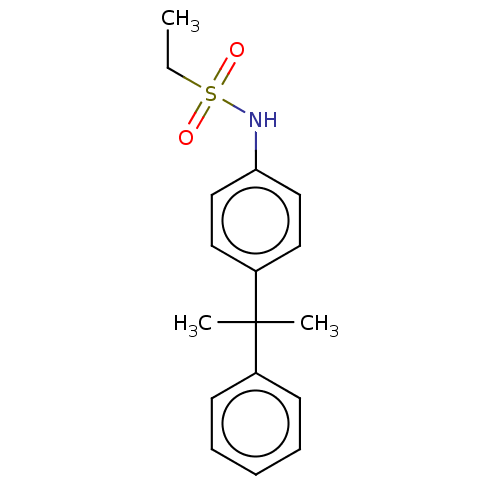
(CHEMBL5186524) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597068

(CHEMBL5201004) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like elastase family member 1/2A
(Sus scrofa (Pig)) | BDBM50226399

(CHEMBL3143932)Show SMILES COC(=O)CCC(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)CN(C(C)C)C(=O)Sc1nnnn1-c1ccccc1 |r| Show InChI InChI=1S/C28H38N8O7S/c1-17(2)35(28(42)44-27-31-32-33-36(27)20-10-7-6-8-11-20)16-22(37)21-12-9-15-34(21)26(41)19(4)30-25(40)18(3)29-23(38)13-14-24(39)43-5/h6-8,10-11,17-19,21H,9,12-16H2,1-5H3,(H,29,38)(H,30,40)/t18-,19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound is evaluated for the inhibition of porcine pancreatic (PP) elastase |
J Med Chem 29: 1468-76 (1986)
BindingDB Entry DOI: 10.7270/Q2NG4ST4 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86752
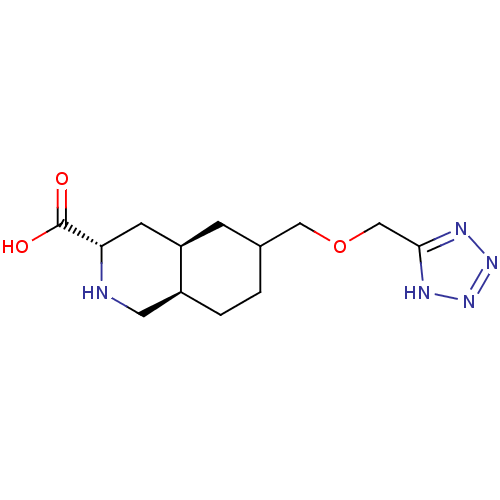
(LY 294486 | LY-294486)Show SMILES OC(=O)[C@@H]1C[C@H]2CC(COCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O3/c19-13(20)11-4-10-3-8(1-2-9(10)5-14-11)6-21-7-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8?,9-,10+,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data