 Found 49 hits with Last Name = 'kramer' and Initial = 'g'
Found 49 hits with Last Name = 'kramer' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36514

(CID46912122 | MDW933, 5)Show SMILES CC1=CC(C)=[N+]2C1=C(CCCCc1cn(C[C@H]3[C@H]4O[C@H]4[C@H](O)[C@@H](O)[C@@H]3O)nn1)c1c(C)cc(C)n1[B-]2(F)F |c:4,7,t:1| Show InChI InChI=1S/C26H34BF2N5O4/c1-13-9-15(3)33-20(13)18(21-14(2)10-16(4)34(21)27(33,28)29)8-6-5-7-17-11-32(31-30-17)12-19-22(35)23(36)24(37)26-25(19)38-26/h9-11,19,22-26,35-37H,5-8,12H2,1-4H3/t19-,22-,23+,24-,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -48.4 | 1.24 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36515
(CID46912120 | MDW941, 6)Show SMILES COc1ccc(cc1)C1=[N+]2C(C=C1)=C(CCCCc1cn(C[C@H]3[C@H]4O[C@H]4[C@H](O)[C@@H](O)[C@@H]3O)nn1)c1ccc(-c3ccc(OC)cc3)n1[B-]2(F)F |c:12,t:9,14| Show InChI InChI=1S/C36H38BF2N5O6/c1-48-24-11-7-21(8-12-24)28-15-17-30-26(31-18-16-29(44(31)37(38,39)43(28)30)22-9-13-25(49-2)14-10-22)6-4-3-5-23-19-42(41-40-23)20-27-32(45)33(46)34(47)36-35(27)50-36/h7-19,27,32-36,45-47H,3-6,20H2,1-2H3/t27-,32-,33+,34-,35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -48.1 | 1.94 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010477
(CHEMBL1233799)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O8P/c1-4-15(2)23(26-16(3)29)25(33)27-21(13-17-5-9-19(30)10-6-17)24(32)28-22(37(34,35)36)14-18-7-11-20(31)12-8-18/h5-12,15,21-23,30-31H,4,13-14H2,1-3H3,(H,26,29)(H,27,33)(H,28,32)(H2,34,35,36)/t15-,21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010479
(CHEMBL3264010)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O7P/c1-4-16(2)23(26-17(3)29)25(32)27-21(14-18-8-6-5-7-9-18)24(31)28-22(36(33,34)35)15-19-10-12-20(30)13-11-19/h5-13,16,21-23,30H,4,14-15H2,1-3H3,(H,26,29)(H,27,32)(H,28,31)(H2,33,34,35)/t16-,21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010478
(CHEMBL3264009)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C22H36N3O7P/c1-6-14(4)20(23-15(5)26)22(29)24-18(11-13(2)3)21(28)25-19(33(30,31)32)12-16-7-9-17(27)10-8-16/h7-10,13-14,18-20,27H,6,11-12H2,1-5H3,(H,23,26)(H,24,29)(H,25,28)(H2,30,31,32)/t14-,18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010478

(CHEMBL3264009)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C22H36N3O7P/c1-6-14(4)20(23-15(5)26)22(29)24-18(11-13(2)3)21(28)25-19(33(30,31)32)12-16-7-9-17(27)10-8-16/h7-10,13-14,18-20,27H,6,11-12H2,1-5H3,(H,23,26)(H,24,29)(H,25,28)(H2,30,31,32)/t14-,18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36513
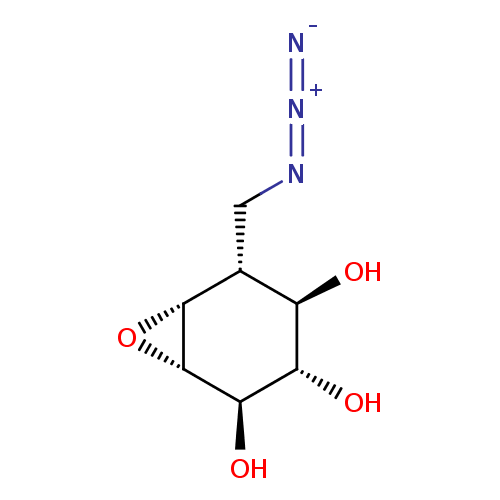
(CID46912128 | KY170, 4 | US9056847, Azido-cyclophe...)Show SMILES O[C@H]1[C@@H]2O[C@@H]2[C@H](CN=[N+]=[N-])[C@@H](O)[C@@H]1O Show InChI InChI=1S/C7H11N3O4/c8-10-9-1-2-3(11)4(12)5(13)7-6(2)14-7/h2-7,11-13H,1H2/t2-,3-,4+,5-,6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 44 | -43.7 | 120 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010477

