

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM126083 (US8779142, 102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human HSP90-beta by fluorescence polarization competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01715 BindingDB Entry DOI: 10.7270/Q2Z323H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM126083 (US8779142, 102) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FITC-GDA binding to human HSP90-alpha incubated for 2 hrs followed by FITC-GDA addition and measured after 5 hrs by fluorescence polari... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01715 BindingDB Entry DOI: 10.7270/Q2Z323H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50582399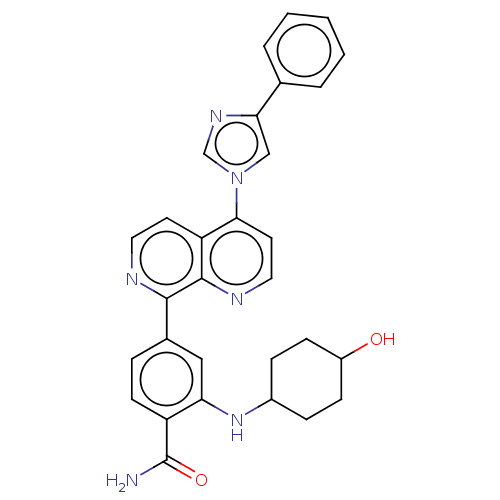 (CHEMBL5094983) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FITC-GDA binding to human HSP90-alpha incubated for 2 hrs followed by FITC-GDA addition and measured after 5 hrs by fluorescence polari... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01715 BindingDB Entry DOI: 10.7270/Q2Z323H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM50582399 (CHEMBL5094983) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human HSP90-beta by fluorescence polarization competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01715 BindingDB Entry DOI: 