 Found 53 hits with Last Name = 'kerwin' and Initial = 'sm'
Found 53 hits with Last Name = 'kerwin' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005397
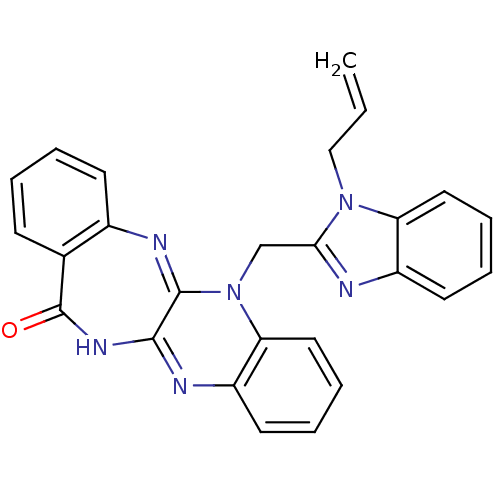
(CHEMBL2206684)Show SMILES C=CCn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:20,t:7| Show InChI InChI=1S/C26H20N6O/c1-2-15-31-21-13-7-5-11-19(21)27-23(31)16-32-22-14-8-6-12-20(22)28-24-25(32)29-18-10-4-3-9-17(18)26(33)30-24/h2-14H,1,15-16H2,(H,28,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402366

(CHEMBL2206696)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccncc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-17(22-25-18-8-4-5-9-19(18)26-22)14-20(15-6-2-1-3-7-15)27(21)16-10-12-24-13-11-16/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005398
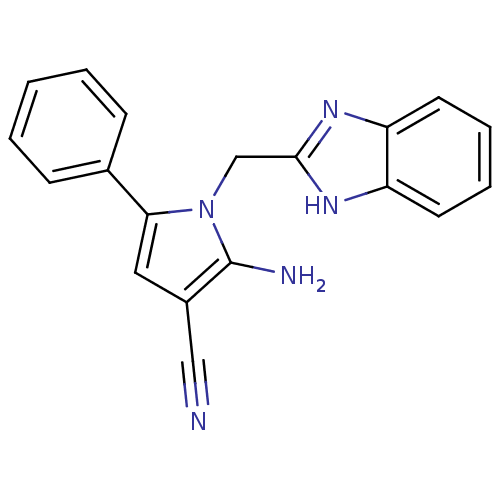
(CHEMBL2206694)Show InChI InChI=1S/C19H15N5/c20-11-14-10-17(13-6-2-1-3-7-13)24(19(14)21)12-18-22-15-8-4-5-9-16(15)23-18/h1-10H,12,21H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402378
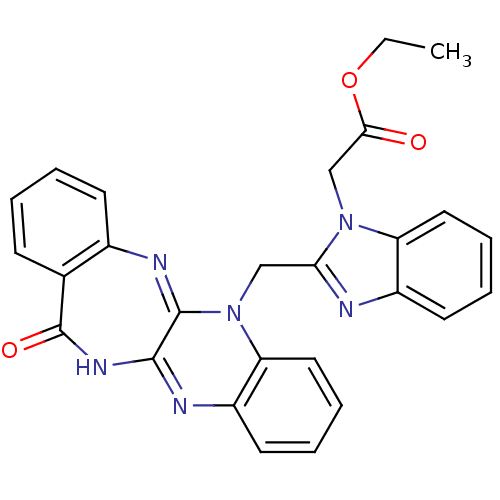
(CHEMBL2206685)Show SMILES CCOC(=O)Cn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:23,t:10| Show InChI InChI=1S/C27H22N6O3/c1-2-36-24(34)16-32-21-13-7-5-11-19(21)28-23(32)15-33-22-14-8-6-12-20(22)29-25-26(33)30-18-10-4-3-9-17(18)27(35)31-25/h3-14H,2,15-16H2,1H3,(H,29,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402361
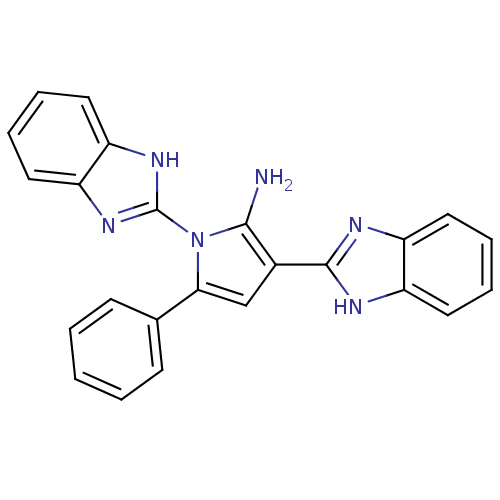
(CHEMBL2206680)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H18N6/c25-22-16(23-26-17-10-4-5-11-18(17)27-23)14-21(15-8-2-1-3-9-15)30(22)24-28-19-12-6-7-13-20(19)29-24/h1-14H,25H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402360

(CHEMBL2206681)Show SMILES Nc1c(cc(-c2ccccc2)n1Cc1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H20N6/c26-24-17(25-29-20-12-6-7-13-21(20)30-25)14-22(16-8-2-1-3-9-16)31(24)15-23-27-18-10-4-5-11-19(18)28-23/h1-14H,15,26H2,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402373
