 Found 41 hits with Last Name = 'keating' and Initial = 'ta'
Found 41 hits with Last Name = 'keating' and Initial = 'ta' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate racemase
(Helicobacter pylori) | BDBM26431
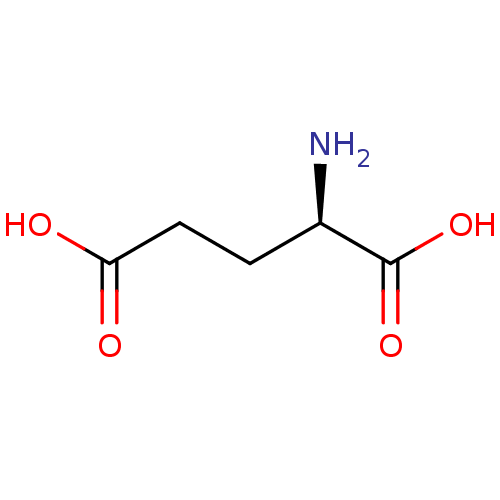
((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori glutamate racemase |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional protein GlmU
(Escherichia coli) | BDBM92474

(Sulfonamide, 5)Show SMILES COc1cc(OC)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C24H22N2O8S/c1-32-20-14-21(33-2)22(13-15(20)25-23(27)11-12-24(28)29)35(30,31)26-16-7-3-5-9-18(16)34-19-10-6-4-8-17(19)26/h3-10,13-14H,11-12H2,1-2H3,(H,25,27)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Bifunctional protein GlmU
(Escherichia coli) | BDBM92472
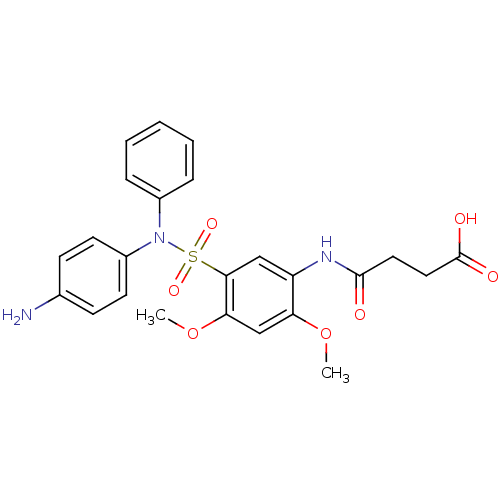
(Sulfonamide, 3)Show SMILES COc1cc(OC)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N(c1ccccc1)c1ccc(N)cc1 Show InChI InChI=1S/C24H25N3O7S/c1-33-20-15-21(34-2)22(14-19(20)26-23(28)12-13-24(29)30)35(31,32)27(17-6-4-3-5-7-17)18-10-8-16(25)9-11-18/h3-11,14-15H,12-13,25H2,1-2H3,(H,26,28)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional protein GlmU
(Haemophilus influenzae) | BDBM92474

