 Found 602 hits with Last Name = 'krier' and Initial = 'm'
Found 602 hits with Last Name = 'krier' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610836

(MSC-4381) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610833

(CHEMBL5276884) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610834
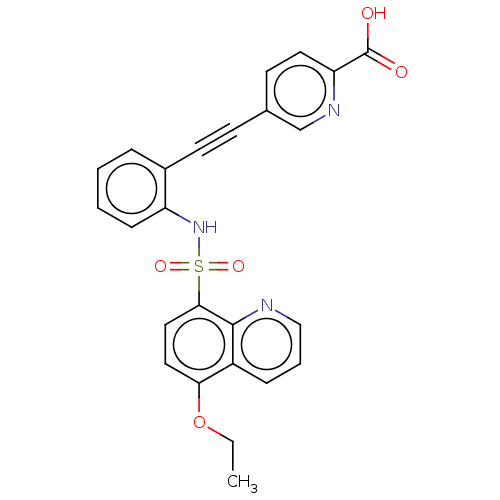
(CHEMBL5279064) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610830
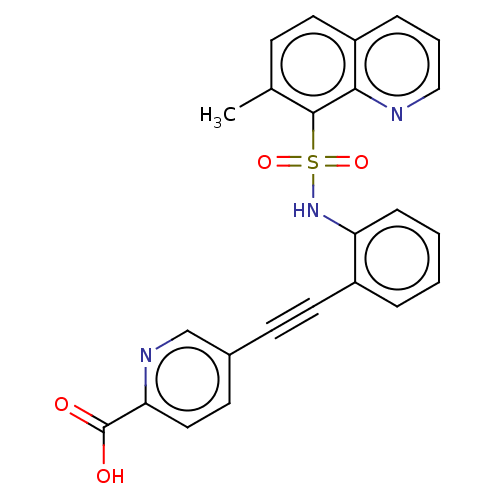
(CHEMBL5267752) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610837
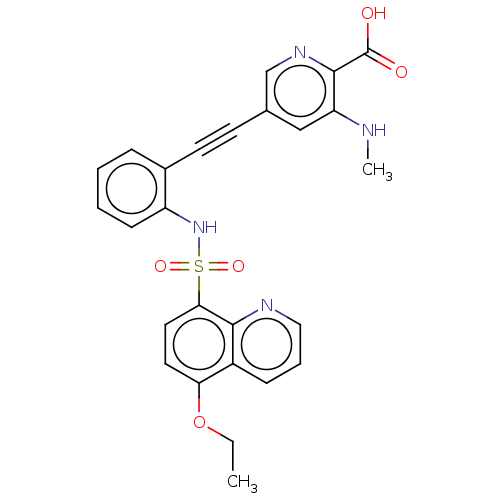
(CHEMBL5281492) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610835

(CHEMBL5267349) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610826

(CHEMBL5287351) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610827
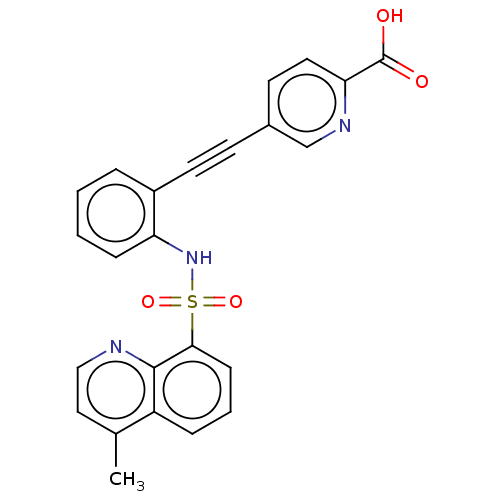
(CHEMBL5287780) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610824