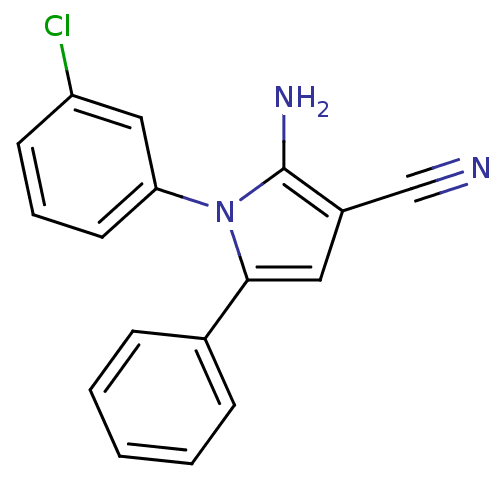
(CHEMBL2206691)Show InChI InChI=1S/C17H12ClN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402372
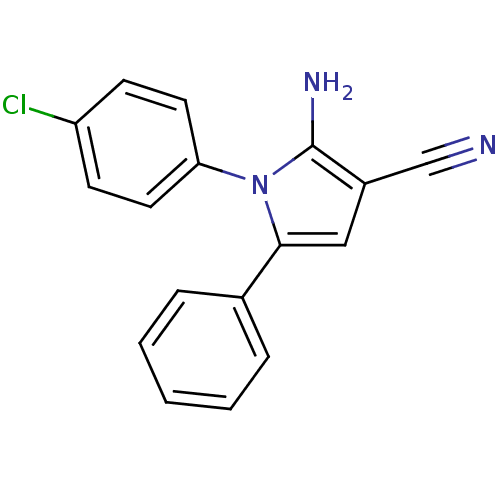
(CHEMBL2206692)Show InChI InChI=1S/C17H12ClN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402371
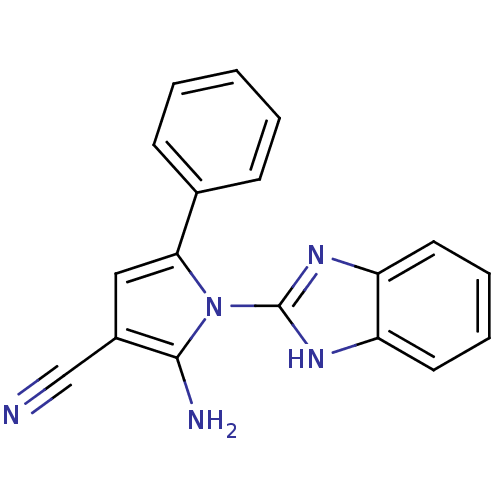
(CHEMBL2206693)Show InChI InChI=1S/C18H13N5/c19-11-13-10-16(12-6-2-1-3-7-12)23(17(13)20)18-21-14-8-4-5-9-15(14)22-18/h1-10H,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402374

(CHEMBL2206690)Show InChI InChI=1S/C17H12BrN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402362
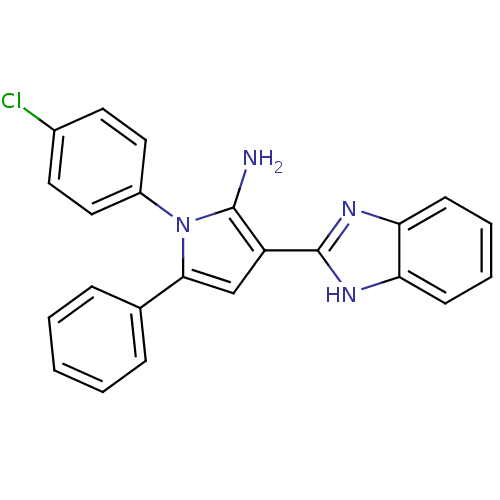
(CHEMBL2206700)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Cl)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402375
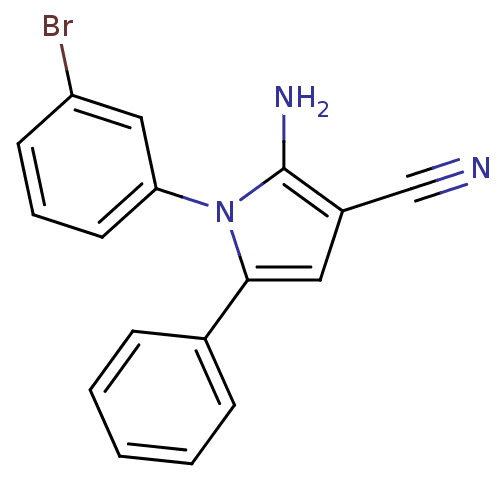
(CHEMBL2206689)Show InChI InChI=1S/C17H12BrN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402363
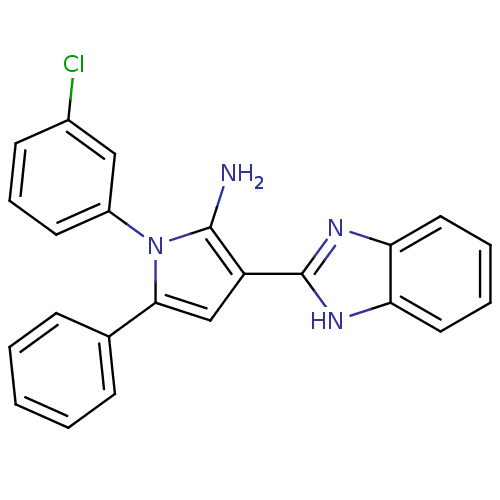
(CHEMBL2206699)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Cl)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402370
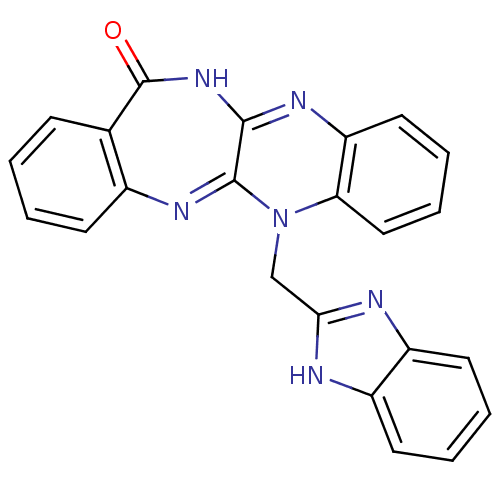
(CHEMBL2206683)Show SMILES O=C1NC2=Nc3ccccc3N(Cc3nc4ccccc4[nH]3)C2=Nc2ccccc12 |c:26,t:3| Show InChI InChI=1S/C23H16N6O/c30-23-14-7-1-2-8-15(14)27-22-21(28-23)26-18-11-5-6-12-19(18)29(22)13-20-24-16-9-3-4-10-17(16)25-20/h1-12H,13H2,(H,24,25)(H,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402367

(CHEMBL2206695)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccccn1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-16(22-25-17-10-4-5-11-18(17)26-22)14-19(15-8-2-1-3-9-15)27(21)20-12-6-7-13-24-20/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402376
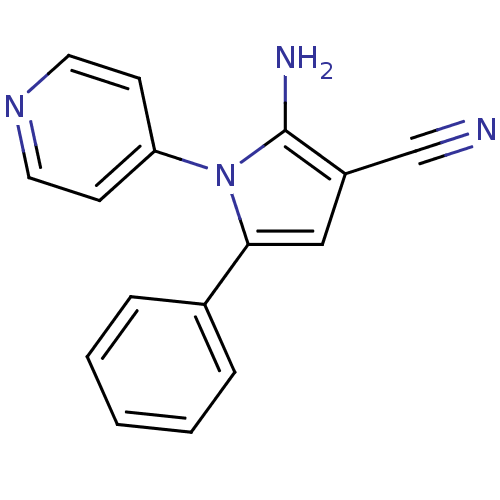