(Sulfonamide, 5)Show SMILES COc1cc(OC)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C24H22N2O8S/c1-32-20-14-21(33-2)22(13-15(20)25-23(27)11-12-24(28)29)35(30,31)26-16-7-3-5-9-18(16)34-19-10-6-4-8-17(19)26/h3-10,13-14H,11-12H2,1-2H3,(H,25,27)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50215445
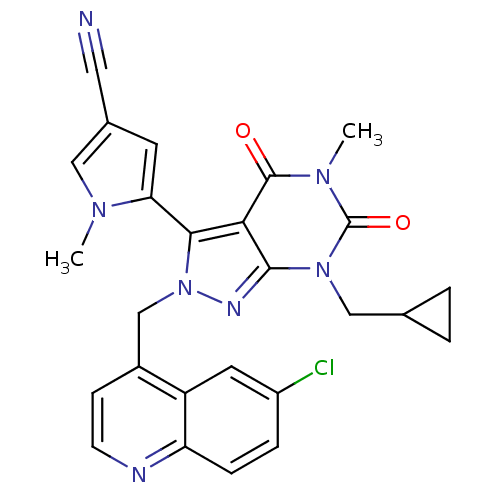
(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(17.56,4.29,;19.1,4.29,;20,5.54,;21.47,5.06,;21.47,3.52,;20,3.04,;19.53,1.58,;20.45,.33,;21.99,.33,;22.76,-1.01,;21.98,-2.34,;22.75,-3.67,;24.29,-3.67,;25.06,-2.32,;26.59,-2.32,;27.35,-.99,;26.57,.34,;27.33,1.68,;25.05,.33,;24.29,-1,;19.53,-.93,;18.06,-.45,;16.72,-1.22,;16.71,-2.76,;15.38,-3.52,;13.84,-3.53,;14.61,-4.86,;15.38,-.45,;14.05,-1.22,;15.38,1.1,;14.05,1.87,;16.72,1.88,;16.71,3.42,;18.06,1.1,;22.71,5.96,;23.96,6.86,)| Show InChI InChI=1S/C26H22ClN7O2/c1-31-12-16(11-28)9-21(31)23-22-24(33(13-15-3-4-15)26(36)32(2)25(22)35)30-34(23)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,12,15H,3-4,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori glutamate racemase |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Escherichia coli) | BDBM92473
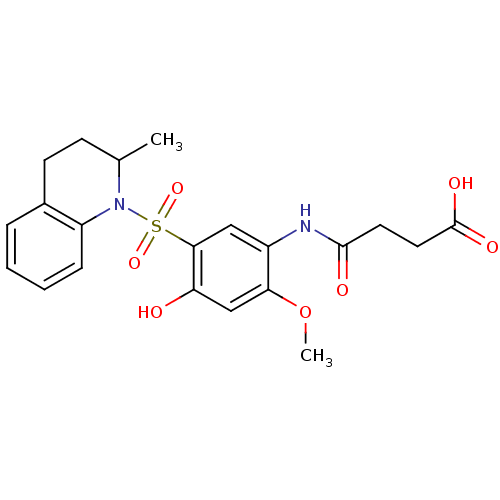
(Sulfonamide, 4)Show SMILES COc1cc(O)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N1C(C)CCc2ccccc12 Show InChI InChI=1S/C21H24N2O7S/c1-13-7-8-14-5-3-4-6-16(14)23(13)31(28,29)19-11-15(18(30-2)12-17(19)24)22-20(25)9-10-21(26)27/h3-6,11-13,24H,7-10H2,1-2H3,(H,22,25)(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Bifunctional protein GlmU
(Haemophilus influenzae) | BDBM92473

(Sulfonamide, 4)Show SMILES COc1cc(O)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N1C(C)CCc2ccccc12 Show InChI InChI=1S/C21H24N2O7S/c1-13-7-8-14-5-3-4-6-16(14)23(13)31(28,29)19-11-15(18(30-2)12-17(19)24)22-20(25)9-10-21(26)27/h3-6,11-13,24H,7-10H2,1-2H3,(H,22,25)(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Haemophilus influenzae) | BDBM92472

