

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50206821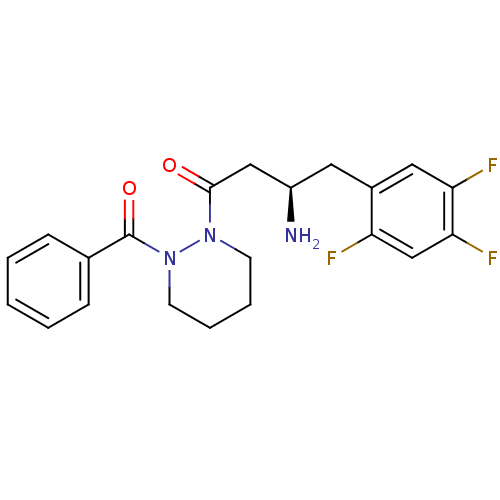 ((R)-3-amino-1-(2-benzoylpiperazin-1-yl)-4-(2,4,5-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of pig kidney DPP4 | Bioorg Med Chem Lett 17: 2622-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.111 BindingDB Entry DOI: 10.7270/Q21V5FS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50206820 ((R)-3-amino-1-(2-benzoyl-1,2-diazepan-1-yl)-4-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of pig kidney DPP4 | Bioorg Med Chem Lett 17: 2622-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.111 BindingDB Entry DOI: 10.7270/Q21V5FS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50220217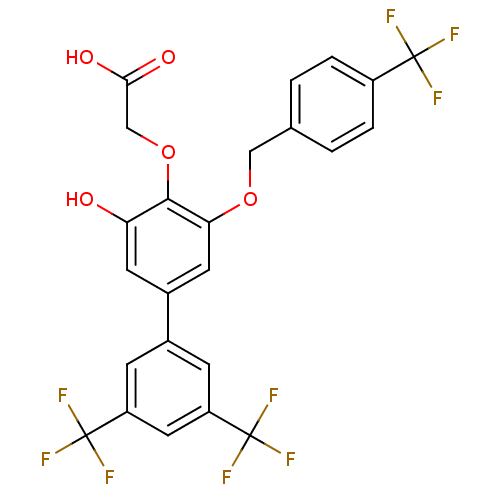 (CHEMBL238138 | [3-hydroxy-3',5'-bis-trifluoromethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of PTP1B after 10 mins | Bioorg Med Chem Lett 17: 5357-60 (2007) Article DOI: 10.1016/j.bmcl.2007.08.019 BindingDB Entry DOI: 10.7270/Q2BK1D55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50220217 (CHEMBL238138 | [3-hydroxy-3',5'-bis-trifluoromethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of TC-PTP after 10 mins | Bioorg Med Chem Lett 17: 5357-60 (2007) Article DOI: 10.1016/j.bmcl.2007.08.019 BindingDB Entry DOI: 10.7270/Q2BK1D55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337787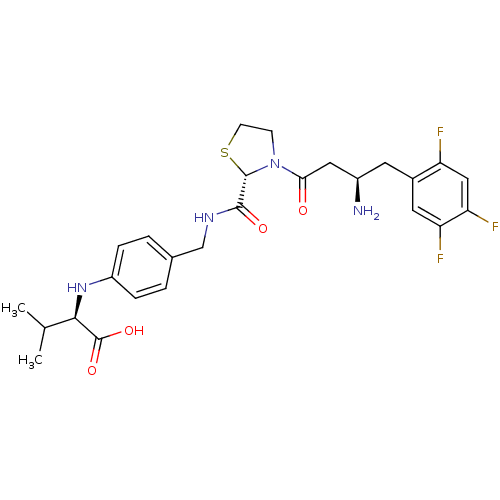 ((R)-2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337789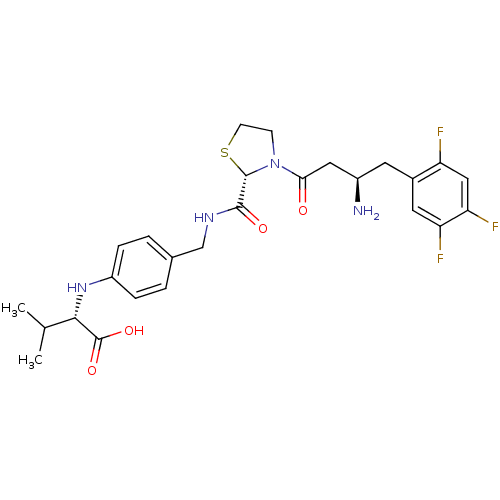 ((S)-2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286099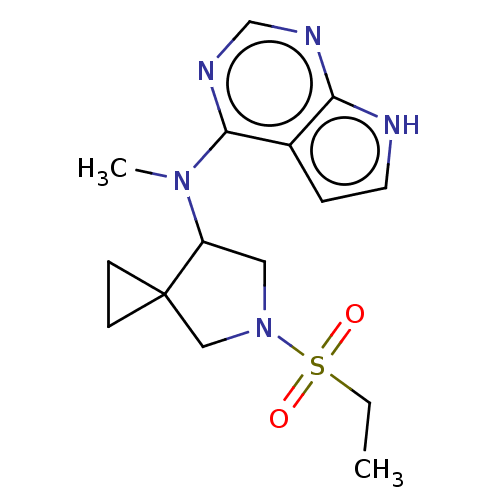 (N-(5-(ethylsulfonyl)-5-azaspiro[2.4]heptan-7-yl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337785 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286098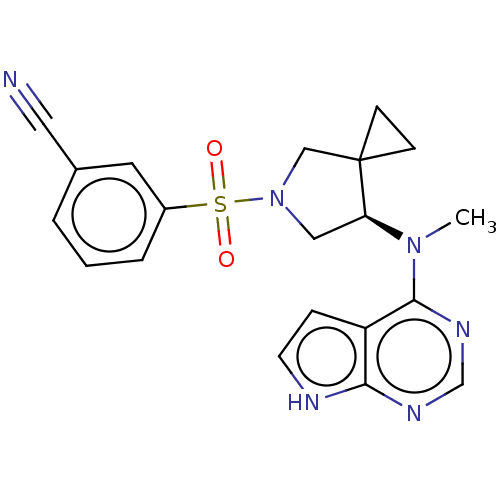 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337779 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286095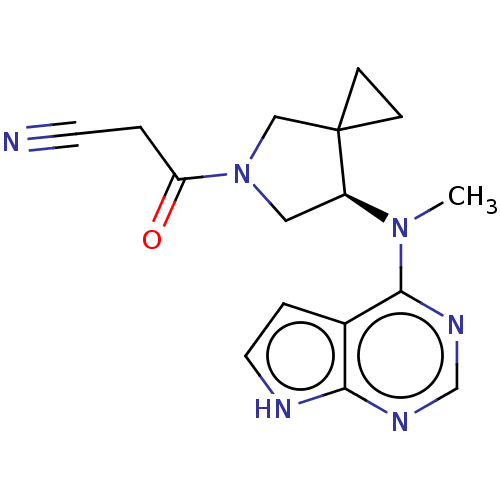 ((R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337791 ((R)-2-(4-(((R)-3-((R)-3-amino-4-(2,4,5-trifluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337783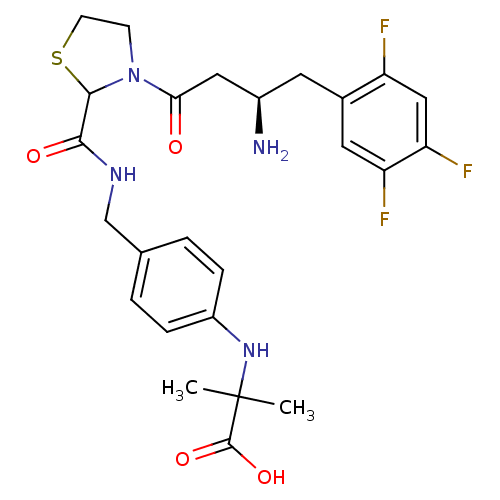 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337793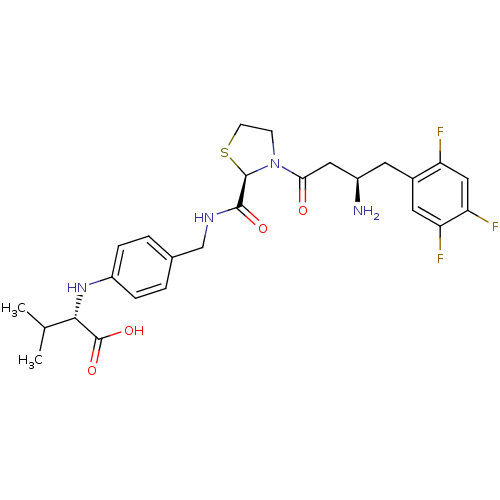 ((S)-2-(4-(((R)-3-((R)-3-amino-4-(2,4,5-trifluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337788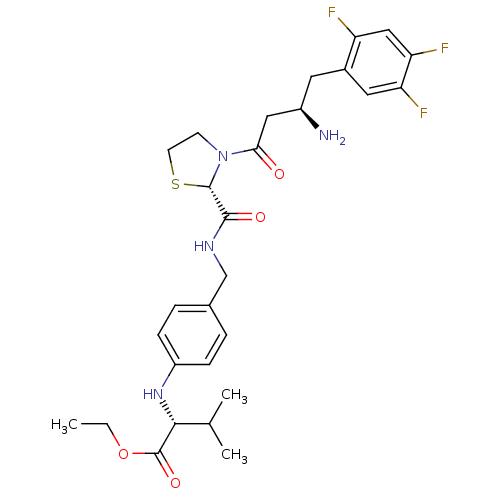 ((R)-ethyl 2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286098 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.7 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337790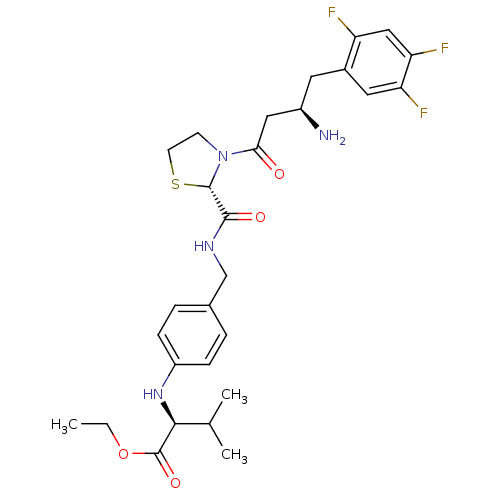 ((S)-ethyl 