(CHEMBL2206688)Show InChI InChI=1S/C16H12N4/c17-11-13-10-15(12-4-2-1-3-5-12)20(16(13)18)14-6-8-19-9-7-14/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402364
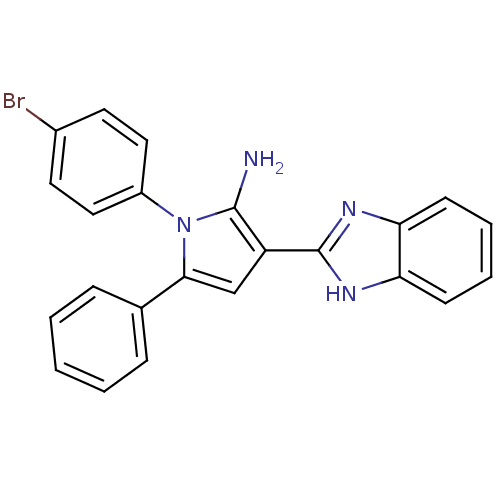
(CHEMBL2206698)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Br)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402365
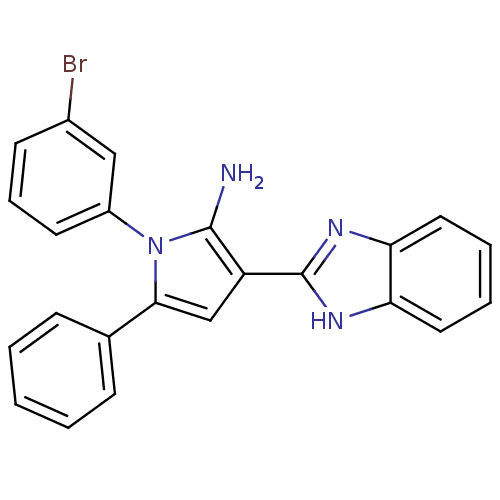
(CHEMBL2206697)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Br)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402377

(CHEMBL2206687)Show InChI InChI=1S/C16H12N4/c17-11-13-10-14(12-6-2-1-3-7-12)20(16(13)18)15-8-4-5-9-19-15/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402379
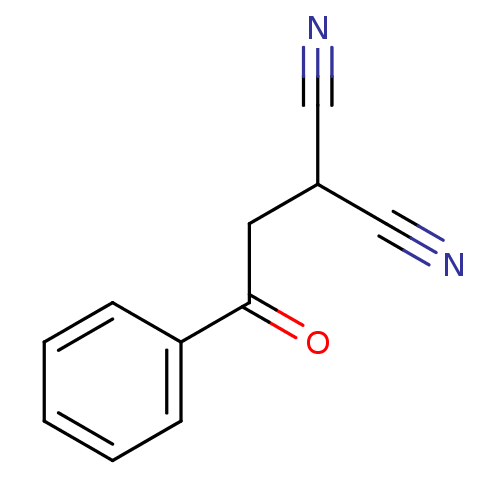
(CHEMBL2206686)Show InChI InChI=1S/C11H8N2O/c12-7-9(8-13)6-11(14)10-4-2-1-3-5-10/h1-5,9H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402368
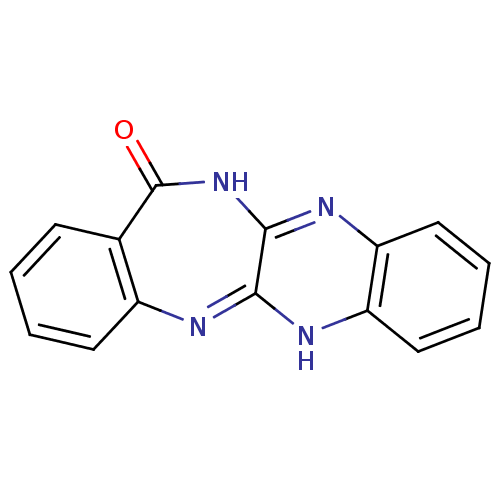
(CHEMBL2206682)Show InChI InChI=1S/C15H10N4O/c20-15-9-5-1-2-6-10(9)16-13-14(19-15)18-12-8-4-3-7-11(12)17-13/h1-8H,(H,16,17)(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402369

(CHEMBL1652555)Show InChI InChI=1S/C8H7N3O/c9-7-8(12)11-6-4-2-1-3-5(6)10-7/h1-4H,(H2,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50302943
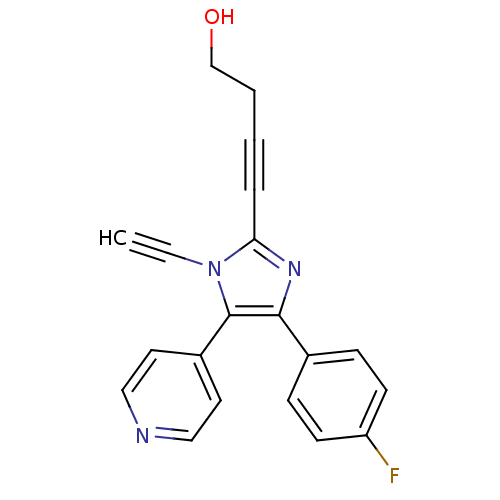
(4-(4-fluorophenyl)-5-(4-pyridyl)-1-(ethynyl)-2-(bu...)Show InChI InChI=1S/C20H14FN3O/c1-2-24-18(5-3-4-14-25)23-19(15-6-8-17(21)9-7-15)20(24)16-10-12-22-13-11-16/h1,6-13,25H,4,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha by FRET assay |
Bioorg Med Chem Lett 19: 6293-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.094
BindingDB Entry DOI: 10.7270/Q25B02JM |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471898
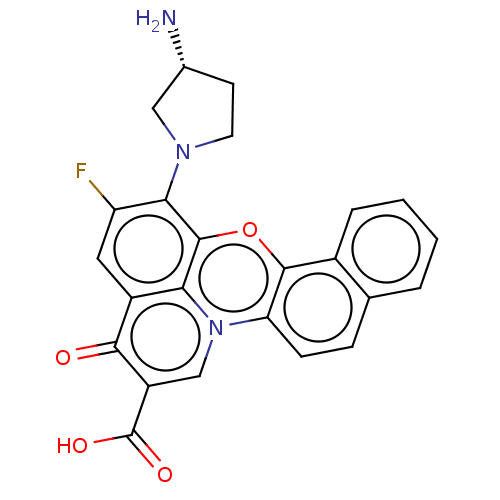