(CHEMBL5268966) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610829
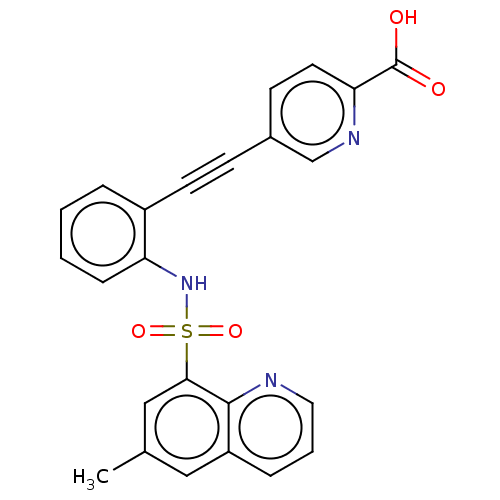
(CHEMBL5288904) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610831
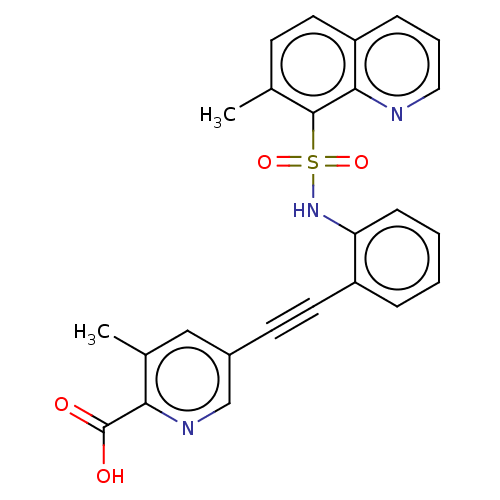
(CHEMBL5265956) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610825

(CHEMBL5266104) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610828
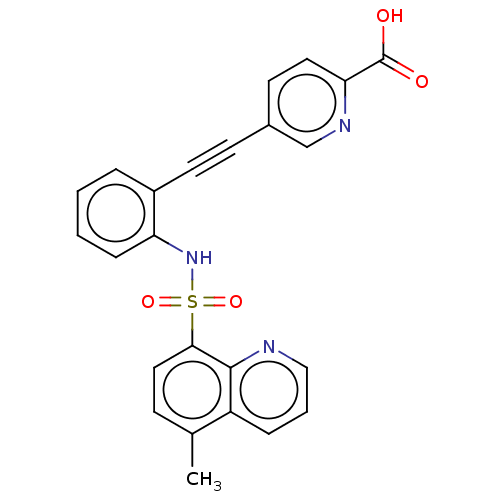
(CHEMBL5271816) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Mus musculus) | BDBM50610868
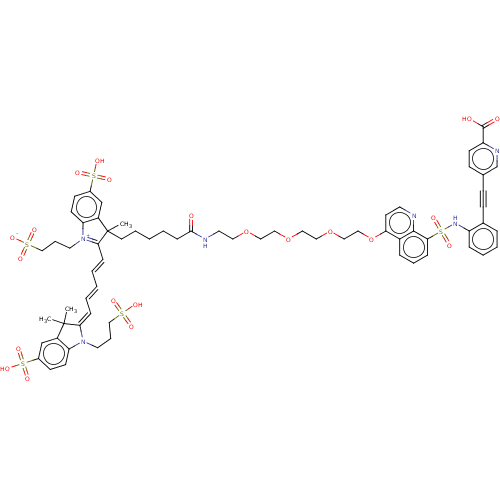
(CHEMBL5282978) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50475194
(CHEMBL195155)Show InChI InChI=1S/C23H26N2O3/c1-27-21-14-12-19(17-22(21)28-2)20-13-15-23(26)25(24-20)16-8-4-7-11-18-9-5-3-6-10-18/h3,5-6,9-10,12-15,17H,4,7-8,11,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7081
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine smooth muscle phosphodiesterase 4 |
J Med Chem 48: 3816-22 (2005)
Article DOI: 10.1021/jm050063y
BindingDB Entry DOI: 10.7270/Q2BK1G4J |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50318884