(Sulfonamide, 3)Show SMILES COc1cc(OC)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N(c1ccccc1)c1ccc(N)cc1 Show InChI InChI=1S/C24H25N3O7S/c1-33-20-15-21(34-2)22(14-19(20)26-23(28)12-13-24(29)30)35(31,32)27(17-6-4-3-5-7-17)18-10-8-16(25)9-11-18/h3-11,14-15H,12-13,25H2,1-2H3,(H,26,28)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50215444
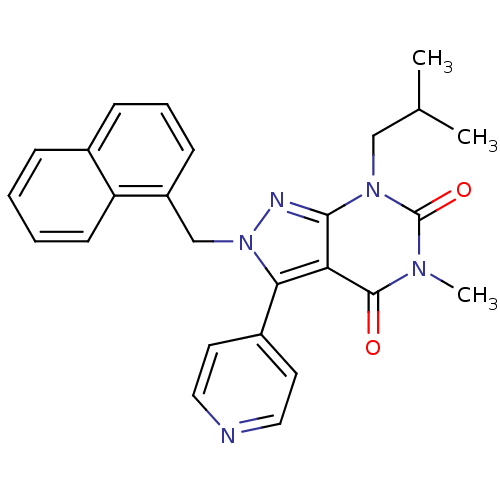
(5-METHYL-7-(2-METHYLPROPYL)-2-(NAPHTHALEN-1-YLMETH...)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3ccncc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C26H25N5O2/c1-17(2)15-30-24-22(25(32)29(3)26(30)33)23(19-11-13-27-14-12-19)31(28-24)16-20-9-6-8-18-7-4-5-10-21(18)20/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori glutamate racemase |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional protein GlmU
(Escherichia coli) | BDBM50365062
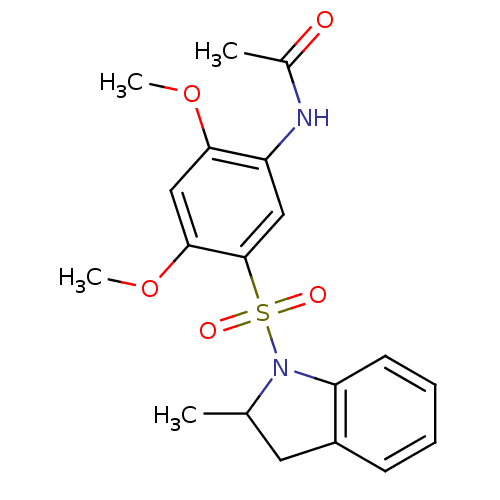
(CHEMBL1951072 | Sulfonamide, 1)Show SMILES COc1cc(OC)c(cc1NC(C)=O)S(=O)(=O)N1C(C)Cc2ccccc12 Show InChI InChI=1S/C19H22N2O5S/c1-12-9-14-7-5-6-8-16(14)21(12)27(23,24)19-10-15(20-13(2)22)17(25-3)11-18(19)26-4/h5-8,10-12H,9H2,1-4H3,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional protein GlmU
(Staphylococcus aureus) | BDBM50365075
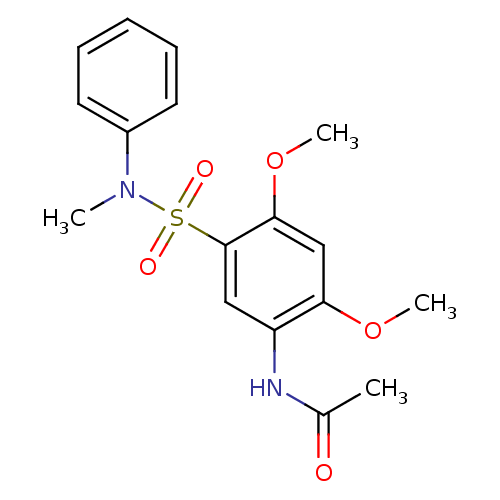
(CHEMBL1951164 | Sulfonamide, 2)Show SMILES COc1cc(OC)c(cc1NC(C)=O)S(=O)(=O)N(C)c1ccccc1 Show InChI InChI=1S/C17H20N2O5S/c1-12(20)18-14-10-17(16(24-4)11-15(14)23-3)25(21,22)19(2)13-8-6-5-7-9-13/h5-11H,1-4H3,(H,18,20) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | 1.90E+3 | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Streptococcus pneumoniae) | BDBM92473

(Sulfonamide, 4)Show SMILES COc1cc(O)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N1C(C)CCc2ccccc12 Show InChI InChI=1S/C21H24N2O7S/c1-13-7-8-14-5-3-4-6-16(14)23(13)31(28,29)19-11-15(18(30-2)12-17(19)24)22-20(25)9-10-21(26)27/h3-6,11-13,24H,7-10H2,1-2H3,(H,22,25)(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Haemophilus influenzae) | BDBM50365075

(CHEMBL1951164 | Sulfonamide, 2)Show SMILES COc1cc(OC)c(cc1NC(C)=O)S(=O)(=O)N(C)c1ccccc1 Show InChI InChI=1S/C17H20N2O5S/c1-12(20)18-14-10-17(16(24-4)11-15(14)23-3)25(21,22)19(2)13-8-6-5-7-9-13/h5-11H,1-4H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Haemophilus influenzae) | BDBM50365062

(CHEMBL1951072 | Sulfonamide, 1)Show SMILES COc1cc(OC)c(cc1NC(C)=O)S(=O)(=O)N1C(C)Cc2ccccc12 Show InChI InChI=1S/C19H22N2O5S/c1-12-9-14-7-5-6-8-16(14)21(12)27(23,24)19-10-15(20-13(2)22)17(25-3)11-18(19)26-4/h5-8,10-12H,9H2,1-4H3,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Streptococcus pneumoniae) | BDBM92474