2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337778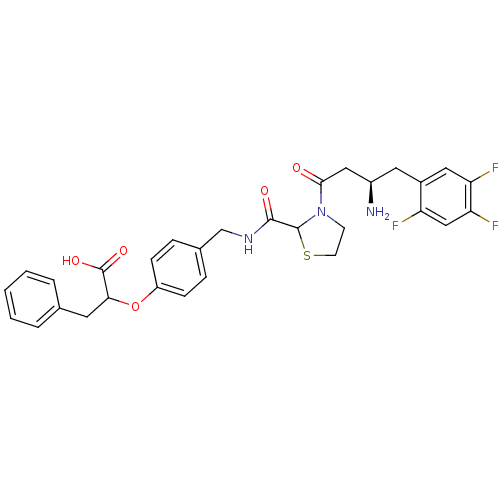 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286096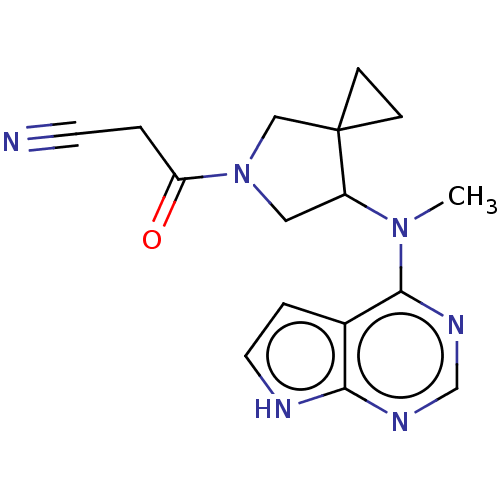 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29.3 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM286096 (3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40.4 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337768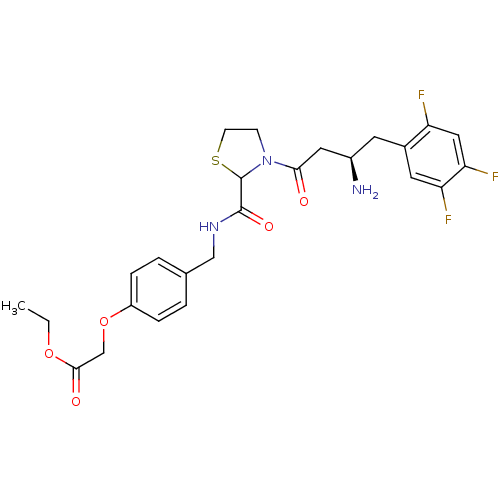 (CHEMBL1683487 | ethyl 2-(4-((3-((R)-3-amino-4-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50256618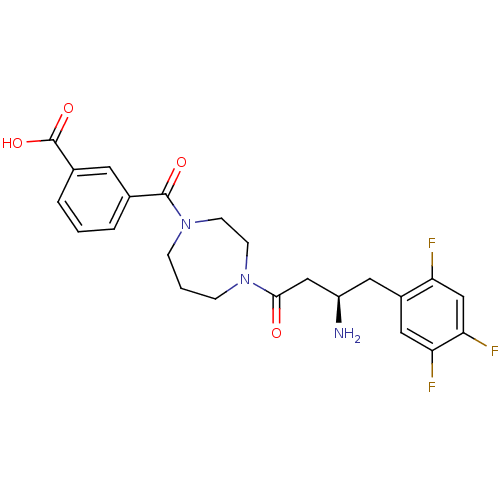 ((R)-3-(4-(3-amino-4-(2,4,5-trifluorophenyl)butanoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP2 (unknown origin) | Bioorg Med Chem Lett 18: 6525-9 (2008) Article DOI: 10.1016/j.bmcl.2008.10.076 BindingDB Entry DOI: 10.7270/Q25D8RQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50437758 (CHEMBL2409565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT-1 expressed in Hep3B cell lysate using didecanoyl glycerol and [14C]decanoyl-CoA as substrate after 60 mins by l... | Bioorg Med Chem Lett 23: 4713-8 (2013) Article DOI: 10.1016/j.bmcl.2013.05.081 BindingDB Entry DOI: 10.7270/Q21J9C63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337786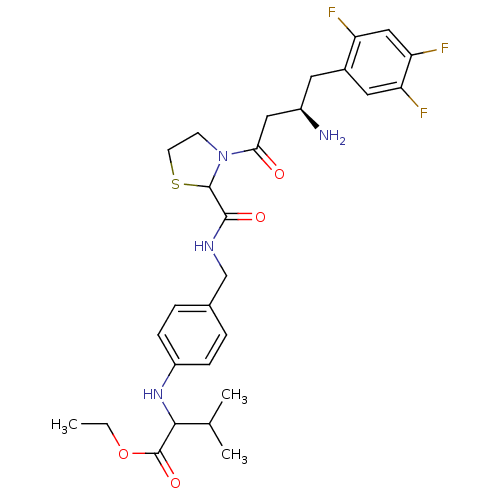 (CHEMBL1683505 | ethyl 