(CHEMBL135778)Show SMILES N[C@@H]1CCN(C1)c1c(F)cc2c3c1oc1c4ccccc4ccc1n3cc(C(O)=O)c2=O |r| Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-19-23(20(17)27-8-7-13(26)10-27)32-22-14-4-2-1-3-12(14)5-6-18(22)28(19)11-16(21(15)29)24(30)31/h1-6,9,11,13H,7-8,10,26H2,(H,30,31)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50098137
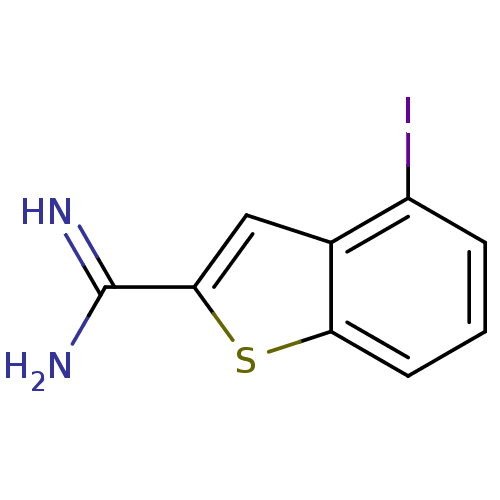
(2-amidino-4-iodobenzothiophene | 4-Iodo-benzo[b]th...)Show InChI InChI=1S/C9H7IN2S/c10-6-2-1-3-7-5(6)4-8(13-7)9(11)12/h1-4H,(H3,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Inhibition of uPA |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50302944
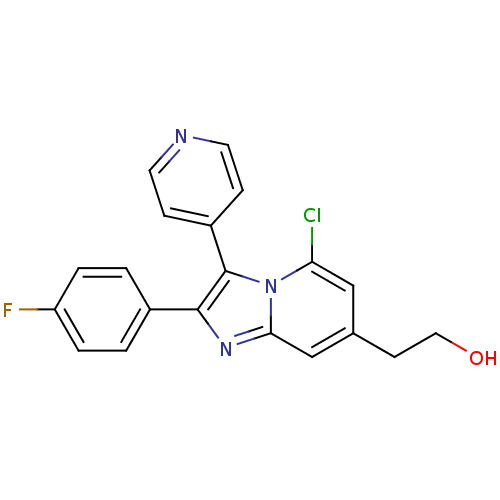
(2-(4-Fluorophenyl)-3(4-pyridinyl)-5-chloro-7-ethan...)Show SMILES OCCc1cc(Cl)n2c(c(nc2c1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C20H15ClFN3O/c21-17-11-13(7-10-26)12-18-24-19(14-1-3-16(22)4-2-14)20(25(17)18)15-5-8-23-9-6-15/h1-6,8-9,11-12,26H,7,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha by FRET assay |
Bioorg Med Chem Lett 19: 6293-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.094
BindingDB Entry DOI: 10.7270/Q25B02JM |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471897

(CHEMBL137098)Show SMILES N[C@@H]1CCN(C1)c1c(F)cc2c3c1oc1cc4ccccc4cc1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-20-23(21(17)27-6-5-14(26)10-27)32-19-8-13-4-2-1-3-12(13)7-18(19)28(20)11-16(22(15)29)24(30)31/h1-4,7-9,11,14H,5-6,10,26H2,(H,30,31)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471907
(CHEMBL337526)Show SMILES N[C@H]1CCN(C1)c1c(F)cc2c3c1oc1cc4ccccc4cc1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-20-23(21(17)27-6-5-14(26)10-27)32-19-8-13-4-2-1-3-12(13)7-18(19)28(20)11-16(22(15)29)24(30)31/h1-4,7-9,11,14H,5-6,10,26H2,(H,30,31)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471900

(CHEMBL335700)Show SMILES N[C@H]1CCN(C1)c1c(F)cc2c3c1oc1c4ccccc4ccc1n3cc(C(O)=O)c2=O |r| Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-19-23(20(17)27-8-7-13(26)10-27)32-22-14-4-2-1-3-12(14)5-6-18(22)28(19)11-16(21(15)29)24(30)31/h1-6,9,11,13H,7-8,10,26H2,(H,30,31)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471895