(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Competitive binding affinity to FAK kinase domain (410 to 689) (unknown origin) assessed as phosphorylation of p(Glu/Tyr) in presence of ATP |
Bioorg Med Chem Lett 23: 5401-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.050
BindingDB Entry DOI: 10.7270/Q2891782 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM21998
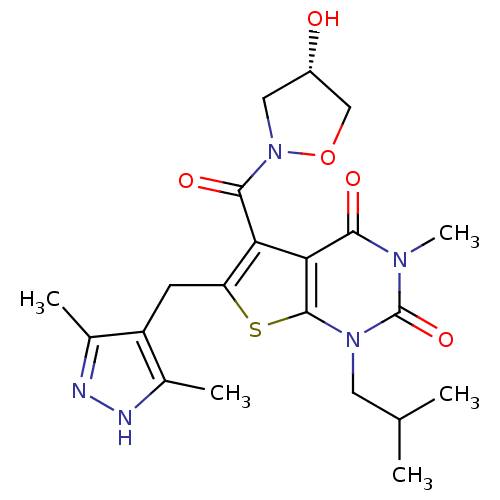
(6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-{[(4S)-...)Show SMILES CC(C)Cn1c2sc(Cc3c(C)n[nH]c3C)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H27N5O5S/c1-10(2)7-25-20-17(18(28)24(5)21(25)30)16(19(29)26-8-13(27)9-31-26)15(32-20)6-14-11(3)22-23-12(14)4/h10,13,27H,6-9H2,1-5H3,(H,22,23)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM21998

(6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-{[(4S)-...)Show SMILES CC(C)Cn1c2sc(Cc3c(C)n[nH]c3C)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H27N5O5S/c1-10(2)7-25-20-17(18(28)24(5)21(25)30)16(19(29)26-8-13(27)9-31-26)15(32-20)6-14-11(3)22-23-12(14)4/h10,13,27H,6-9H2,1-5H3,(H,22,23)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50103571
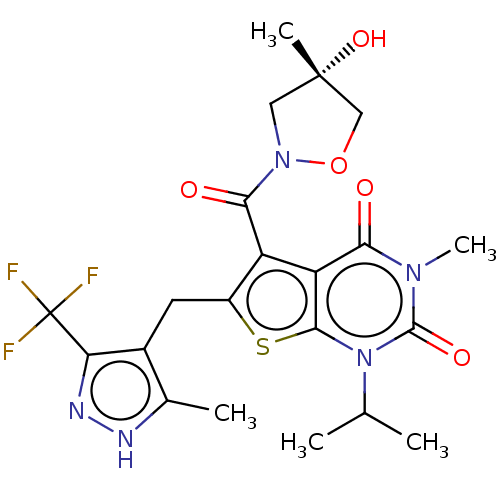
(CHEMBL3335793)Show SMILES CC(C)n1c2sc(Cc3c(C)[nH]nc3C(F)(F)F)c(C(=O)N3C[C@](C)(O)CO3)c2c(=O)n(C)c1=O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610832
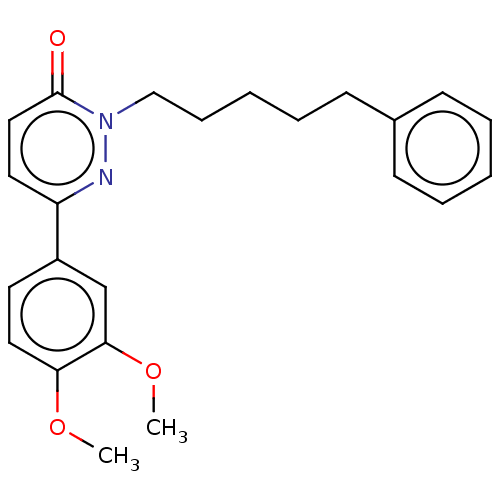
(CHEMBL5282741) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610830

(CHEMBL5267752) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50475193
(CHEMBL363562)Show InChI InChI=1S/C21H22N2O3/c1-25-19-12-10-17(15-20(19)26-2)18-11-13-21(24)23(22-18)14-6-9-16-7-4-3-5-8-16/h3-5,7-8,10-13,15H,6,9,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7081
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine smooth muscle phosphodiesterase 4 |
J Med Chem 48: 3816-22 (2005)
Article DOI: 10.1021/jm050063y
BindingDB Entry DOI: 10.7270/Q2BK1G4J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50475199