(CHEMBL1233799)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O8P/c1-4-15(2)23(26-16(3)29)25(33)27-21(13-17-5-9-19(30)10-6-17)24(32)28-22(37(34,35)36)14-18-7-11-20(31)12-8-18/h5-12,15,21-23,30-31H,4,13-14H2,1-3H3,(H,26,29)(H,27,33)(H,28,32)(H2,34,35,36)/t15-,21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM36512
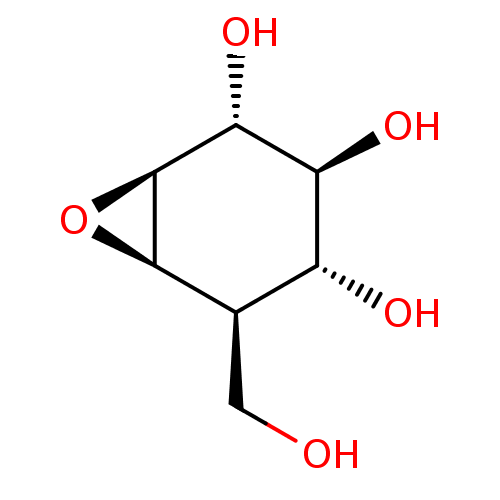
(CID164227 | Cylcophellitol, 3 | US11826435, Compou...)Show SMILES OC[C@H]1[C@H]2O[C@H]2[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C7H12O5/c8-1-2-3(9)4(10)5(11)7-6(2)12-7/h2-11H,1H2/t2-,3-,4+,5-,6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 152 | -40.5 | 150 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010479

(CHEMBL3264010)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O7P/c1-4-16(2)23(26-17(3)29)25(32)27-21(14-18-8-6-5-7-9-18)24(31)28-22(36(33,34)35)15-19-10-12-20(30)13-11-19/h5-13,16,21-23,30H,4,14-15H2,1-3H3,(H,26,29)(H,27,32)(H,28,31)(H2,33,34,35)/t16-,21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50078114

((1S,2R,3S,4S,5R,6R)-7-Oxa-bicyclo[4.1.0]heptane-2,...)Show SMILES O[C@H]1[C@H]2O[C@H]2[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C6H10O5/c7-1-2(8)4(10)6-5(11-6)3(1)9/h1-10H/t1-,2-,3+,4+,5-,6+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+4 | -25.4 | 9.49E+3 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University
| Assay Description
Enzymatic activity assay using fluorescent activity-based labeling method. |
Nat Chem Biol 6: 907-13 (2010)
Article DOI: 10.1038/nchembio.466
BindingDB Entry DOI: 10.7270/Q2FX77ST |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50137113
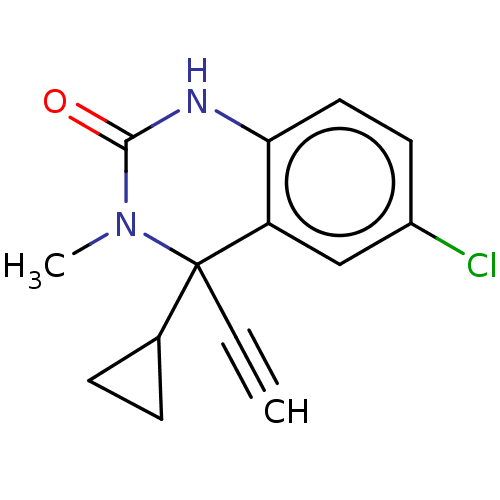
(CHEMBL3753096)Show InChI InChI=1S/C14H13ClN2O/c1-3-14(9-4-5-9)11-8-10(15)6-7-12(11)16-13(18)17(14)2/h1,6-9H,4-5H2,2H3,(H,16,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010479

(CHEMBL3264010)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O7P/c1-4-16(2)23(26-17(3)29)25(32)27-21(14-18-8-6-5-7-9-18)24(31)28-22(36(33,34)35)15-19-10-12-20(30)13-11-19/h5-13,16,21-23,30H,4,14-15H2,1-3H3,(H,26,29)(H,27,32)(H,28,31)(H2,33,34,35)/t16-,21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010477