(A-62176 | CHEMBL101299)Show SMILES N[C@H]1CCN(C1)c1c(F)cc2c3c1oc1ccccc1n3cc(C(O)=O)c2=O |r| Show InChI InChI=1S/C20H16FN3O4/c21-13-7-11-16-19(17(13)23-6-5-10(22)8-23)28-15-4-2-1-3-14(15)24(16)9-12(18(11)25)20(26)27/h1-4,7,9-10H,5-6,8,22H2,(H,26,27)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471905
(CHEMBL334497)Show SMILES NC1CCN(C1)c1c(F)cc2c3c1oc1c4ccccc4ccc1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-19-23(20(17)27-8-7-13(26)10-27)32-22-14-4-2-1-3-12(14)5-6-18(22)28(19)11-16(21(15)29)24(30)31/h1-6,9,11,13H,7-8,10,26H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471906

(CHEMBL337205)Show SMILES OC(=O)c1cn2c3cc4ccccc4cc3oc3c(N4CCNCC4)c(F)cc(c23)c1=O Show InChI InChI=1S/C24H18FN3O4/c25-17-11-15-20-23(21(17)27-7-5-26-6-8-27)32-19-10-14-4-2-1-3-13(14)9-18(19)28(20)12-16(22(15)29)24(30)31/h1-4,9-12,26H,5-8H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471903
(CHEMBL335387)Show SMILES N[C@H]1CCN(C1)c1c(F)cc2c3c1oc1ccc4ccccc4c1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-20-23(21(17)27-8-7-13(26)10-27)32-18-6-5-12-3-1-2-4-14(12)19(18)28(20)11-16(22(15)29)24(30)31/h1-6,9,11,13H,7-8,10,26H2,(H,30,31)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471902

(CHEMBL335643)Show SMILES NC1CCN(C1)c1c(F)cc2c3c1oc1ccc4ccccc4c1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-20-23(21(17)27-8-7-13(26)10-27)32-18-6-5-12-3-1-2-4-14(12)19(18)28(20)11-16(22(15)29)24(30)31/h1-6,9,11,13H,7-8,10,26H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471896
(CHEMBL423534)Show SMILES NC1CCN(C1)c1c(F)cc2c3c1oc1cc4ccccc4cc1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-20-23(21(17)27-6-5-14(26)10-27)32-19-8-13-4-2-1-3-12(13)7-18(19)28(20)11-16(22(15)29)24(30)31/h1-4,7-9,11,14H,5-6,10,26H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471901
