

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of COX2 | Bioorg Med Chem Lett 19: 3271-4 (2009) Article DOI: 10.1016/j.bmcl.2009.04.078 BindingDB Entry DOI: 10.7270/Q2222TSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX2 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-2 in human HNSCC 1483 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured a... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50076267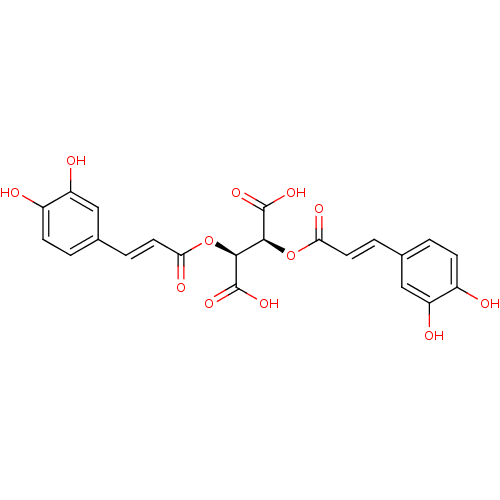 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073630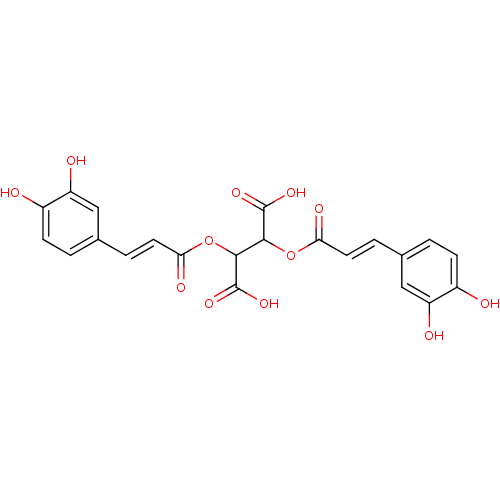 (2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acryloyloxy]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073630 (2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acryloyloxy]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 [18-604,R106Q] (Mus musculus (Mouse)) | BDBM50312668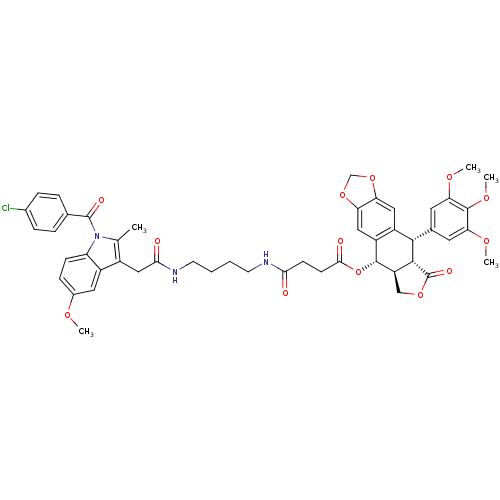 (CHEMBL1076638 | Chemocoxib A (12) | N-{(Succinylpo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205489 (Cytotoxic conjugates, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50392945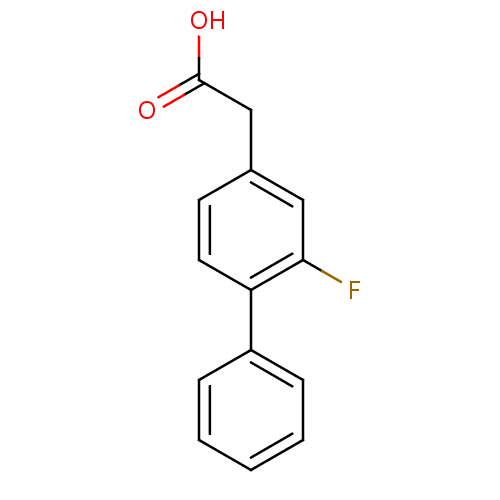 (CHEMBL2152245) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse COX2-mediated 2-arachidonoylglycerol oxygenation preincubated for 3 mins before 2-arachidonoylglycerol addition measured after 30... | ACS Med Chem Lett 3: 759-763 (2012) Article DOI: 10.1021/ml3001616 BindingDB Entry DOI: 10.7270/Q2FJ2HWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110164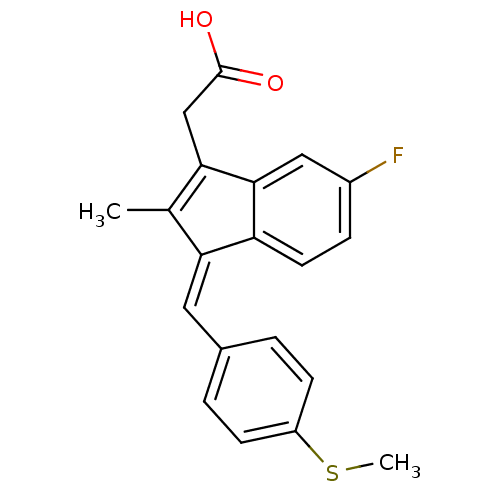 ((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50272862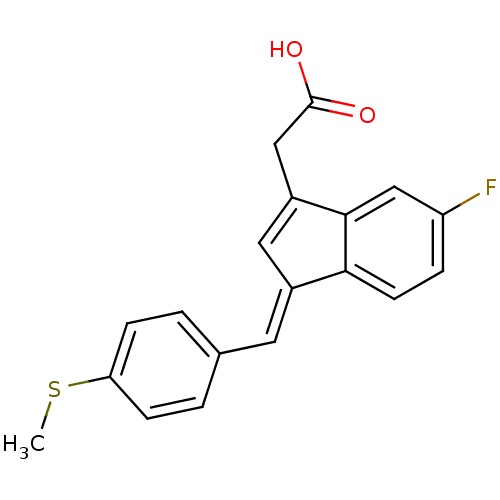 ((E)-2'-des-methyl sulindac sulfide | 2-desmethylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured after... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110164 ((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX2 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205496 (Cytotoxic conjugates, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50293599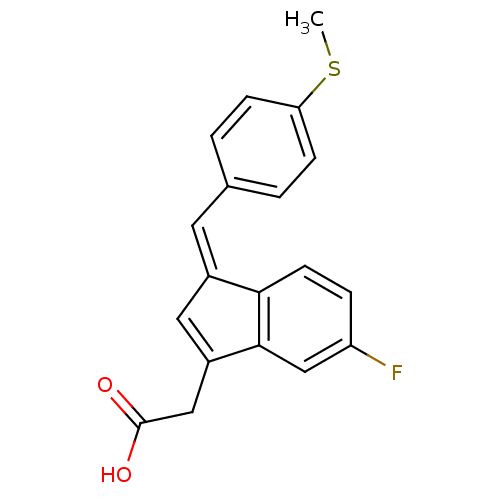 ((Z)-2'-des-methyl sulindac sulfide | CHEMBL561959) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of mouse COX2 under reduced substrate concentration by TLC | Bioorg Med Chem Lett 19: 3271-4 (2009) Article DOI: 10.1016/j.bmcl.2009.04.078 BindingDB Entry DOI: 10.7270/Q2222TSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured after... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205497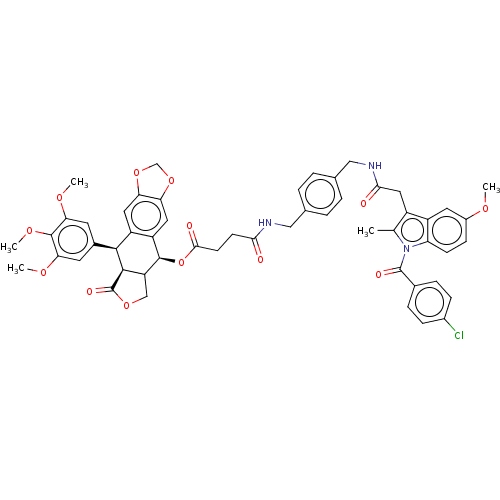 (Cytotoxic conjugates, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 [18-604,S516A] (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205495 (Cytotoxic conjugates, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 19: 3271-4 (2009) Article DOI: 10.1016/j.bmcl.2009.04.078 BindingDB Entry DOI: 10.7270/Q2222TSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205487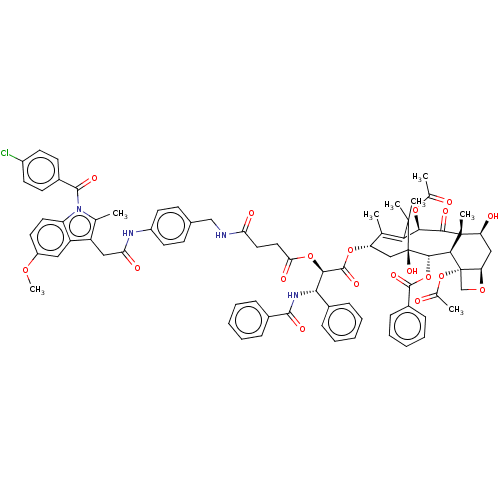 (Cytotoxic conjugates, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50312668 (CHEMBL1076638 | Chemocoxib A (12) | N-{(Succinylpo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50312668 (CHEMBL1076638 | Chemocoxib A (12) | N-{(Succinylpo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 [18-604,R106Q] (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50273065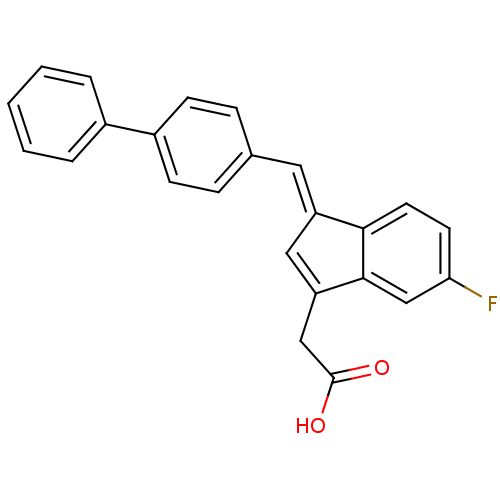 ((E)-2-(1-(Biphenyl-4-ylmethylene)-5-fluoro-1H-inde...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured after... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX2 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50293599 ((Z)-2'-des-methyl sulindac sulfide | CHEMBL561959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of ovine COX1 under reduced substrate concentration by TLC | Bioorg Med Chem Lett 19: 3271-4 (2009) Article DOI: 10.1016/j.bmcl.2009.04.078 BindingDB Entry DOI: 10.7270/Q2222TSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073626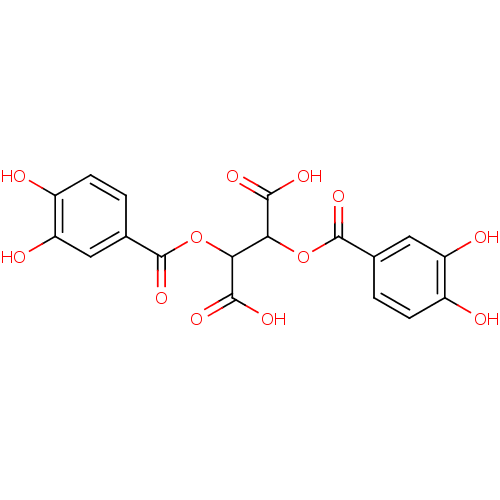 (2,3-Bis-(3,4-dihydroxy-benzoyloxy)-succinic acid |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205488 (Cytotoxic conjugates, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 [18-604,V509I] (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205494 (Cytotoxic conjugates, 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50388954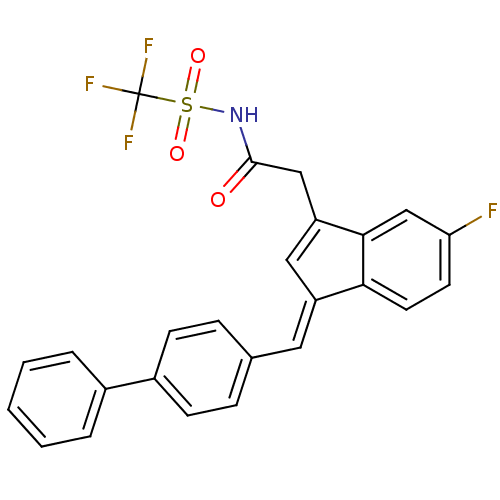 (CHEMBL2063559) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using [14C] arachidonic acid as substrate preincubated for 17 mins before substrate addition measured after 3 mins by thin-... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205498 (Cytotoxic conjugates, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50388954 (CHEMBL2063559) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 495 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured after... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50295286 (2-(3-benzoylphenyl)acetic acid | CHEMBL561718) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse COX2-mediated 2-arachidonoylglycerol oxygenation preincubated for 3 mins before 2-arachidonoylglycerol addition measured after 30... | ACS Med Chem Lett 3: 759-763 (2012) Article DOI: 10.1021/ml3001616 BindingDB Entry DOI: 10.7270/Q2FJ2HWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073631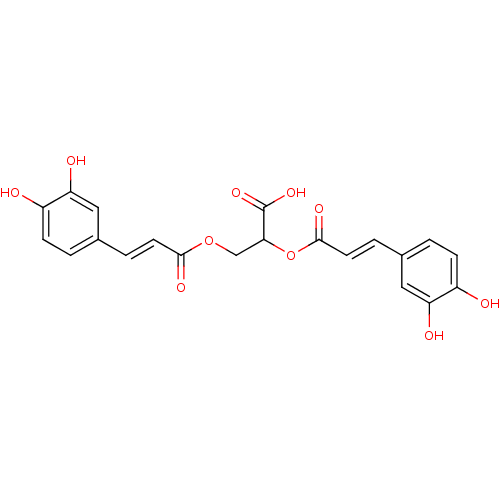 ((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid 1-carbox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50273065 ((E)-2-(1-(Biphenyl-4-ylmethylene)-5-fluoro-1H-inde...