(CHEMBL1233799)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O8P/c1-4-15(2)23(26-16(3)29)25(33)27-21(13-17-5-9-19(30)10-6-17)24(32)28-22(37(34,35)36)14-18-7-11-20(31)12-8-18/h5-12,15,21-23,30-31H,4,13-14H2,1-3H3,(H,26,29)(H,27,33)(H,28,32)(H2,34,35,36)/t15-,21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010478

(CHEMBL3264009)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C22H36N3O7P/c1-6-14(4)20(23-15(5)26)22(29)24-18(11-13(2)3)21(28)25-19(33(30,31)32)12-16-7-9-17(27)10-8-16/h7-10,13-14,18-20,27H,6,11-12H2,1-5H3,(H,23,26)(H,24,29)(H,25,28)(H2,30,31,32)/t14-,18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50137112
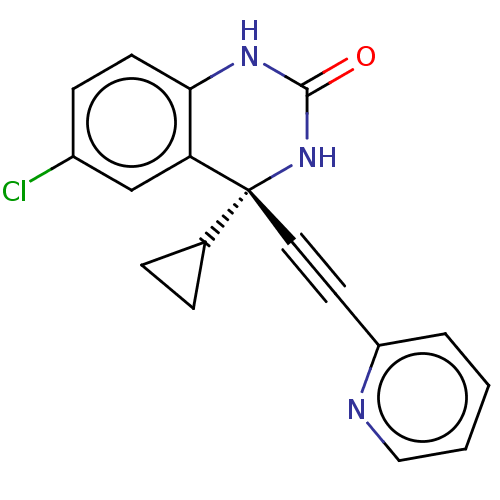
(CHEMBL309896)Show SMILES Clc1ccc2NC(=O)N[C@](C#Cc3ccccn3)(C3CC3)c2c1 Show InChI InChI=1S/C18H14ClN3O/c19-13-6-7-16-15(11-13)18(12-4-5-12,22-17(23)21-16)9-8-14-3-1-2-10-20-14/h1-3,6-7,10-12H,4-5H2,(H2,21,22,23)/t18-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM3107
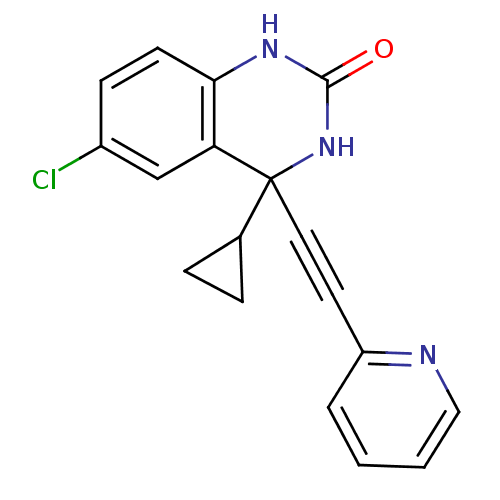
(3,4-Dihydroquinazolinon 4a | 6-Chloro-4-cyclopyrop...)Show InChI InChI=1S/C18H14ClN3O/c19-13-6-7-16-15(11-13)18(12-4-5-12,22-17(23)21-16)9-8-14-3-1-2-10-20-14/h1-3,6-7,10-12H,4-5H2,(H2,21,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50010477

(CHEMBL1233799)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O8P/c1-4-15(2)23(26-16(3)29)25(33)27-21(13-17-5-9-19(30)10-6-17)24(32)28-22(37(34,35)36)14-18-7-11-20(31)12-8-18/h5-12,15,21-23,30-31H,4,13-14H2,1-3H3,(H,26,29)(H,27,33)(H,28,32)(H2,34,35,36)/t15-,21-,22+,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung somatic ACE using FAPGG substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50010479