(Sulfonamide, 5)Show SMILES COc1cc(OC)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C24H22N2O8S/c1-32-20-14-21(33-2)22(13-15(20)25-23(27)11-12-24(28)29)35(30,31)26-16-7-3-5-9-18(16)34-19-10-6-4-8-17(19)26/h3-10,13-14H,11-12H2,1-2H3,(H,25,27)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50428613
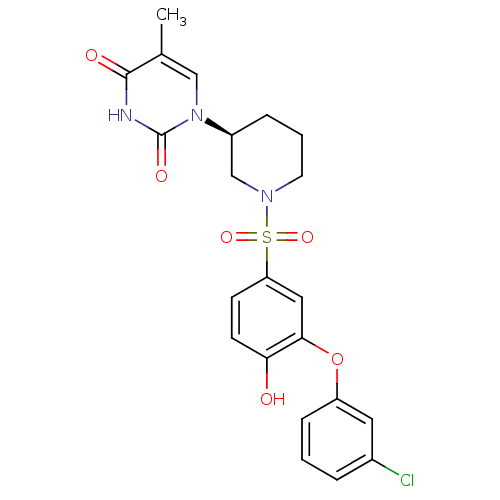
(CHEMBL2331747)Show SMILES Cc1cn([C@H]2CCCN(C2)S(=O)(=O)c2ccc(O)c(Oc3cccc(Cl)c3)c2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H22ClN3O6S/c1-14-12-26(22(29)24-21(14)28)16-5-3-9-25(13-16)33(30,31)18-7-8-19(27)20(11-18)32-17-6-2-4-15(23)10-17/h2,4,6-8,10-12,16,27H,3,5,9,13H2,1H3,(H,24,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 23: 169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.128
BindingDB Entry DOI: 10.7270/Q22J6D74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50428613

(CHEMBL2331747)Show SMILES Cc1cn([C@H]2CCCN(C2)S(=O)(=O)c2ccc(O)c(Oc3cccc(Cl)c3)c2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H22ClN3O6S/c1-14-12-26(22(29)24-21(14)28)16-5-3-9-25(13-16)33(30,31)18-7-8-19(27)20(11-18)32-17-6-2-4-15(23)10-17/h2,4,6-8,10-12,16,27H,3,5,9,13H2,1H3,(H,24,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 23: 169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.128
BindingDB Entry DOI: 10.7270/Q22J6D74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50428613

(CHEMBL2331747)Show SMILES Cc1cn([C@H]2CCCN(C2)S(=O)(=O)c2ccc(O)c(Oc3cccc(Cl)c3)c2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H22ClN3O6S/c1-14-12-26(22(29)24-21(14)28)16-5-3-9-25(13-16)33(30,31)18-7-8-19(27)20(11-18)32-17-6-2-4-15(23)10-17/h2,4,6-8,10-12,16,27H,3,5,9,13H2,1H3,(H,24,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 23: 169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.128
BindingDB Entry DOI: 10.7270/Q22J6D74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50428613

(CHEMBL2331747)Show SMILES Cc1cn([C@H]2CCCN(C2)S(=O)(=O)c2ccc(O)c(Oc3cccc(Cl)c3)c2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H22ClN3O6S/c1-14-12-26(22(29)24-21(14)28)16-5-3-9-25(13-16)33(30,31)18-7-8-19(27)20(11-18)32-17-6-2-4-15(23)10-17/h2,4,6-8,10-12,16,27H,3,5,9,13H2,1H3,(H,24,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 23: 169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.128
BindingDB Entry DOI: 10.7270/Q22J6D74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50428613

(CHEMBL2331747)Show SMILES Cc1cn([C@H]2CCCN(C2)S(=O)(=O)c2ccc(O)c(Oc3cccc(Cl)c3)c2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H22ClN3O6S/c1-14-12-26(22(29)24-21(14)28)16-5-3-9-25(13-16)33(30,31)18-7-8-19(27)20(11-18)32-17-6-2-4-15(23)10-17/h2,4,6-8,10-12,16,27H,3,5,9,13H2,1H3,(H,24,28,29)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 23: 169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.128
BindingDB Entry DOI: 10.7270/Q22J6D74 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50428613

(CHEMBL2331747)Show SMILES Cc1cn([C@H]2CCCN(C2)S(=O)(=O)c2ccc(O)c(Oc3cccc(Cl)c3)c2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H22ClN3O6S/c1-14-12-26(22(29)24-21(14)28)16-5-3-9-25(13-16)33(30,31)18-7-8-19(27)20(11-18)32-17-6-2-4-15(23)10-17/h2,4,6-8,10-12,16,27H,3,5,9,13H2,1H3,(H,24,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.128
BindingDB Entry DOI: 10.7270/Q22J6D74 |
More data for this
Ligand-Target Pair | |
Thymidylate kinase
(Homo sapiens (Human)) | BDBM50398957

(CHEMBL2179281)Show SMILES CCCCC[C@@H](N1CCC[C@@H](C1)n1cc(C)c(=O)[nH]c1=O)c1ccc(C(O)=O)c(Oc2cccc(Br)c2)c1 |r| Show InChI InChI=1S/C29H34BrN3O5/c1-3-4-5-11-25(32-14-7-9-22(18-32)33-17-19(2)27(34)31-29(33)37)20-12-13-24(28(35)36)26(15-20)38-23-10-6-8-21(30)16-23/h6,8,10,12-13,15-17,22,25H,3-5,7,9,11,14,18H2,1-2H3,(H,35,36)(H,31,34,37)/t22-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate kinase assessed as effect on ATP production by Escherichia coli DdlA and Malachite green reagent based assay |
J Med Chem 55: 10010-21 (2012)
Article DOI: 10.1021/jm3011806
BindingDB Entry DOI: 10.7270/Q2BR8T99 |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Streptococcus pneumoniae) | BDBM92472