(CHEMBL195446)Show InChI InChI=1S/C18H25N3O3/c1-23-16-9-7-14(13-17(16)24-2)15-8-10-18(22)21(20-15)12-6-4-3-5-11-19/h7-10,13H,3-6,11-12,19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7081
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine smooth muscle phosphodiesterase 4 |
J Med Chem 48: 3816-22 (2005)
Article DOI: 10.1021/jm050063y
BindingDB Entry DOI: 10.7270/Q2BK1G4J |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610852
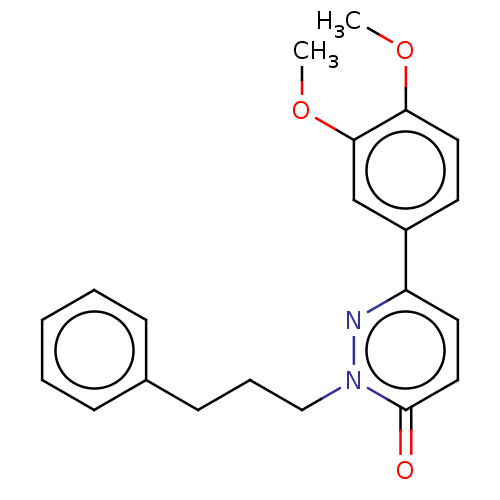
(CHEMBL5287835) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50103571