(CHEMBL3264010)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O7P/c1-4-16(2)23(26-17(3)29)25(32)27-21(14-18-8-6-5-7-9-18)24(31)28-22(36(33,34)35)15-19-10-12-20(30)13-11-19/h5-13,16,21-23,30H,4,14-15H2,1-3H3,(H,26,29)(H,27,32)(H,28,31)(H2,33,34,35)/t16-,21-,22+,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung somatic ACE using FAPGG substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1517
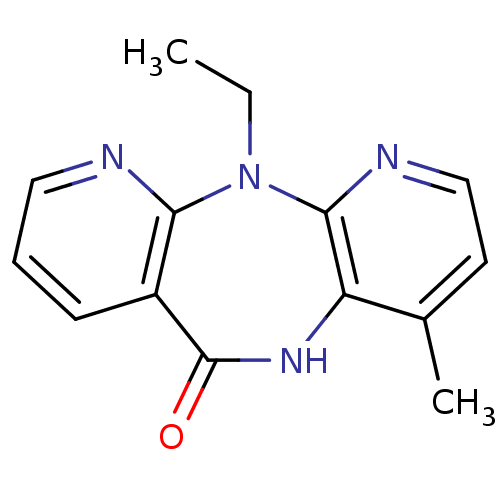
(2-ethyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...)Show InChI InChI=1S/C14H14N4O/c1-3-18-12-10(5-4-7-15-12)14(19)17-11-9(2)6-8-16-13(11)18/h4-8H,3H2,1-2H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524092
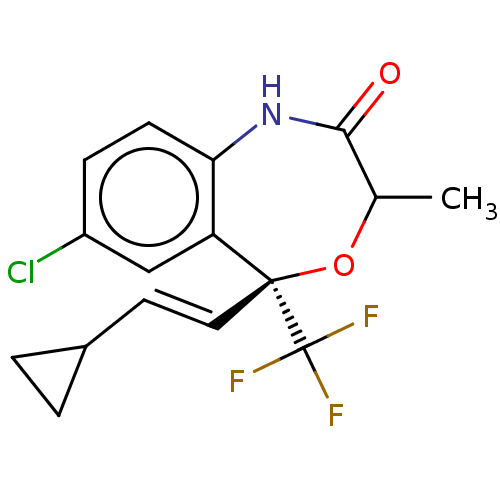
(CHEMBL4436313)Show SMILES CC1O[C@@](\C=C\C2CC2)(c2cc(Cl)ccc2NC1=O)C(F)(F)F |r| Show InChI InChI=1S/C16H15ClF3NO2/c1-9-14(22)21-13-5-4-11(17)8-12(13)15(23-9,16(18,19)20)7-6-10-2-3-10/h4-10H,2-3H2,1H3,(H,21,22)/b7-6+/t9?,15-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010480
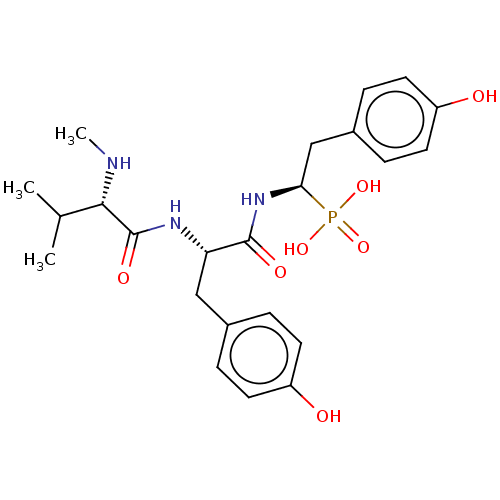
(CHEMBL3264008)Show SMILES CN[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C23H32N3O7P/c1-14(2)21(24-3)23(30)25-19(12-15-4-8-17(27)9-5-15)22(29)26-20(34(31,32)33)13-16-6-10-18(28)11-7-16/h4-11,14,19-21,24,27-28H,12-13H2,1-3H3,(H,25,30)(H,26,29)(H2,31,32,33)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010480

(CHEMBL3264008)Show SMILES CN[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C23H32N3O7P/c1-14(2)21(24-3)23(30)25-19(12-15-4-8-17(27)9-5-15)22(29)26-20(34(31,32)33)13-16-6-10-18(28)11-7-16/h4-11,14,19-21,24,27-28H,12-13H2,1-3H3,(H,25,30)(H,26,29)(H2,31,32,33)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524088
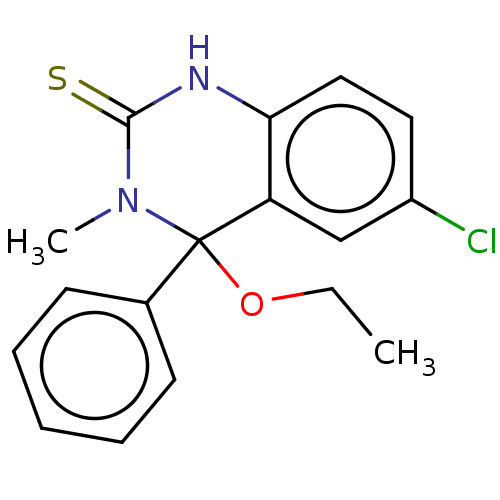
(CHEMBL4483481)Show InChI InChI=1S/C17H17ClN2OS/c1-3-21-17(12-7-5-4-6-8-12)14-11-13(18)9-10-15(14)19-16(22)20(17)2/h4-11H,3H2,1-2H3,(H,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010478