2-(4-((3-((R)-3-amino-4-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50256618 ((R)-3-(4-(3-amino-4-(2,4,5-trifluorophenyl)butanoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) | Bioorg Med Chem Lett 18: 6525-9 (2008) Article DOI: 10.1016/j.bmcl.2008.10.076 BindingDB Entry DOI: 10.7270/Q25D8RQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50206821 ((R)-3-amino-1-(2-benzoylpiperazin-1-yl)-4-(2,4,5-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 17: 2622-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.111 BindingDB Entry DOI: 10.7270/Q21V5FS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 17: 2622-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.111 BindingDB Entry DOI: 10.7270/Q21V5FS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337769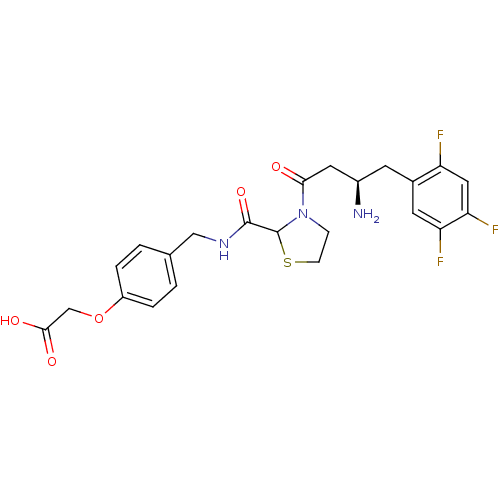 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337777 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM286098 ((R)-3-((7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 58.6 | n/a | n/a | n/a | n/a | n/a | n/a |
YANG JI CHEMICAL CO., LTD.; HAN WHA PHARMA CO., LTD. US Patent | Assay Description Kinases used were human-derived JAK1, JAK2, JAK3, and TYK2 (Millipore, Germany). Each of these kinases was diluted with a suitable buffer solution as... | US Patent US10081635 (2018) BindingDB Entry DOI: 