(CHEMBL3335793)Show SMILES CC(C)n1c2sc(Cc3c(C)[nH]nc3C(F)(F)F)c(C(=O)N3C[C@](C)(O)CO3)c2c(=O)n(C)c1=O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
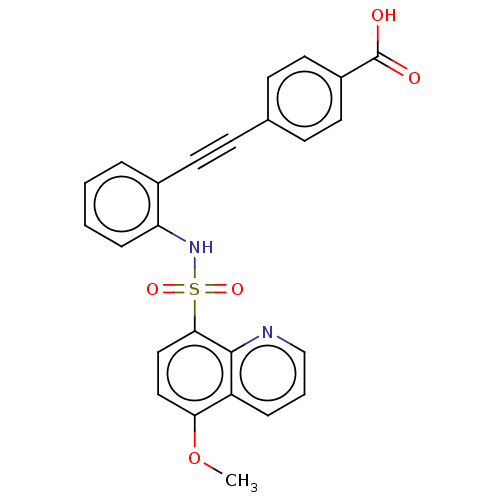
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425672
(CHEMBL2315584)Show SMILES CN(c1ncccc1CNc1c(cnc2[nH]c(cc12)-c1ccc(F)cc1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H19F4N5O2S/c1-31(34(2,32)33)21-14(4-3-9-27-21)11-28-19-16-10-18(13-5-7-15(23)8-6-13)30-20(16)29-12-17(19)22(24,25)26/h3-10,12H,11H2,1-2H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
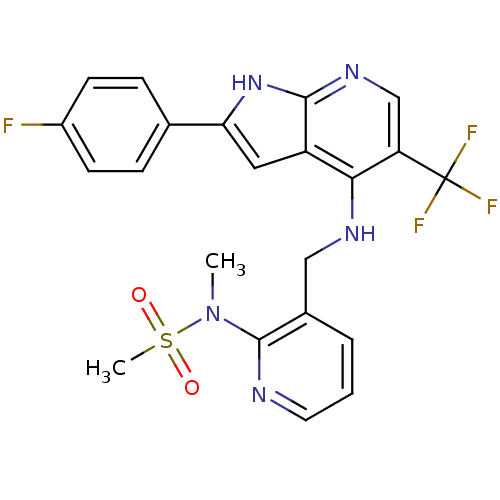
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425681
(CHEMBL2315564)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(F)cc1)C#N Show InChI InChI=1S/C22H16FN5O/c1-25-22(29)16-4-2-3-5-18(16)27-20-14(11-24)12-26-21-17(20)10-19(28-21)13-6-8-15(23)9-7-13/h2-10,12H,1H3,(H,25,29)(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein 2
(Homo sapiens (Human)) | BDBM50234032
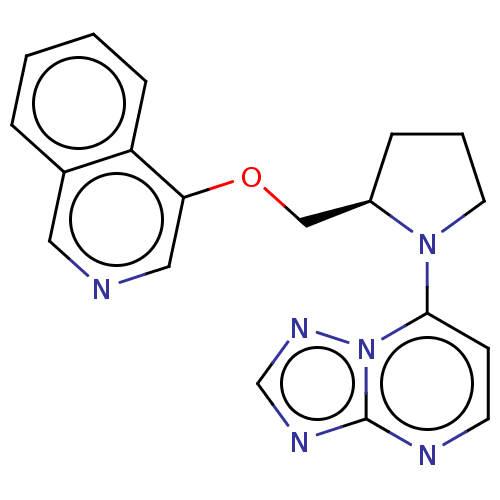
(CHEMBL4060668)Show SMILES C(Oc1cncc2ccccc12)[C@H]1CCCN1c1ccnc2ncnn12 |r| Show InChI InChI=1S/C19H18N6O/c1-2-6-16-14(4-1)10-20-11-17(16)26-12-15-5-3-9-24(15)18-7-8-21-19-22-13-23-25(18)19/h1-2,4,6-8,10-11,13,15H,3,5,9,12H2/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His tagged MetAP-2 using tripeptide Met-Ala-Ser as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem Lett 27: 551-556 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.019
BindingDB Entry DOI: 10.7270/Q2V98BB4 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610863
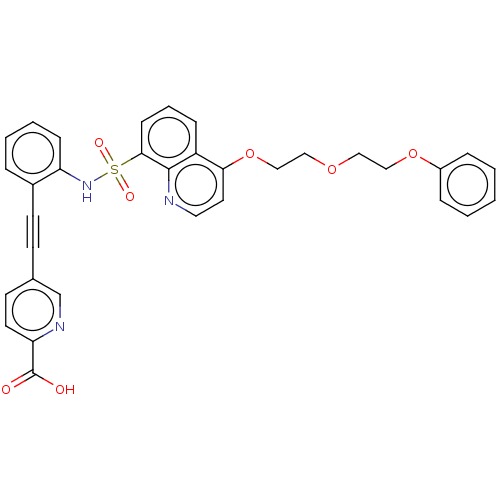
(CHEMBL5280923) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono
Curated by ChEMBL
| Assay Description
Inhibition of human cRAF using [gamma-33P-ATP] as a substrate by scintillation counting |
Bioorg Med Chem Lett 21: 2264-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.108
BindingDB Entry DOI: 10.7270/Q2MS3T23 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425686
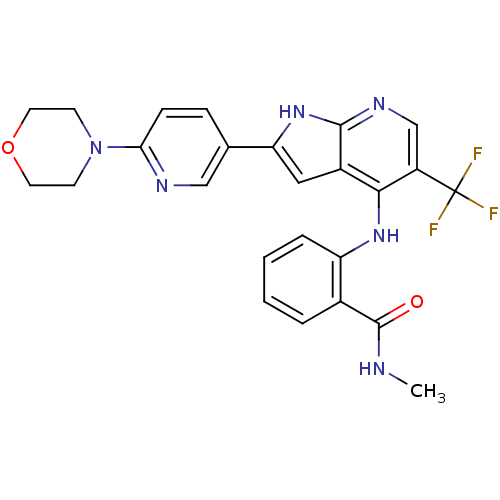
(CHEMBL2315566)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(nc1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C25H23F3N6O2/c1-29-24(35)16-4-2-3-5-19(16)32-22-17-12-20(33-23(17)31-14-18(22)25(26,27)28)15-6-7-21(30-13-15)34-8-10-36-11-9-34/h2-7,12-14H,8-11H2,1H3,(H,29,35)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425687
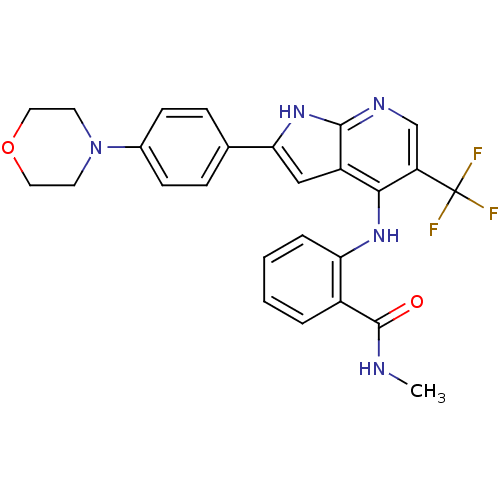
(CHEMBL2315565)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(cc1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C26H24F3N5O2/c1-30-25(35)18-4-2-3-5-21(18)32-23-19-14-22(33-24(19)31-15-20(23)26(27,28)29)16-6-8-17(9-7-16)34-10-12-36-13-11-34/h2-9,14-15H,10-13H2,1H3,(H,30,35)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446652
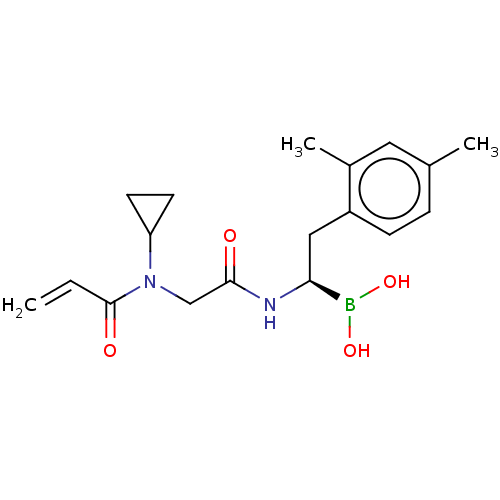
(US10669289, Compound 59)Show SMILES Cc1ccc(C[C@H](NC(=O)CN(C2CC2)C(=O)C=C)B(O)O)c(C)c1 |r| Show InChI InChI=1S/C18H25BN2O4/c1-4-18(23)21(15-7-8-15)11-17(22)20-16(19(24)25)10-14-6-5-12(2)9-13(14)3/h4-6,9,15-16,24-25H,1,7-8,10-11H2,2-3H3,(H,20,22)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446653
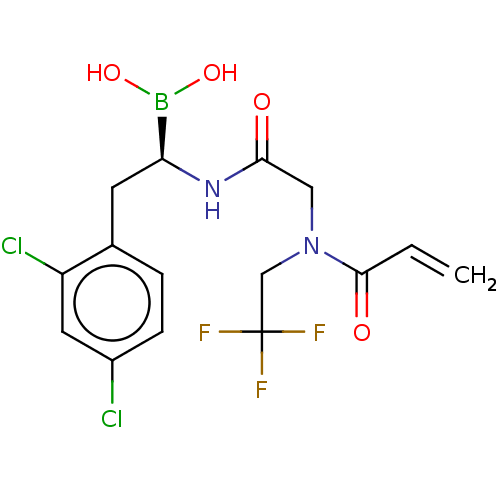
(US10669289, Compound 60)Show SMILES OB(O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)CN(CC(F)(F)F)C(=O)C=C |r| Show InChI InChI=1S/C15H16BCl2F3N2O4/c1-2-14(25)23(8-15(19,20)21)7-13(24)22-12(16(26)27)5-9-3-4-10(17)6-11(9)18/h2-4,6,12,26-27H,1,5,7-8H2,(H,22,24)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446654
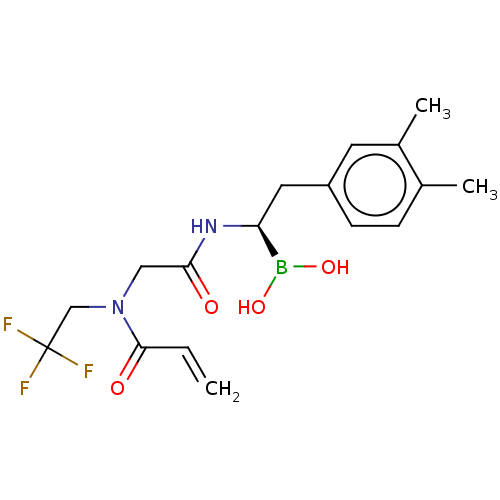
(US10669289, Compound 61)Show SMILES Cc1ccc(C[C@H](NC(=O)CN(CC(F)(F)F)C(=O)C=C)B(O)O)cc1C |r| Show InChI InChI=1S/C17H22BF3N2O4/c1-4-16(25)23(10-17(19,20)21)9-15(24)22-14(18(26)27)8-13-6-5-11(2)12(3)7-13/h4-7,14,26-27H,1,8-10H2,2-3H3,(H,22,24)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446608
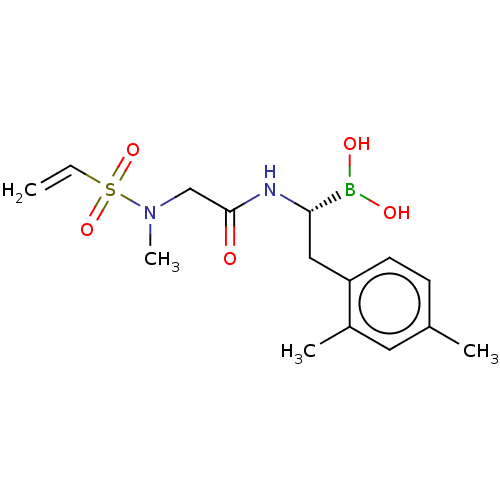
(US10669289, Compound 15)Show SMILES CN(CC(=O)N[C@@H](Cc1ccc(C)cc1C)B(O)O)S(=O)(=O)C=C |r| Show InChI InChI=1S/C15H23BN2O5S/c1-5-24(22,23)18(4)10-15(19)17-14(16(20)21)9-13-7-6-11(2)8-12(13)3/h5-8,14,20-21H,1,9-10H2,2-4H3,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446609