(Sulfonamide, 3)Show SMILES COc1cc(OC)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N(c1ccccc1)c1ccc(N)cc1 Show InChI InChI=1S/C24H25N3O7S/c1-33-20-15-21(34-2)22(14-19(20)26-23(28)12-13-24(29)30)35(31,32)27(17-6-4-3-5-7-17)18-10-8-16(25)9-11-18/h3-11,14-15H,12-13,25H2,1-2H3,(H,26,28)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Thymidylate kinase
(Homo sapiens (Human)) | BDBM50398958
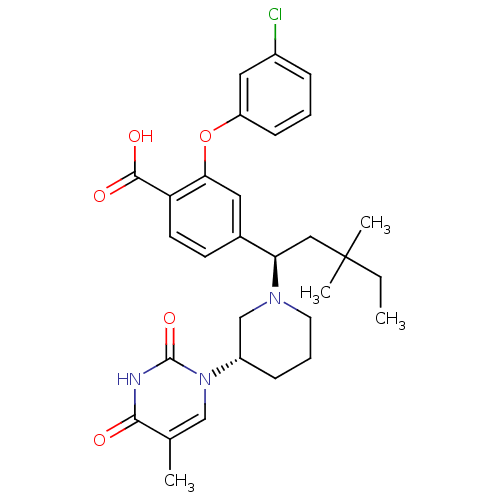
(CHEMBL2179279)Show SMILES CCC(C)(C)C[C@@H](N1CCC[C@@H](C1)n1cc(C)c(=O)[nH]c1=O)c1ccc(C(O)=O)c(Oc2cccc(Cl)c2)c1 |r| Show InChI InChI=1S/C30H36ClN3O5/c1-5-30(3,4)16-25(33-13-7-9-22(18-33)34-17-19(2)27(35)32-29(34)38)20-11-12-24(28(36)37)26(14-20)39-23-10-6-8-21(31)15-23/h6,8,10-12,14-15,17,22,25H,5,7,9,13,16,18H2,1-4H3,(H,36,37)(H,32,35,38)/t22-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate kinase assessed as effect on ATP production by Escherichia coli DdlA and Malachite green reagent based assay |
J Med Chem 55: 10010-21 (2012)
Article DOI: 10.1021/jm3011806
BindingDB Entry DOI: 10.7270/Q2BR8T99 |
More data for this
Ligand-Target Pair | |
Thymidylate kinase
(Homo sapiens (Human)) | BDBM50398959
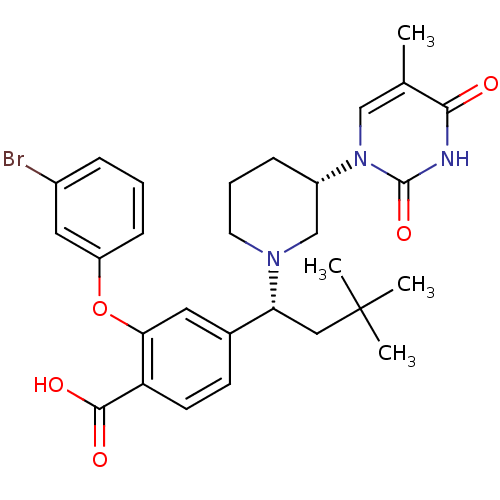
(CHEMBL2179277)Show SMILES Cc1cn([C@H]2CCCN(C2)[C@H](CC(C)(C)C)c2ccc(C(O)=O)c(Oc3cccc(Br)c3)c2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C29H34BrN3O5/c1-18-16-33(28(37)31-26(18)34)21-8-6-12-32(17-21)24(15-29(2,3)4)19-10-11-23(27(35)36)25(13-19)38-22-9-5-7-20(30)14-22/h5,7,9-11,13-14,16,21,24H,6,8,12,15,17H2,1-4H3,(H,35,36)(H,31,34,37)/t21-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate kinase assessed as effect on ATP production by Escherichia coli DdlA and Malachite green reagent based assay |