10.7270/Q2R213D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150870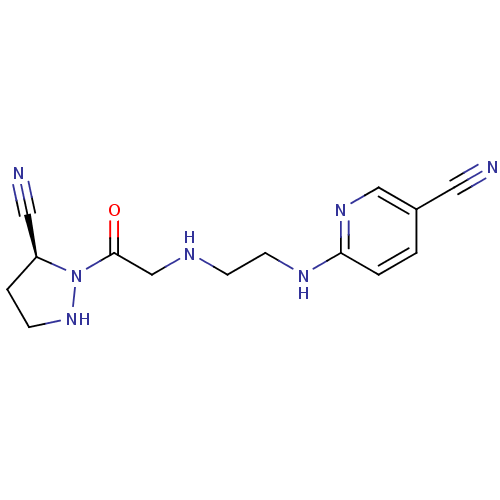 (6-{2-[2-((S)-5-Cyano-pyrazolidin-1-yl)-2-oxo-ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337774 (CHEMBL1683493 | ethyl 5-((3-((R)-3-amino-4-(2,4,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337775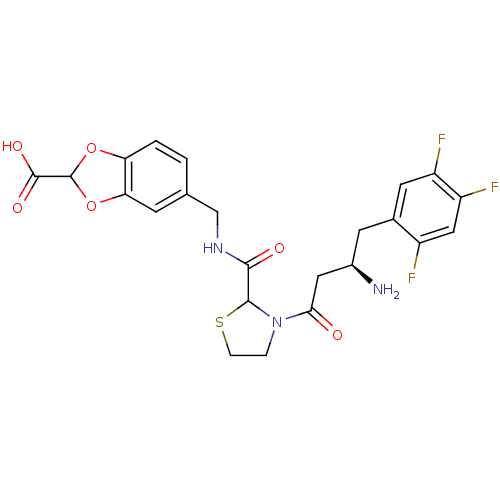 (5-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50256619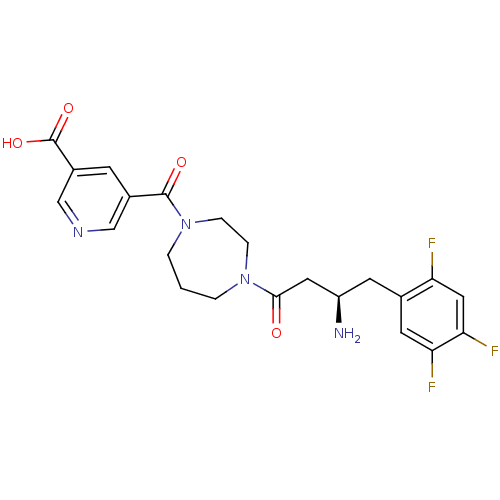 ((R)-5-(4-(3-amino-4-(2,4,5-trifluorophenyl)butanoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) | Bioorg Med Chem Lett 18: 6525-9 (2008) Article DOI: 10.1016/j.bmcl.2008.10.076 BindingDB Entry DOI: 10.7270/Q25D8RQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337784 (CHEMBL1683503 | ethyl 2-(4-((3-((R)-3-amino-4-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50256266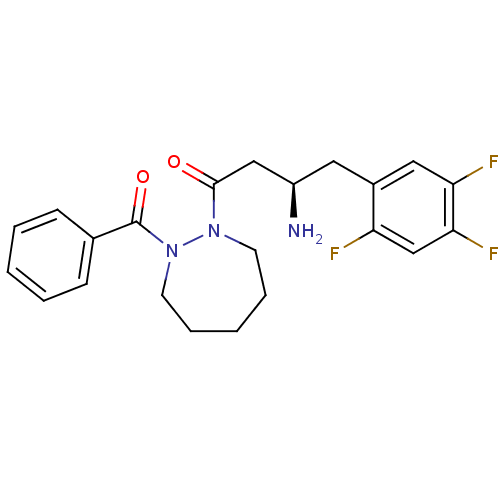 ((2R)-4-(2-BENZOYL-1,2-DIAZEPAN-1-YL)-4-OXO-1-(2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) | Bioorg Med Chem Lett 18: 6525-9 (2008) Article DOI: 10.1016/j.bmcl.2008.10.076 BindingDB Entry DOI: 10.7270/Q25D8RQV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50206820 ((R)-3-amino-1-(2-benzoyl-1,2-diazepan-1-yl)-4-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 17: 2622-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.111 BindingDB Entry DOI: 