(US10669289, Compound 16)Show SMILES CN(CC(=O)N[C@@H](Cc1ccc(C)c(C)c1)B(O)O)C(=O)C=C |r| Show InChI InChI=1S/C16H23BN2O4/c1-5-16(21)19(4)10-15(20)18-14(17(22)23)9-13-7-6-11(2)12(3)8-13/h5-8,14,22-23H,1,9-10H2,2-4H3,(H,18,20)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446610
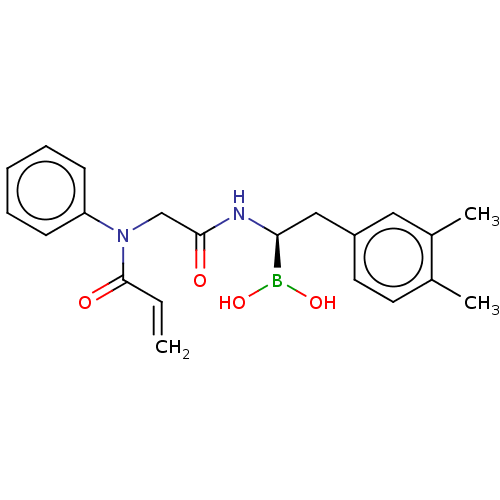
(US10669289, Compound 17)Show SMILES Cc1ccc(C[C@H](NC(=O)CN(C(=O)C=C)c2ccccc2)B(O)O)cc1C |r| Show InChI InChI=1S/C21H25BN2O4/c1-4-21(26)24(18-8-6-5-7-9-18)14-20(25)23-19(22(27)28)13-17-11-10-15(2)16(3)12-17/h4-12,19,27-28H,1,13-14H2,2-3H3,(H,23,25)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446612