J Med Chem 55: 10010-21 (2012)
Article DOI: 10.1021/jm3011806
BindingDB Entry DOI: 10.7270/Q2BR8T99 |
More data for this
Ligand-Target Pair | |
Thymidylate kinase
(Homo sapiens (Human)) | BDBM50398961
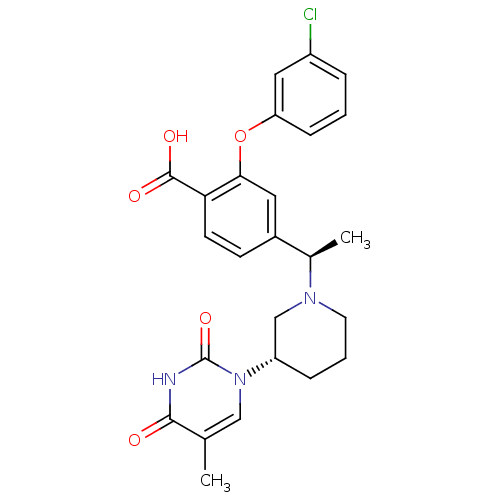
(CHEMBL2179269)Show SMILES C[C@@H](N1CCC[C@@H](C1)n1cc(C)c(=O)[nH]c1=O)c1ccc(C(O)=O)c(Oc2cccc(Cl)c2)c1 |r| Show InChI InChI=1S/C25H26ClN3O5/c1-15-13-29(25(33)27-23(15)30)19-6-4-10-28(14-19)16(2)17-8-9-21(24(31)32)22(11-17)34-20-7-3-5-18(26)12-20/h3,5,7-9,11-13,16,19H,4,6,10,14H2,1-2H3,(H,31,32)(H,27,30,33)/t16-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate kinase assessed as effect on ATP production by Escherichia coli DdlA and Malachite green reagent based assay |
J Med Chem 55: 10010-21 (2012)
Article DOI: 10.1021/jm3011806
BindingDB Entry DOI: 10.7270/Q2BR8T99 |
More data for this
Ligand-Target Pair | |
Thymidylate kinase
(Homo sapiens (Human)) | BDBM50398960
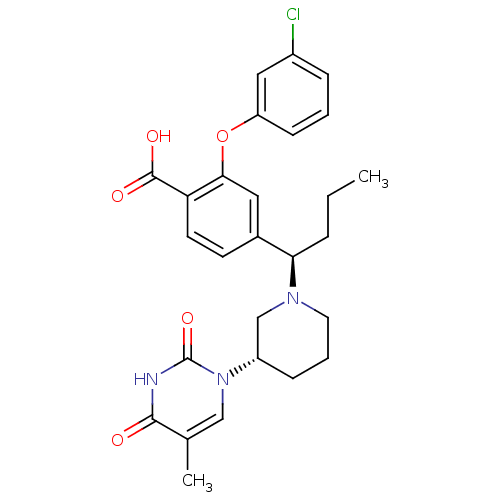
(CHEMBL2179273)Show SMILES CCC[C@@H](N1CCC[C@@H](C1)n1cc(C)c(=O)[nH]c1=O)c1ccc(C(O)=O)c(Oc2cccc(Cl)c2)c1 |r| Show InChI InChI=1S/C27H30ClN3O5/c1-3-6-23(30-12-5-8-20(16-30)31-15-17(2)25(32)29-27(31)35)18-10-11-22(26(33)34)24(13-18)36-21-9-4-7-19(28)14-21/h4,7,9-11,13-15,20,23H,3,5-6,8,12,16H2,1-2H3,(H,33,34)(H,29,32,35)/t20-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate kinase assessed as effect on ATP production by Escherichia coli DdlA and Malachite green reagent based assay |
J Med Chem 55: 10010-21 (2012)
Article DOI: 10.1021/jm3011806
BindingDB Entry DOI: 10.7270/Q2BR8T99 |
More data for this
Ligand-Target Pair | |
Thymidylate kinase
(Homo sapiens (Human)) | BDBM50428613