(CHEMBL3264009)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C22H36N3O7P/c1-6-14(4)20(23-15(5)26)22(29)24-18(11-13(2)3)21(28)25-19(33(30,31)32)12-16-7-9-17(27)10-8-16/h7-10,13-14,18-20,27H,6,11-12H2,1-5H3,(H,23,26)(H,24,29)(H,25,28)(H2,30,31,32)/t14-,18-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524089
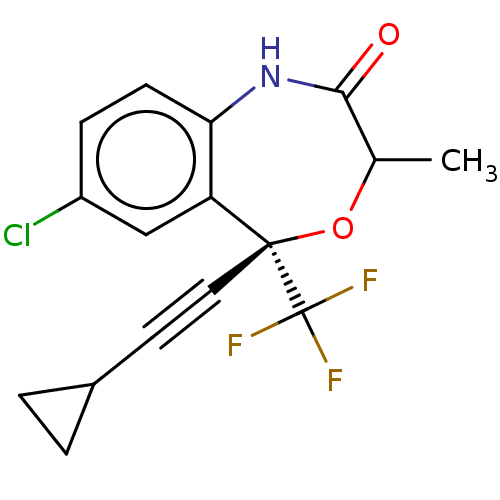
(CHEMBL4529500)Show SMILES CC1O[C@@](C#CC2CC2)(c2cc(Cl)ccc2NC1=O)C(F)(F)F |r| Show InChI InChI=1S/C16H13ClF3NO2/c1-9-14(22)21-13-5-4-11(17)8-12(13)15(23-9,16(18,19)20)7-6-10-2-3-10/h4-5,8-10H,2-3H2,1H3,(H,21,22)/t9?,15-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010479

(CHEMBL3264010)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O7P/c1-4-16(2)23(26-17(3)29)25(32)27-21(14-18-8-6-5-7-9-18)24(31)28-22(36(33,34)35)15-19-10-12-20(30)13-11-19/h5-13,16,21-23,30H,4,14-15H2,1-3H3,(H,26,29)(H,27,32)(H,28,31)(H2,33,34,35)/t16-,21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50010478

(CHEMBL3264009)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C22H36N3O7P/c1-6-14(4)20(23-15(5)26)22(29)24-18(11-13(2)3)21(28)25-19(33(30,31)32)12-16-7-9-17(27)10-8-16/h7-10,13-14,18-20,27H,6,11-12H2,1-5H3,(H,23,26)(H,24,29)(H,25,28)(H2,30,31,32)/t14-,18-,19+,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung somatic ACE using FAPGG substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010477

(CHEMBL1233799)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O8P/c1-4-15(2)23(26-16(3)29)25(33)27-21(13-17-5-9-19(30)10-6-17)24(32)28-22(37(34,35)36)14-18-7-11-20(31)12-8-18/h5-12,15,21-23,30-31H,4,13-14H2,1-3H3,(H,26,29)(H,27,33)(H,28,32)(H2,34,35,36)/t15-,21-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1518
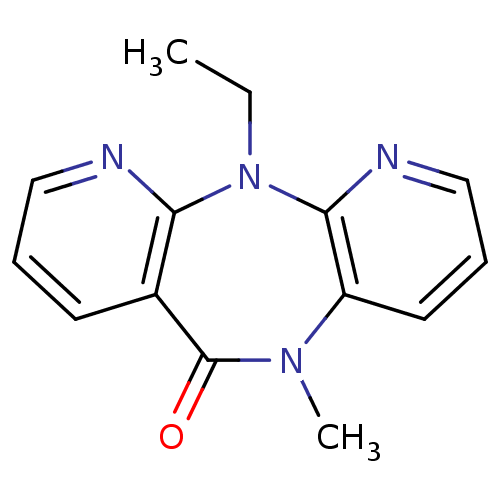
(2-ethyl-9-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...)Show InChI InChI=1S/C14H14N4O/c1-3-18-12-10(6-4-8-15-12)14(19)17(2)11-7-5-9-16-13(11)18/h4-9H,3H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50010480