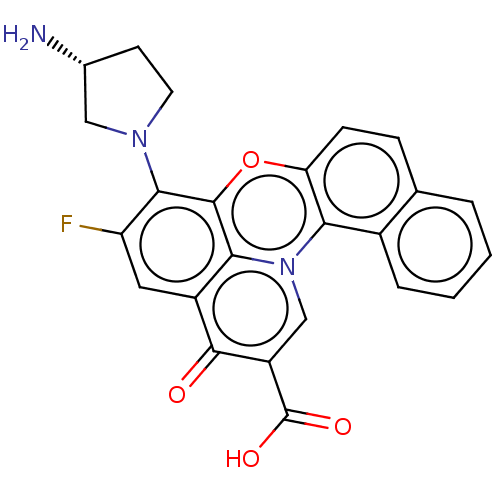
(CHEMBL422988)Show SMILES N[C@@H]1CCN(C1)c1c(F)cc2c3c1oc1ccc4ccccc4c1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-20-23(21(17)27-8-7-13(26)10-27)32-18-6-5-12-3-1-2-4-14(12)19(18)28(20)11-16(22(15)29)24(30)31/h1-6,9,11,13H,7-8,10,26H2,(H,30,31)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471899
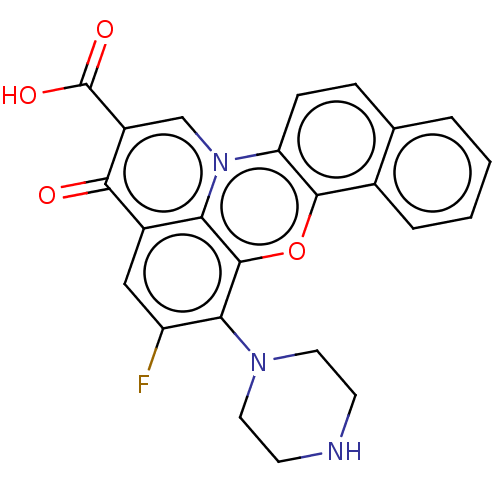
(CHEMBL137263)Show SMILES OC(=O)c1cn2c3ccc4ccccc4c3oc3c(N4CCNCC4)c(F)cc(c23)c1=O Show InChI InChI=1S/C24H18FN3O4/c25-17-11-15-19-23(20(17)27-9-7-26-8-10-27)32-22-14-4-2-1-3-13(14)5-6-18(22)28(19)12-16(21(15)29)24(30)31/h1-6,11-12,26H,7-10H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50302943

(4-(4-fluorophenyl)-5-(4-pyridyl)-1-(ethynyl)-2-(bu...)Show InChI InChI=1S/C20H14FN3O/c1-2-24-18(5-3-4-14-25)23-19(15-6-8-17(21)9-7-15)20(24)16-10-12-22-13-11-16/h1,6-13,25H,4,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of human MAP4K4 by FRET assay |
Bioorg Med Chem Lett 19: 6293-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.094
BindingDB Entry DOI: 10.7270/Q25B02JM |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50302943

(4-(4-fluorophenyl)-5-(4-pyridyl)-1-(ethynyl)-2-(bu...)Show InChI InChI=1S/C20H14FN3O/c1-2-24-18(5-3-4-14-25)23-19(15-6-8-17(21)9-7-15)20(24)16-10-12-22-13-11-16/h1,6-13,25H,4,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of p38beta by FRET assay |
Bioorg Med Chem Lett 19: 6293-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.094
BindingDB Entry DOI: 10.7270/Q25B02JM |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471904
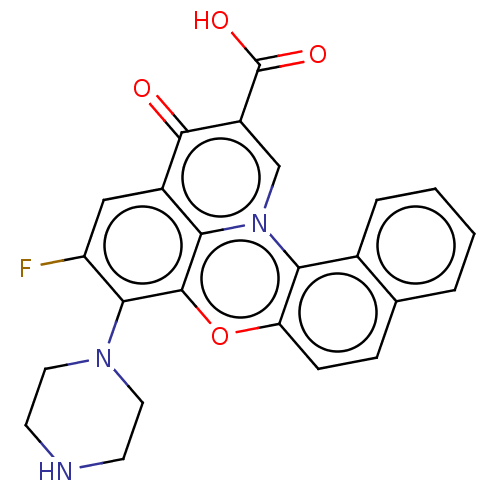
(CHEMBL339379)Show SMILES OC(=O)c1cn2c3c(ccc4ccccc34)oc3c(N4CCNCC4)c(F)cc(c23)c1=O Show InChI InChI=1S/C24H18FN3O4/c25-17-11-15-20-23(21(17)27-9-7-26-8-10-27)32-18-6-5-13-3-1-2-4-14(13)19(18)28(20)12-16(22(15)29)24(30)31/h1-6,11-12,26H,7-10H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50058933