(CHEMBL2331747)Show SMILES Cc1cn([C@H]2CCCN(C2)S(=O)(=O)c2ccc(O)c(Oc3cccc(Cl)c3)c2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H22ClN3O6S/c1-14-12-26(22(29)24-21(14)28)16-5-3-9-25(13-16)33(30,31)18-7-8-19(27)20(11-18)32-17-6-2-4-15(23)10-17/h2,4,6-8,10-12,16,27H,3,5,9,13H2,1H3,(H,24,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate kinase |
Bioorg Med Chem Lett 23: 169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.128
BindingDB Entry DOI: 10.7270/Q22J6D74 |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Streptococcus pneumoniae) | BDBM50365075

(CHEMBL1951164 | Sulfonamide, 2)Show SMILES COc1cc(OC)c(cc1NC(C)=O)S(=O)(=O)N(C)c1ccccc1 Show InChI InChI=1S/C17H20N2O5S/c1-12(20)18-14-10-17(16(24-4)11-15(14)23-3)25(21,22)19(2)13-8-6-5-7-9-13/h5-11H,1-4H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Staphylococcus aureus) | BDBM50365075

(CHEMBL1951164 | Sulfonamide, 2)Show SMILES COc1cc(OC)c(cc1NC(C)=O)S(=O)(=O)N(C)c1ccccc1 Show InChI InChI=1S/C17H20N2O5S/c1-12(20)18-14-10-17(16(24-4)11-15(14)23-3)25(21,22)19(2)13-8-6-5-7-9-13/h5-11H,1-4H3,(H,18,20) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Staphylococcus aureus) | BDBM50365062

(CHEMBL1951072 | Sulfonamide, 1)Show SMILES COc1cc(OC)c(cc1NC(C)=O)S(=O)(=O)N1C(C)Cc2ccccc12 Show InChI InChI=1S/C19H22N2O5S/c1-12-9-14-7-5-6-8-16(14)21(12)27(23,24)19-10-15(20-13(2)22)17(25-3)11-18(19)26-4/h5-8,10-12H,9H2,1-4H3,(H,20,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Staphylococcus aureus) | BDBM92472

(Sulfonamide, 3)Show SMILES COc1cc(OC)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N(c1ccccc1)c1ccc(N)cc1 Show InChI InChI=1S/C24H25N3O7S/c1-33-20-15-21(34-2)22(14-19(20)26-23(28)12-13-24(29)30)35(31,32)27(17-6-4-3-5-7-17)18-10-8-16(25)9-11-18/h3-11,14-15H,12-13,25H2,1-2H3,(H,26,28)(H,29,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Staphylococcus aureus) | BDBM92473

(Sulfonamide, 4)Show SMILES COc1cc(O)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N1C(C)CCc2ccccc12 Show InChI InChI=1S/C21H24N2O7S/c1-13-7-8-14-5-3-4-6-16(14)23(13)31(28,29)19-11-15(18(30-2)12-17(19)24)22-20(25)9-10-21(26)27/h3-6,11-13,24H,7-10H2,1-2H3,(H,22,25)(H,26,27) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Staphylococcus aureus) | BDBM92474

(Sulfonamide, 5)Show SMILES COc1cc(OC)c(cc1NC(=O)CCC(O)=O)S(=O)(=O)N1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C24H22N2O8S/c1-32-20-14-21(33-2)22(13-15(20)25-23(27)11-12-24(28)29)35(30,31)26-16-7-3-5-9-18(16)34-19-10-6-4-8-17(19)26/h3-10,13-14H,11-12H2,1-2H3,(H,25,27)(H,28,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Thymidylate kinase
(Homo sapiens (Human)) | BDBM50398962
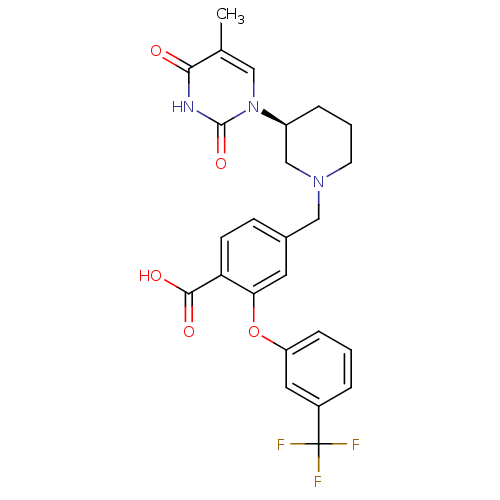
(CHEMBL2179669)Show SMILES Cc1cn([C@H]2CCCN(Cc3ccc(C(O)=O)c(Oc4cccc(c4)C(F)(F)F)c3)C2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C25H24F3N3O5/c1-15-12-31(24(35)29-22(15)32)18-5-3-9-30(14-18)13-16-7-8-20(23(33)34)21(10-16)36-19-6-2-4-17(11-19)25(26,27)28/h2,4,6-8,10-12,18H,3,5,9,13-14H2,1H3,(H,33,34)(H,29,32,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate kinase assessed as effect on ATP production by Escherichia coli DdlA and Malachite green reagent based assay |
J Med Chem 55: 10010-21 (2012)
Article DOI: 10.1021/jm3011806
BindingDB Entry DOI: 10.7270/Q2BR8T99 |
More data for this
Ligand-Target Pair | |
Bifunctional protein GlmU
(Streptococcus pneumoniae) | BDBM50365062