(CHEMBL3264008)Show SMILES CN[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C23H32N3O7P/c1-14(2)21(24-3)23(30)25-19(12-15-4-8-17(27)9-5-15)22(29)26-20(34(31,32)33)13-16-6-10-18(28)11-7-16/h4-11,14,19-21,24,27-28H,12-13H2,1-3H3,(H,25,30)(H,26,29)(H2,31,32,33)/t19-,20+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung somatic ACE using FAPGG substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010481
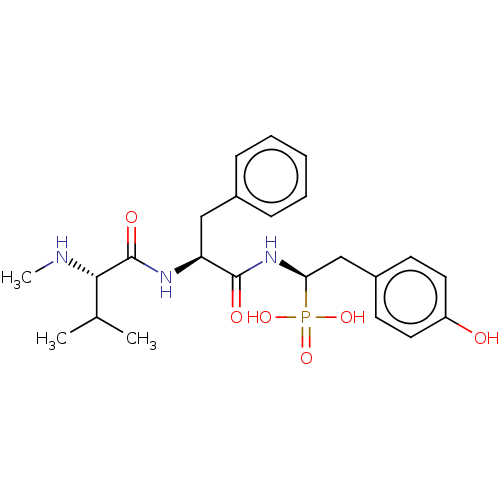
(CHEMBL3264007)Show SMILES CN[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C23H32N3O6P/c1-15(2)21(24-3)23(29)25-19(13-16-7-5-4-6-8-16)22(28)26-20(33(30,31)32)14-17-9-11-18(27)12-10-17/h4-12,15,19-21,24,27H,13-14H2,1-3H3,(H,25,29)(H,26,28)(H2,30,31,32)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant HIV1 reverse transcriptase using poly(rA)-oligo(dT) as template/primer |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1633

(2-ethyl-10-methyl-2,4,10-triazatricyclo[9.4.0.0^{3...)Show InChI InChI=1S/C15H15N3O/c1-3-18-13-9-5-4-8-12(13)17(2)15(19)11-7-6-10-16-14(11)18/h4-10H,3H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50010481

(CHEMBL3264007)Show SMILES CN[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C23H32N3O6P/c1-15(2)21(24-3)23(29)25-19(13-16-7-5-4-6-8-16)22(28)26-20(33(30,31)32)14-17-9-11-18(27)12-10-17/h4-12,15,19-21,24,27H,13-14H2,1-3H3,(H,25,29)(H,26,28)(H2,30,31,32)/t19-,20+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung somatic ACE using FAPGG substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010481