10.7270/Q21V5FS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337764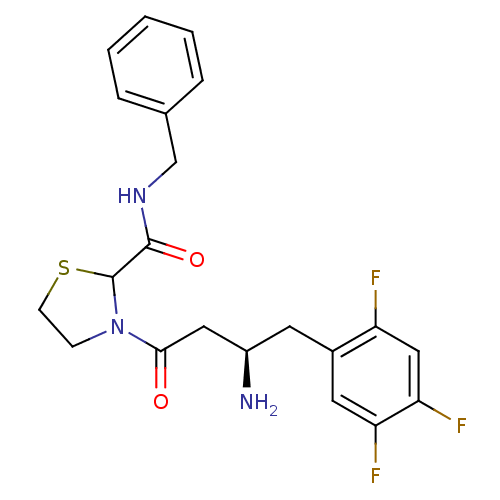 (3-((R)-3-amino-4-(2,4,5-trifluorophenyl)butanoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337780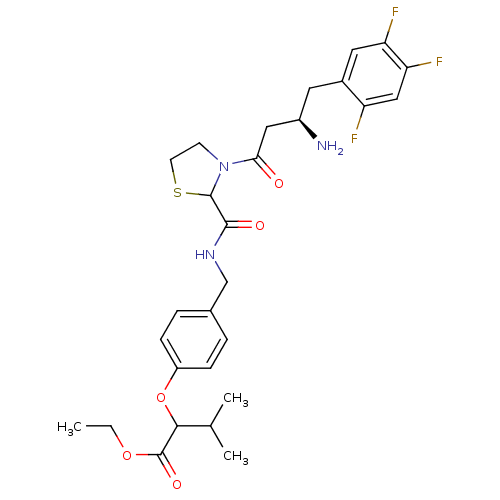 (CHEMBL1683499 | ethyl 2-(4-((3-((R)-3-amino-4-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337781 (2-((4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337782 (CHEMBL1683501 | ethyl 2-((4-((3-((R)-3-amino-4-(2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50150851 (2-((R)-2-Amino-3-methyl-pentanoyl)-3,4-dihydro-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against dipeptidyl peptidase IV in Caco-2 cell assay | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) | Bioorg Med Chem Lett 18: 6525-9 (2008) Article DOI: 10.1016/j.bmcl.2008.10.076 BindingDB Entry DOI: 10.7270/Q25D8RQV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM50355142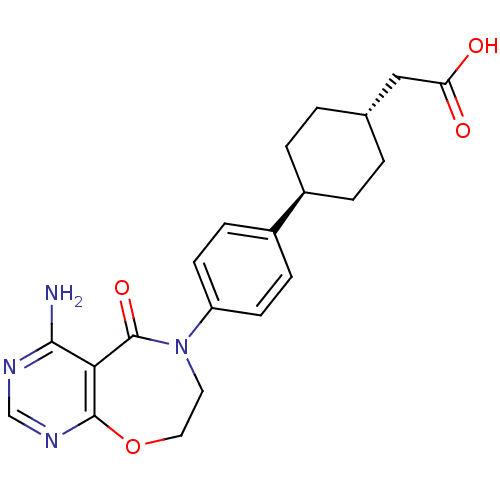 (CHEMBL1835919) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DGAT-1 in mouse liver microsomes using didecanoyl glycerol and [14C]decanoyl-CoA as substrate after 60 mins by liquid scintillation cou... | Bioorg Med Chem Lett 23: 4713-8 (2013) Article DOI: 10.1016/j.bmcl.2013.05.081 BindingDB Entry DOI: 10.7270/Q21J9C63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337763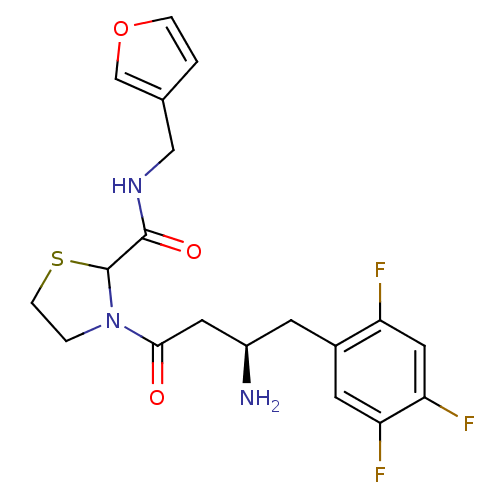 (3-((R)-3-amino-4-(2,4,5-trifluorophenyl)butanoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50355142 (CHEMBL1835919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT-1 expressed in Hep3B cell lysate using didecanoyl glycerol and [14C]decanoyl-CoA as substrate after 60 mins by l... | Bioorg Med Chem Lett 23: 4713-8 (2013) Article DOI: 10.1016/j.bmcl.2013.05.081 BindingDB Entry DOI: 10.7270/Q21J9C63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50437757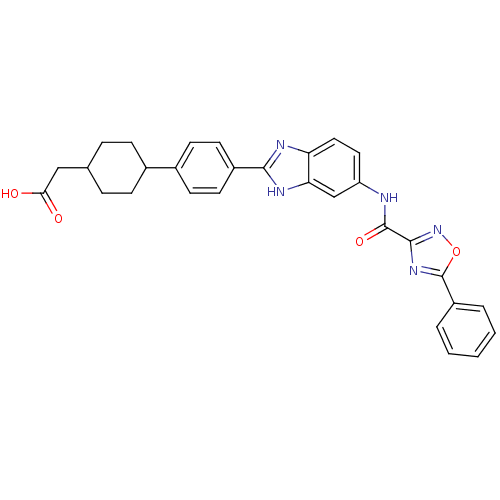 (CHEMBL2409563) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT-1 expressed in Hep3B cell lysate using didecanoyl glycerol and [14C]decanoyl-CoA as substrate after 60 mins by l... | Bioorg Med Chem Lett 23: 4713-8 (2013) Article DOI: 10.1016/j.bmcl.2013.05.081 BindingDB Entry DOI: 10.7270/Q21J9C63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337770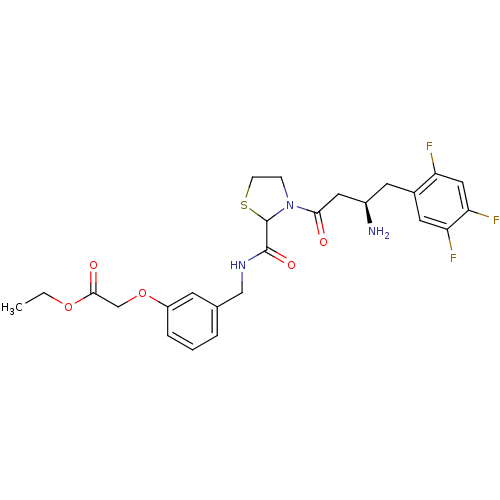 (CHEMBL1683489 | ethyl 2-(3-((3-((R)-3-amino-4-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50256619 ((R)-5-(4-(3-amino-4-(2,4,5-trifluorophenyl)butanoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DPP2 (unknown origin) | Bioorg Med Chem Lett 18: 6525-9 (2008) Article DOI: 10.1016/j.bmcl.2008.10.076 BindingDB Entry DOI: 10.7270/Q25D8RQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 386 total ) | Next | Last >> |