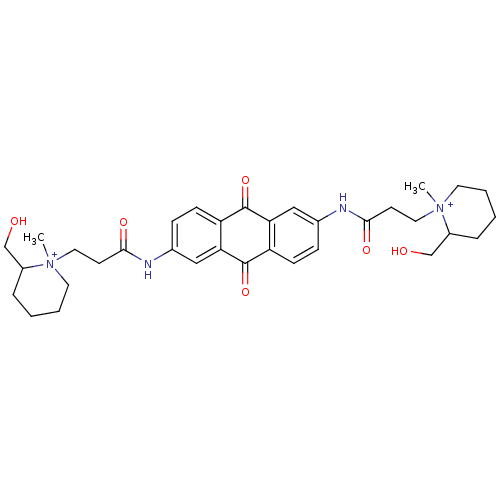
(3-(2-Hydroxymethyl-1-methyl-piperidin-1-yl)-N-{6-[...)Show SMILES C[N+]1(CCC(=O)Nc2ccc3C(=O)c4cc(NC(=O)CC[N+]5(C)CCCCC5CO)ccc4C(=O)c3c2)CCCCC1CO Show InChI InChI=1S/C34H44N4O6/c1-37(15-5-3-7-25(37)21-39)17-13-31(41)35-23-9-11-27-29(19-23)33(43)28-12-10-24(20-30(28)34(27)44)36-32(42)14-18-38(2)16-6-4-8-26(38)22-40/h9-12,19-20,25-26,39-40H,3-8,13-18,21-22H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Drug Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human telomerase. |
J Med Chem 40: 2113-6 (1997)
Article DOI: 10.1021/jm970199z
BindingDB Entry DOI: 10.7270/Q2028QNJ |
More data for this
Ligand-Target Pair | |
Ricin
(Ricinus communis) | BDBM50108008
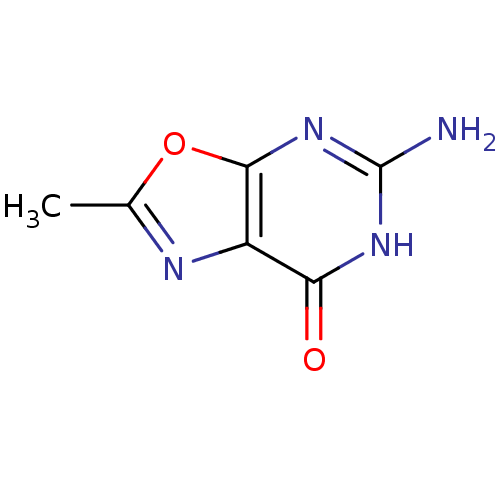
(5-AMINO-2-METHYL-6H-OXAZOLO[5,4-D]PYRIMIDIN-7-ONE ...)Show InChI InChI=1S/C6H6N4O2/c1-2-8-3-4(11)9-6(7)10-5(3)12-2/h1H3,(H3,7,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ricin
(Ricinus communis) | BDBM50108009
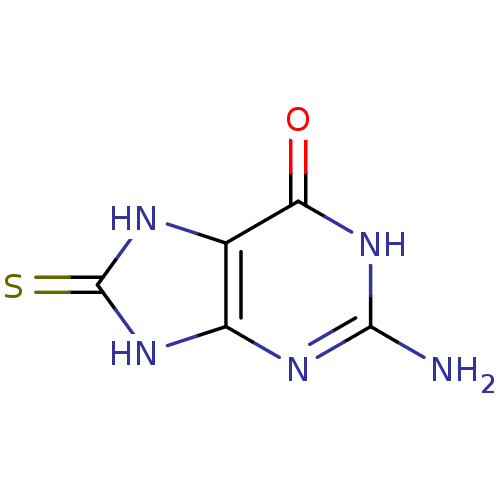
(2-Amino-8-mercapto-1,9-dihydro-purin-6-one | 2-ami...)Show InChI InChI=1S/C5H5N5OS/c6-4-8-2-1(3(11)10-4)7-5(12)9-2/h(H5,6,7,8,9,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | |
Ricin
(Ricinus communis) | BDBM6645
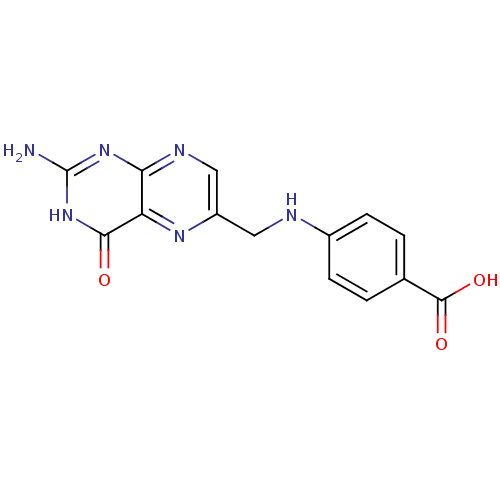
(4-{[(2-amino-4-hydroxypteridin-6-yl)methyl]amino}b...)Show InChI InChI=1S/C14H12N6O3/c15-14-19-11-10(12(21)20-14)18-9(6-17-11)5-16-8-3-1-7(2-4-8)13(22)23/h1-4,6,16H,5H2,(H,22,23)(H3,15,17,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Ricin
(Ricinus communis) | BDBM50108004
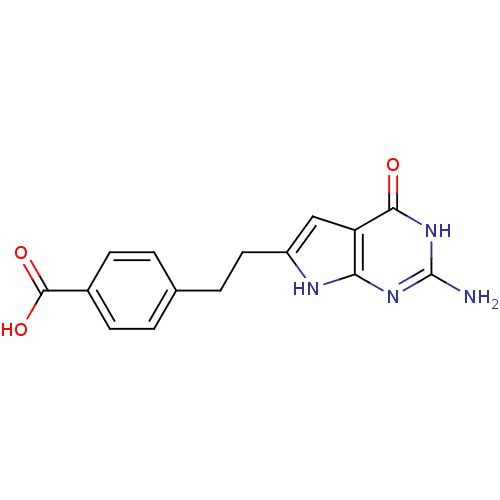