(CHEMBL3264007)Show SMILES CN[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C23H32N3O6P/c1-15(2)21(24-3)23(29)25-19(13-16-7-5-4-6-8-16)22(28)26-20(33(30,31)32)14-17-9-11-18(27)12-10-17/h4-12,15,19-21,24,27H,13-14H2,1-3H3,(H,25,29)(H,26,28)(H2,30,31,32)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain using Cbz-Phe-His-Leu-OH substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50137120
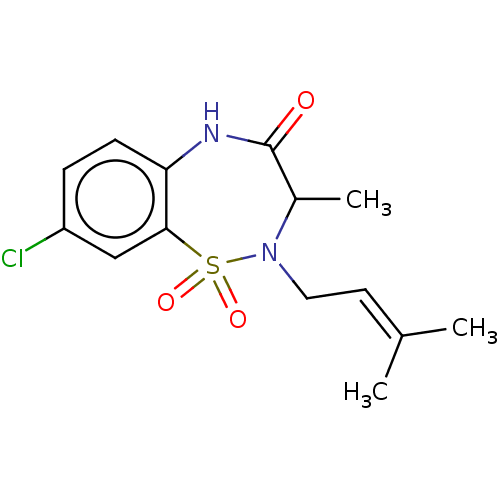
(CHEMBL3753006)Show SMILES [#6]-[#6]-1-[#7](-[#6]\[#6]=[#6](\[#6])-[#6])S(=O)(=O)c2cc(Cl)ccc2-[#7]-[#6]-1=O Show InChI InChI=1S/C14H17ClN2O3S/c1-9(2)6-7-17-10(3)14(18)16-12-5-4-11(15)8-13(12)21(17,19)20/h4-6,8,10H,7H2,1-3H3,(H,16,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50137115
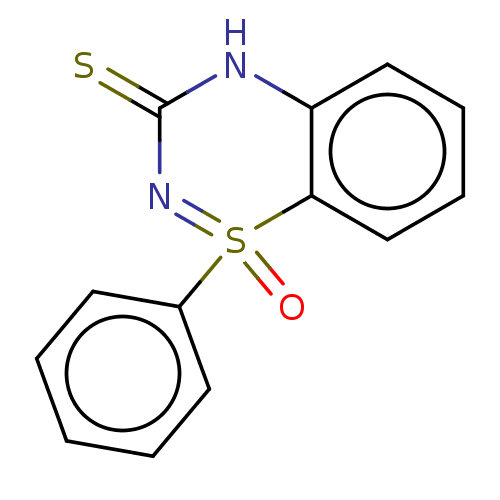
(CHEMBL3754170)Show InChI InChI=1S/C13H10N2OS2/c16-18(10-6-2-1-3-7-10)12-9-5-4-8-11(12)14-13(17)15-18/h1-9H,(H,14,15,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50010476
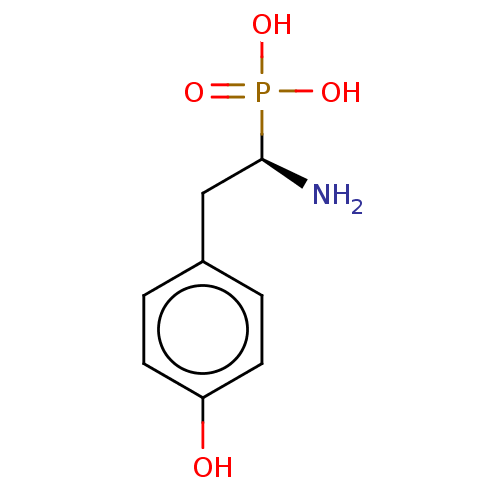
(CHEMBL3264357)Show InChI InChI=1S/C8H12NO4P/c9-8(14(11,12)13)5-6-1-3-7(10)4-2-6/h1-4,8,10H,5,9H2,(H2,11,12,13)/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1437
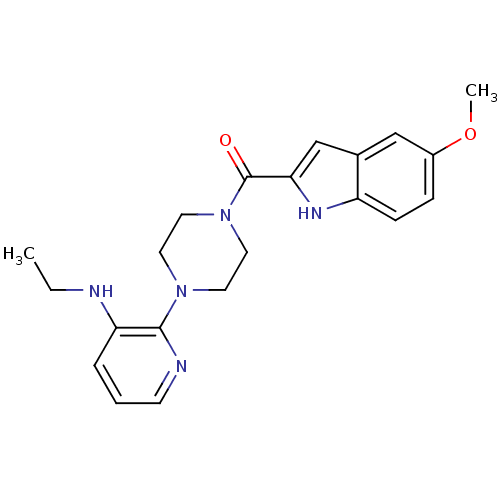
(5-methoxyindole-2-carboxylic acid [N -[3-(aminoeth...)Show SMILES CCNc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(OC)ccc2[nH]1 Show InChI InChI=1S/C21H25N5O2/c1-3-22-18-5-4-8-23-20(18)25-9-11-26(12-10-25)21(27)19-14-15-13-16(28-2)6-7-17(15)24-19/h4-8,13-14,22,24H,3,9-12H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant HIV1 reverse transcriptase using poly(rA)-oligo(dT) as template/primer |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50010482
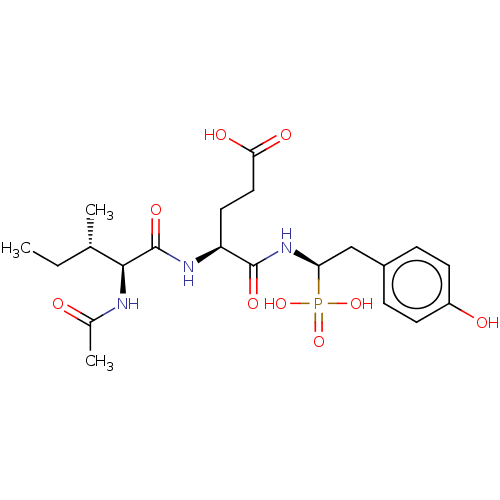
(CHEMBL3264356)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C21H32N3O9P/c1-4-12(2)19(22-13(3)25)21(30)23-16(9-10-18(27)28)20(29)24-17(34(31,32)33)11-14-5-7-15(26)8-6-14/h5-8,12,16-17,19,26H,4,9-11H2,1-3H3,(H,22,25)(H,23,30)(H,24,29)(H,27,28)(H2,31,32,33)/t12-,16-,17+,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of rabbit lung somatic ACE using FAPGG substrate |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524091