(CHEMBL1951072 | Sulfonamide, 1)Show SMILES COc1cc(OC)c(cc1NC(C)=O)S(=O)(=O)N1C(C)Cc2ccccc12 Show InChI InChI=1S/C19H22N2O5S/c1-12-9-14-7-5-6-8-16(14)21(12)27(23,24)19-10-15(20-13(2)22)17(25-3)11-18(19)26-4/h5-8,10-12H,9H2,1-4H3,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.35 | n/a |
AstraZeneca
| Assay Description
Inhibition assay using GlmU bifuncation enzyme with various bacterial strains. |
J Biol Chem 286: 40734-42 (2011)
Article DOI: 10.1074/jbc.M111.274068
BindingDB Entry DOI: 10.7270/Q2BP01DD |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Streptococcus pneumoniae) | BDBM50215444

(5-METHYL-7-(2-METHYLPROPYL)-2-(NAPHTHALEN-1-YLMETH...)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3ccncc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C26H25N5O2/c1-17(2)15-30-24-22(25(32)29(3)26(30)33)23(19-11-13-27-14-12-19)31(28-24)16-20-9-6-8-18-7-4-5-10-21(18)20/h4-14,17H,15-16H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of MurI in Streptococcus pneumoniae |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Streptococcus pneumoniae) | BDBM50215445

(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(17.56,4.29,;19.1,4.29,;20,5.54,;21.47,5.06,;21.47,3.52,;20,3.04,;19.53,1.58,;20.45,.33,;21.99,.33,;22.76,-1.01,;21.98,-2.34,;22.75,-3.67,;24.29,-3.67,;25.06,-2.32,;26.59,-2.32,;27.35,-.99,;26.57,.34,;27.33,1.68,;25.05,.33,;24.29,-1,;19.53,-.93,;18.06,-.45,;16.72,-1.22,;16.71,-2.76,;15.38,-3.52,;13.84,-3.53,;14.61,-4.86,;15.38,-.45,;14.05,-1.22,;15.38,1.1,;14.05,1.87,;16.72,1.88,;16.71,3.42,;18.06,1.1,;22.71,5.96,;23.96,6.86,)| Show InChI InChI=1S/C26H22ClN7O2/c1-31-12-16(11-28)9-21(31)23-22-24(33(13-15-3-4-15)26(36)32(2)25(22)35)30-34(23)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,12,15H,3-4,13-14H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of MurI in Streptococcus pneumoniae |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50215444

(5-METHYL-7-(2-METHYLPROPYL)-2-(NAPHTHALEN-1-YLMETH...)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3ccncc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C26H25N5O2/c1-17(2)15-30-24-22(25(32)29(3)26(30)33)23(19-11-13-27-14-12-19)31(28-24)16-20-9-6-8-18-7-4-5-10-21(18)20/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Helicobacter pylori MurI by protein fluorescence binding assay |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate racemase
(Helicobacter pylori) | BDBM50215445

(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(17.56,4.29,;19.1,4.29,;20,5.54,;21.47,5.06,;21.47,3.52,;20,3.04,;19.53,1.58,;20.45,.33,;21.99,.33,;22.76,-1.01,;21.98,-2.34,;22.75,-3.67,;24.29,-3.67,;25.06,-2.32,;26.59,-2.32,;27.35,-.99,;26.57,.34,;27.33,1.68,;25.05,.33,;24.29,-1,;19.53,-.93,;18.06,-.45,;16.72,-1.22,;16.71,-2.76,;15.38,-3.52,;13.84,-3.53,;14.61,-4.86,;15.38,-.45,;14.05,-1.22,;15.38,1.1,;14.05,1.87,;16.72,1.88,;16.71,3.42,;18.06,1.1,;22.71,5.96,;23.96,6.86,)| Show InChI InChI=1S/C26H22ClN7O2/c1-31-12-16(11-28)9-21(31)23-22-24(33(13-15-3-4-15)26(36)32(2)25(22)35)30-34(23)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,12,15H,3-4,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Helicobacter pylori MurI by protein fluorescence binding assay |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50215444

(5-METHYL-7-(2-METHYLPROPYL)-2-(NAPHTHALEN-1-YLMETH...)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3ccncc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C26H25N5O2/c1-17(2)15-30-24-22(25(32)29(3)26(30)33)23(19-11-13-27-14-12-19)31(28-24)16-20-9-6-8-18-7-4-5-10-21(18)20/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Helicobacter pylori MurI by isothermal titration calorimetry |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data