(4-[2-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]p...)Show SMILES Nc1nc2[nH]c(CCc3ccc(cc3)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C15H14N4O3/c16-15-18-12-11(13(20)19-15)7-10(17-12)6-3-8-1-4-9(5-2-8)14(21)22/h1-2,4-5,7H,3,6H2,(H,21,22)(H4,16,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | |
Ricin
(Ricinus communis) | BDBM50108006
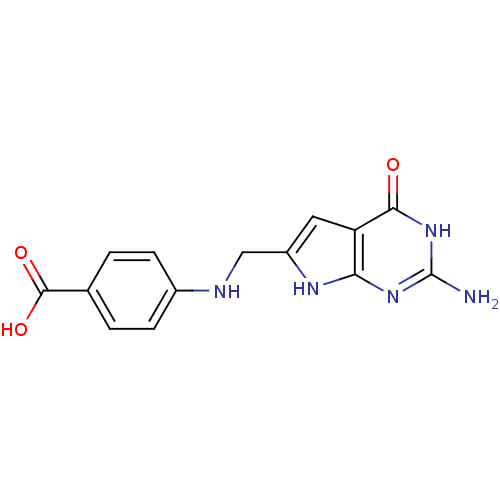
(4-[(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyr...)Show SMILES Nc1nc2[nH]c(CNc3ccc(cc3)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C14H13N5O3/c15-14-18-11-10(12(20)19-14)5-9(17-11)6-16-8-3-1-7(2-4-8)13(21)22/h1-5,16H,6H2,(H,21,22)(H4,15,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Ricin
(Ricinus communis) | BDBM50200094

(2-amino-1,9-dihydro-6H-purin-6-one | CHEMBL219568 ...)Show InChI InChI=1S/C5H5N5O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H4,6,7,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Ricin
(Ricinus communis) | BDBM50108005
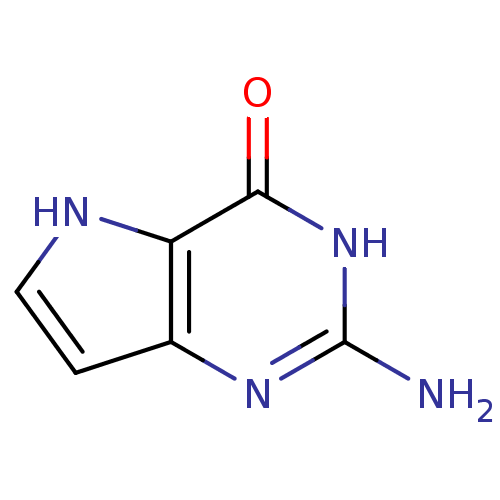
(2-Amino-3,5-dihydro-pyrrolo[3,2-d]pyrimidin-4-one ...)Show InChI InChI=1S/C6H6N4O/c7-6-9-3-1-2-8-4(3)5(11)10-6/h1-2,8H,(H3,7,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
PubMed
| n/a | n/a | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ricin
(Ricinus communis) | BDBM50022241
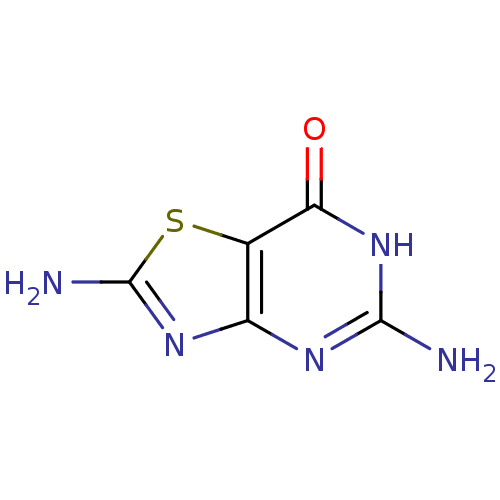
(2,5-Diamino-6H-thiazolo[4,5-d]pyrimidin-7-one | CH...)Show InChI InChI=1S/C5H5N5OS/c6-4-8-2-1(3(11)10-4)12-5(7)9-2/h(H5,6,7,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | |
Ricin
(Ricinus communis) | BDBM50108007
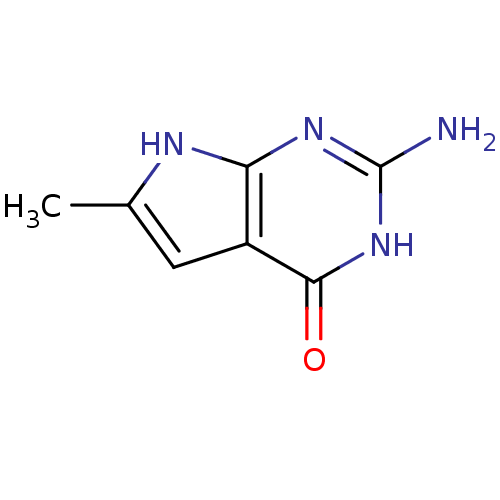
(2-Amino-6-methyl-3,7-dihydro-pyrrolo[2,3-d]pyrimid...)Show InChI InChI=1S/C7H8N4O/c1-3-2-4-5(9-3)10-7(8)11-6(4)12/h2H,1H3,(H4,8,9,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Enzymatic A chain of ricin (RTA) using Artemia salina ribosomes |
J Med Chem 45: 90-8 (2001)
BindingDB Entry DOI: 10.7270/Q2F190F7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data