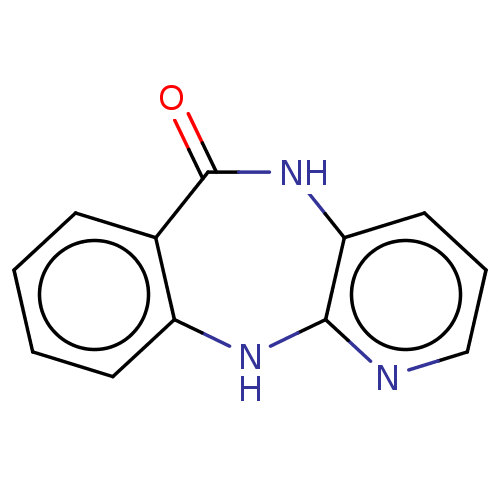
(CHEMBL307792)Show InChI InChI=1S/C12H9N3O/c16-12-8-4-1-2-5-9(8)14-11-10(15-12)6-3-7-13-11/h1-7H,(H,13,14)(H,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant HIV1 reverse transcriptase Y181C mutant using poly(rA)-oligo(dT) as template/primer |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524093
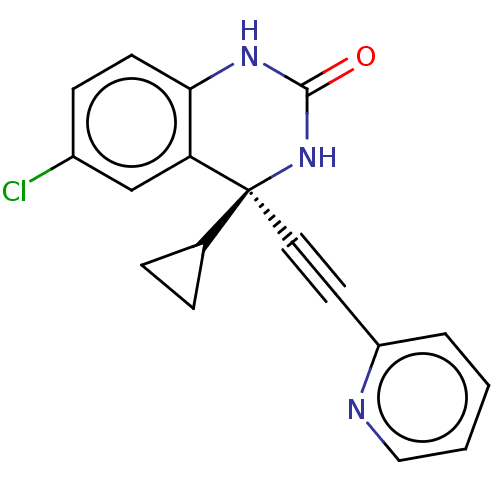
(CHEMBL311050)Show SMILES Clc1ccc2NC(=O)N[C@@](C#Cc3ccccn3)(C3CC3)c2c1 Show InChI InChI=1S/C18H14ClN3O/c19-13-6-7-16-15(11-13)18(12-4-5-12,22-17(23)21-16)9-8-14-3-1-2-10-20-14/h1-3,6-7,10-12H,4-5H2,(H2,21,22,23)/t18-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524090
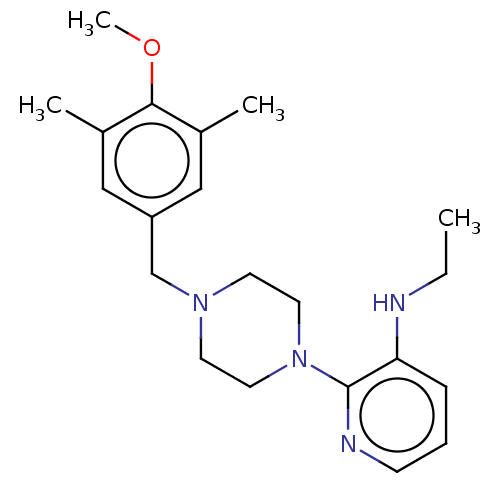
(CHEMBL1192060)Show InChI InChI=1S/C21H30N4O/c1-5-22-19-7-6-8-23-21(19)25-11-9-24(10-12-25)15-18-13-16(2)20(26-4)17(3)14-18/h6-8,13-14,22H,5,9-12,15H2,1-4H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant HIV1 reverse transcriptase using poly(rA)-oligo(dT) as template/primer |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Dipeptidyl carboxypeptidase
(Escherichia coli (strain K12)) | BDBM50010477

(CHEMBL1233799)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C25H34N3O8P/c1-4-15(2)23(26-16(3)29)25(33)27-21(13-17-5-9-19(30)10-6-17)24(32)28-22(37(34,35)36)14-18-7-11-20(31)12-8-18/h5-12,15,21-23,30-31H,4,13-14H2,1-3H3,(H,26,29)(H,27,33)(H,28,32)(H2,34,35,36)/t15-,21-,22+,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Department of Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli dipeptidyl carboxypeptidase assessed as FAPGG substrate hydrolysis |
ACS Med Chem Lett 5: 346-51 (2014)
Article DOI: 10.1021/ml4004588
BindingDB Entry DOI: 10.7270/Q20C4X8P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data