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using [14C] arachidonic acid as substrate preincubated for 17 mins before substrate addition measured after 3 mins by thin-... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073627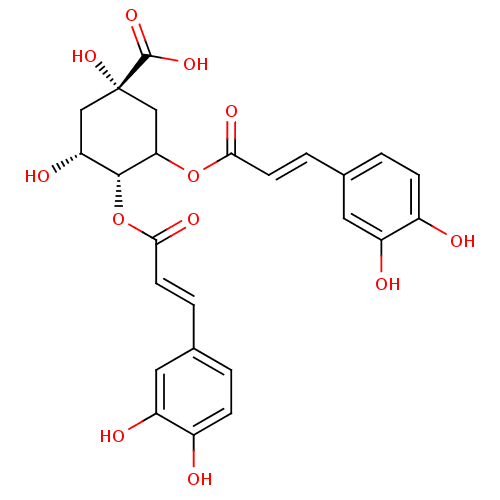 ((1S,4R,5R)-3,4-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205490 (Cytotoxic conjugates, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50392945 (CHEMBL2152245) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of COX2-mediated 2-arachidonoylglycerol oxygenation in LPS/IFN-gamma-stimulated mouse RAW264.7 cells assessed as 2-AG to PGE2-G/PGD2-G con... | ACS Med Chem Lett 3: 759-763 (2012) Article DOI: 10.1021/ml3001616 BindingDB Entry DOI: 10.7270/Q2FJ2HWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX2 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073643 ((1R,3R,4S)-3,5-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073638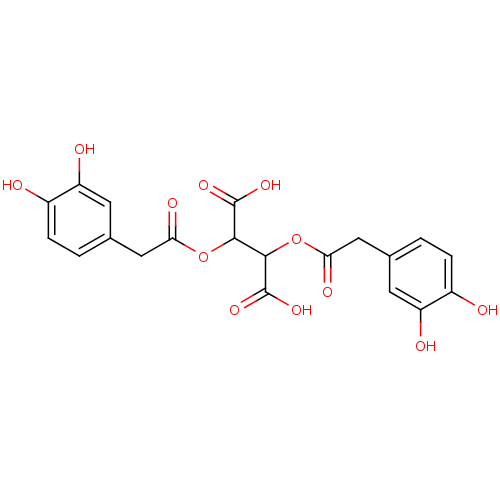 (2,3-Bis-[2-(3,4-dihydroxy-phenyl)-acetoxy]-succini...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205491 (Cytotoxic conjugates, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073634 (2,3-Bis-(3,4,5-trihydroxy-benzoyloxy)-succinic aci...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Human Immunodeficiency Virus Type 1 integrase (HIV-1 IN) in the disintegration assay. | J Med Chem 42: 497-509 (1999) Article DOI: 10.1021/jm9804735 BindingDB Entry DOI: 10.7270/Q2G161J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 118 total ) | Next | Last >> |