10.7270/Q2Z323H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmin (Homo sapiens (Human)) | BDBM126083 (US8779142, 102) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to GRP94 (unknown origin) by fluorescence polarization competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01715 BindingDB Entry DOI: 10.7270/Q2Z323H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein 75 kDa, mitochondrial (Homo sapiens (Human)) | BDBM126083 (US8779142, 102) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to TRAP1 (unknown origin) by fluorescence polarization competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01715 BindingDB Entry DOI: 10.7270/Q2Z323H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmin (Homo sapiens (Human)) | BDBM50582399 (CHEMBL5094983) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to GRP94 (unknown origin) by fluorescence polarization competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01715 BindingDB Entry DOI: 10.7270/Q2Z323H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein 75 kDa, mitochondrial (Homo sapiens (Human)) | BDBM50582399 (CHEMBL5094983) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to TRAP1 (unknown origin) by fluorescence polarization competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01715 BindingDB Entry DOI: 10.7270/Q2Z323H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309489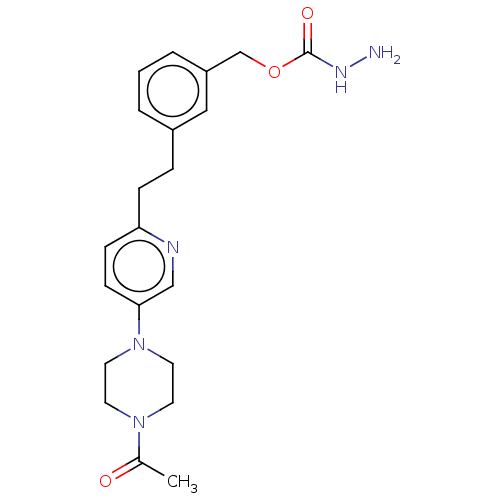 (US9603833, Example 111) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM309489 (US9603833, Example 111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in the Production Examples were examined for the inhibitory effect on human monoamineoxydase enzymes ... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309497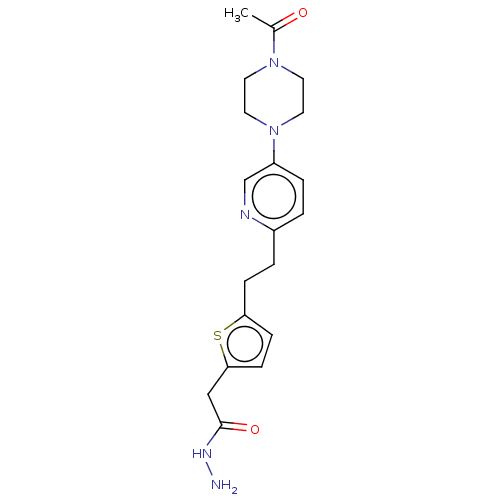 (US9603833, Example 120) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309491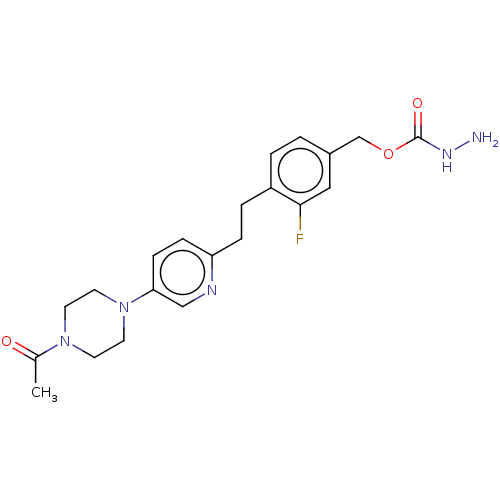 (US9603833, Example 113) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309490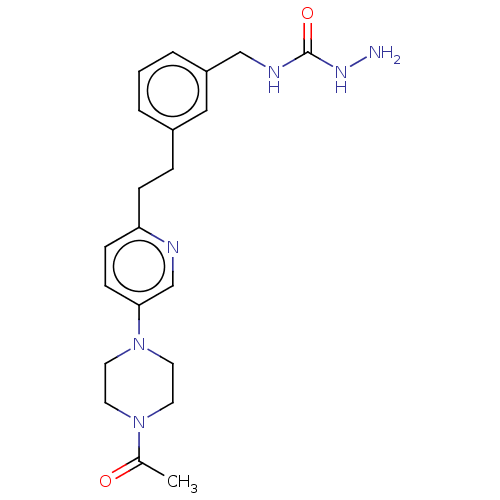 (US9603833, Example 112) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM309490 (US9603833, Example 112) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM50576728 (CHEMBL4849611 | US20230348495, Example 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM50576730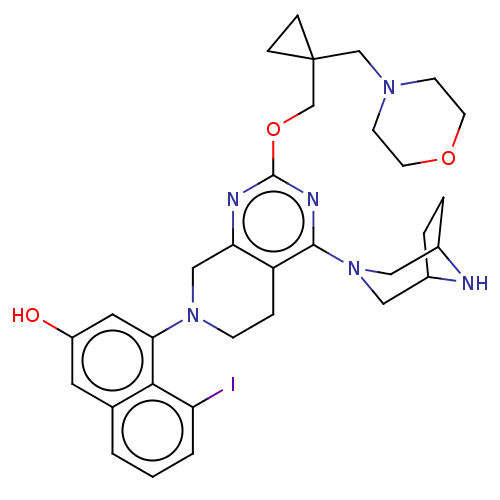 (CHEMBL4869200 | US20230348495, Example 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309486 (US9603833, Example 108) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM633148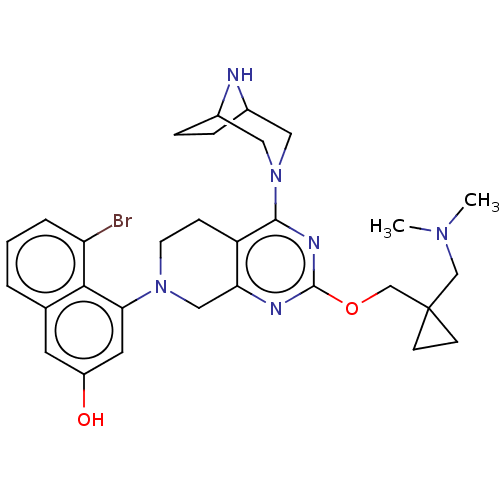 (US20230348495, Example 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309479 (US9603833, Example 92) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309477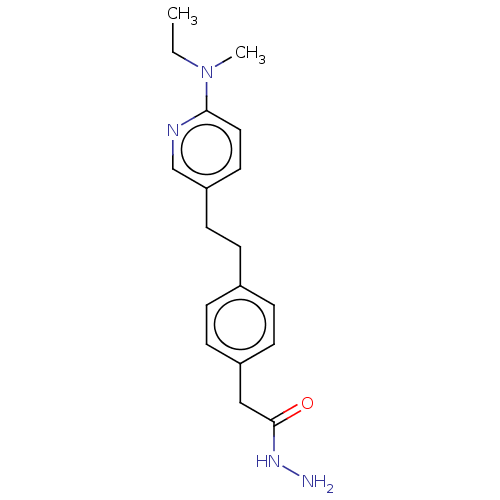 (US9603833, Example 89) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309476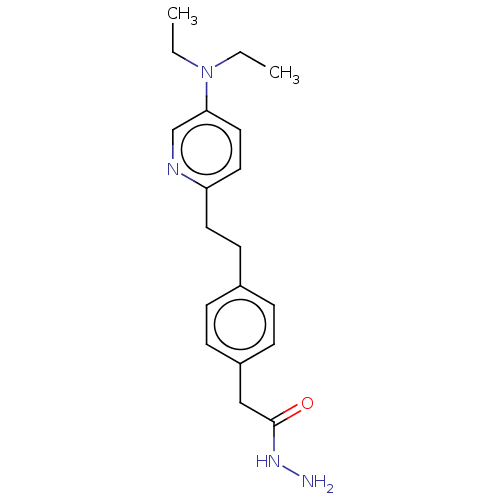 (US9603833, Example 88) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM50576729 (CHEMBL4876274 | US20230348495, Example 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM309491 (US9603833, Example 113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM309486 (US9603833, Example 108) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309474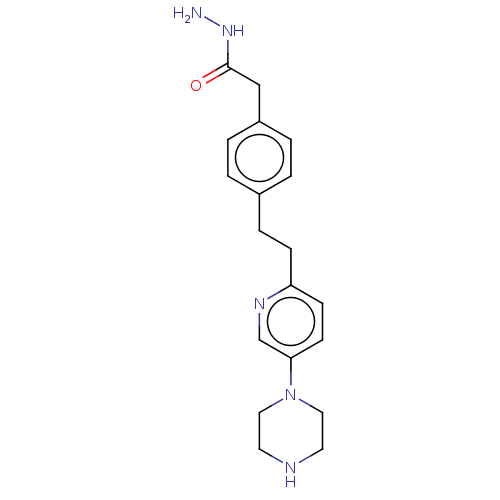 (US9603833, Example 86) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309459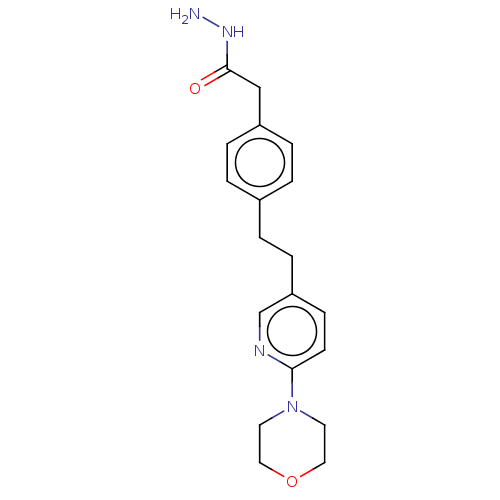 (US9603833, Example 12) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309492 (US9603833, Example 114) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309464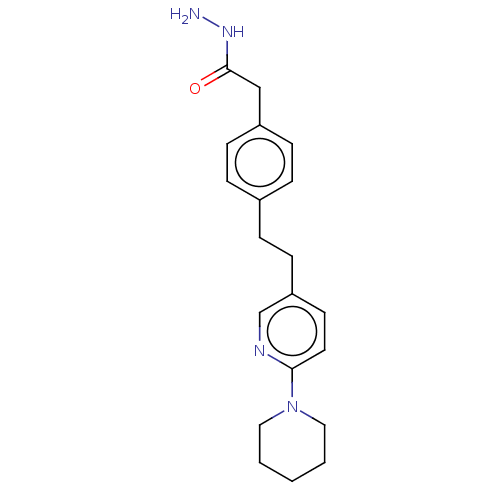 (US9603833, Example 45) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM50576726 (CHEMBL4872714 | US20230348495, Example 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309470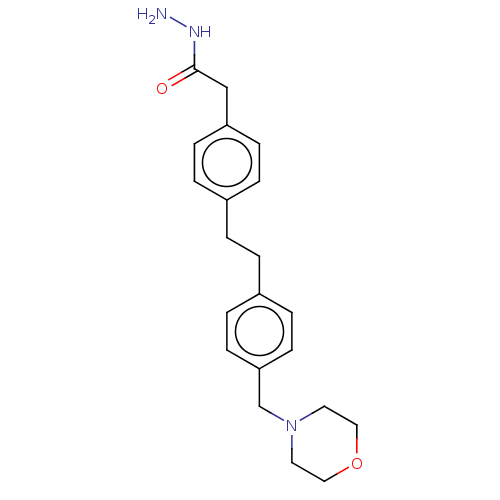 (US9603833, Example 70) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309478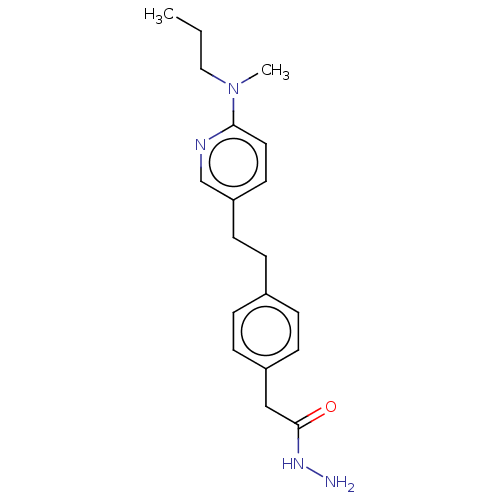 (US9603833, Example 90) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309475 (US9603833, Example 87) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309487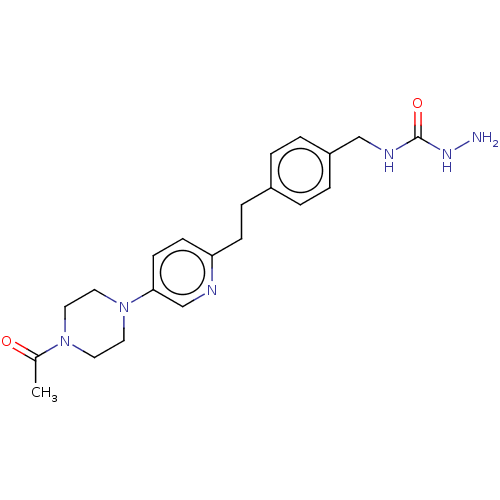 (US9603833, Example 109) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM633208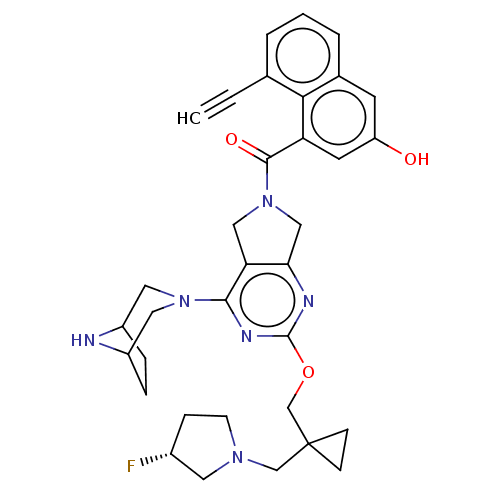 (US20230348495, Example 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM50576725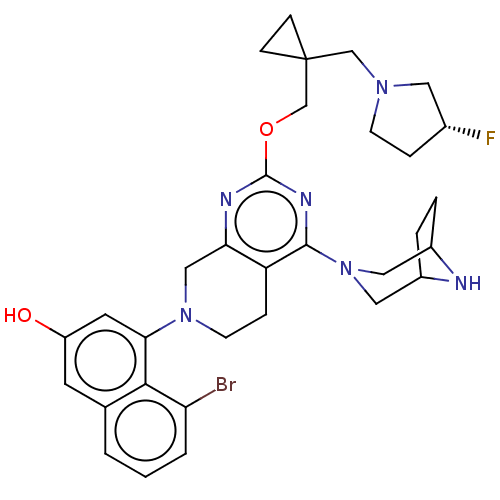 (CHEMBL4875572 | US20230348495, Example 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM633194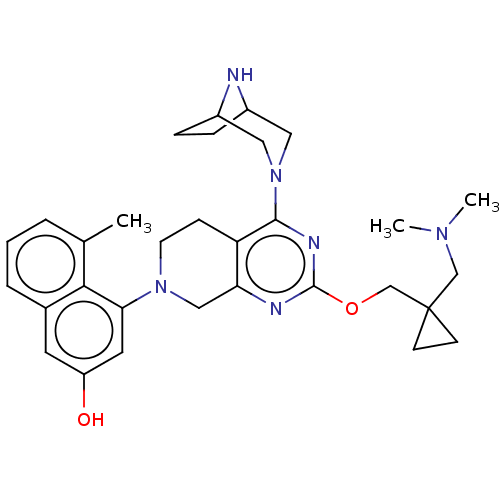 (US20230348495, Example 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM633207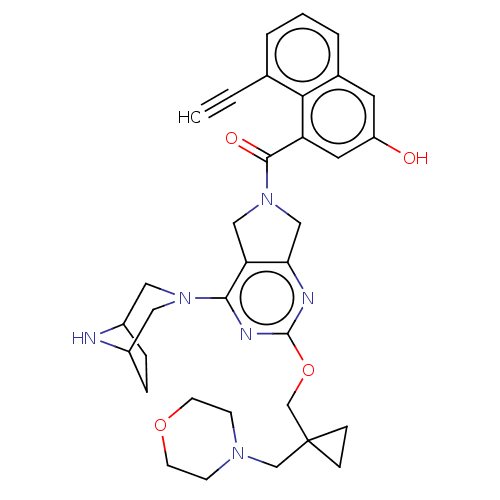 (US20230348495, Example 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309498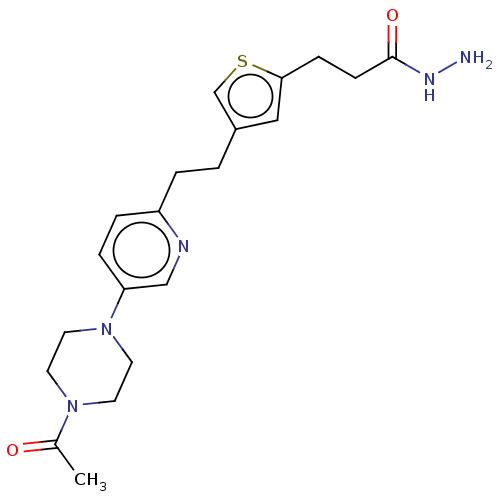 (US9603833, Example 121) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309488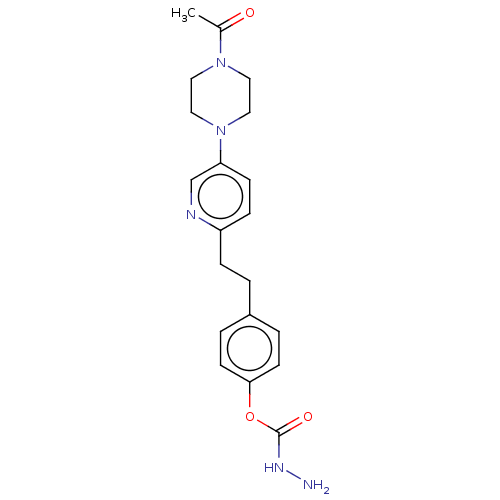 (US9603833, Example 110) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309473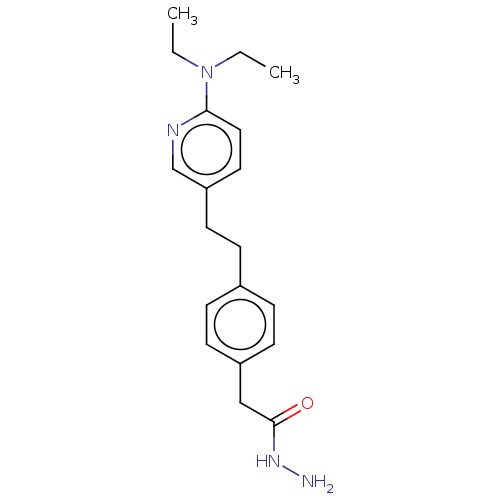 (US9603833, Example 85) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM309492 (US9603833, Example 114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309482 (US9603833, Example 101) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM309498 (US9603833, Example 121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309461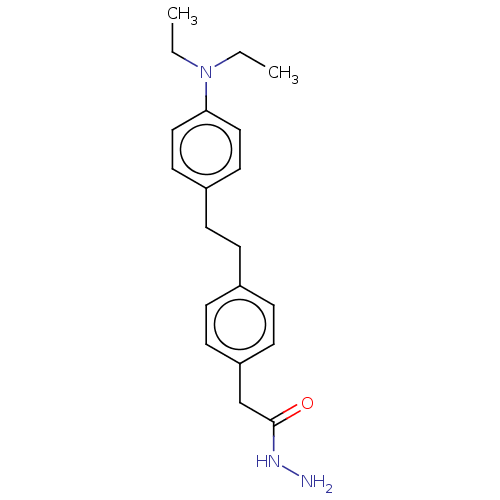 (US9603833, Example 30) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309481 (US9603833, Example 99) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM309495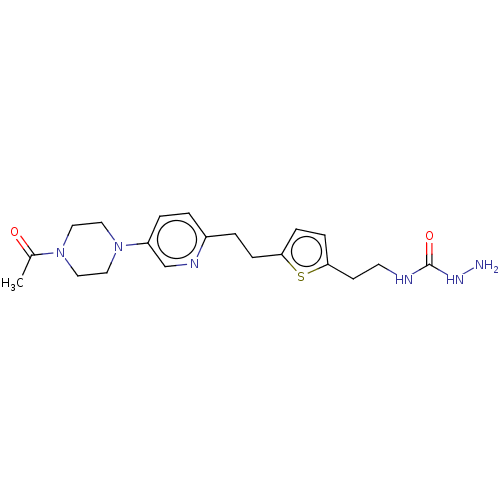 (US9603833, Example 117) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd. US Patent | Assay Description The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... | US Patent US9603833 (2017) BindingDB Entry DOI: 10.7270/Q2W37ZCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM376962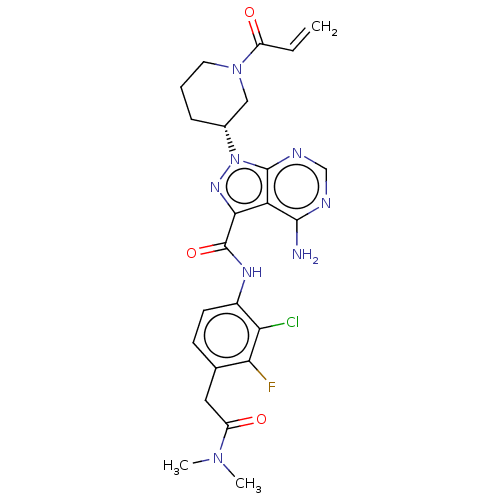 (US10329300, Example 50 | US11696917, Example 50 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot | Assay Description For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... | Bioorg Med Chem 16: 1242-53 (2008) BindingDB Entry DOI: 10.7270/Q2BR8VHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM376962 (US10329300, Example 50 | US11696917, Example 50 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... | US Patent US10329300 (2019) BindingDB Entry DOI: 10.7270/Q2MG7RWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G12D] (Homo sapiens (Human)) | BDBM633226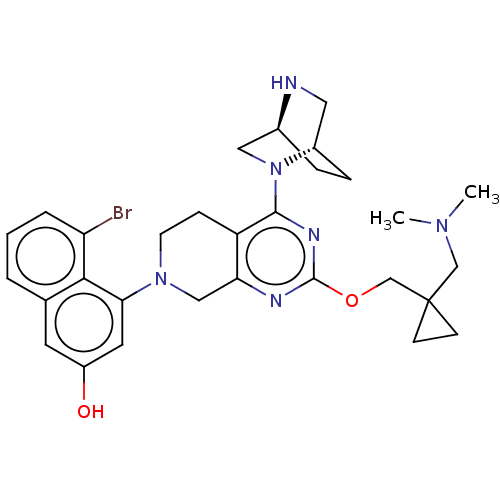 (US20230348495, Example 20) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 514 total ) | Next | Last >> |