(US10669289, Compound 19)Show SMILES CCN(CC(=O)N[C@@H](Cc1ccc(C)cc1C)B(O)O)C(=O)C#CC |r| Show InChI InChI=1S/C18H25BN2O4/c1-5-7-18(23)21(6-2)12-17(22)20-16(19(24)25)11-15-9-8-13(3)10-14(15)4/h8-10,16,24-25H,6,11-12H2,1-4H3,(H,20,22)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446613
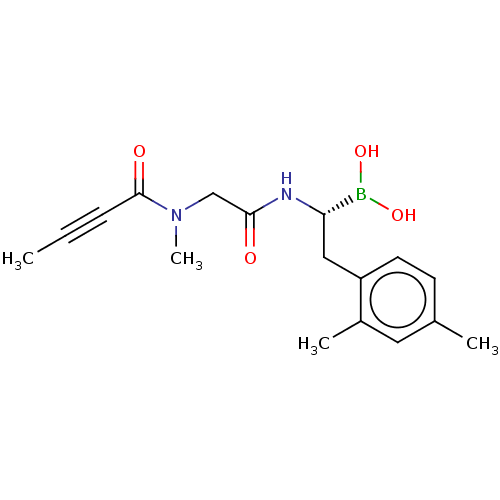
(US10669289, Compound 20)Show SMILES CC#CC(=O)N(C)CC(=O)N[C@@H](Cc1ccc(C)cc1C)B(O)O |r| Show InChI InChI=1S/C17H23BN2O4/c1-5-6-17(22)20(4)11-16(21)19-15(18(23)24)10-14-8-7-12(2)9-13(14)3/h7-9,15,23-24H,10-11H2,1-4H3,(H,19,21)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446616
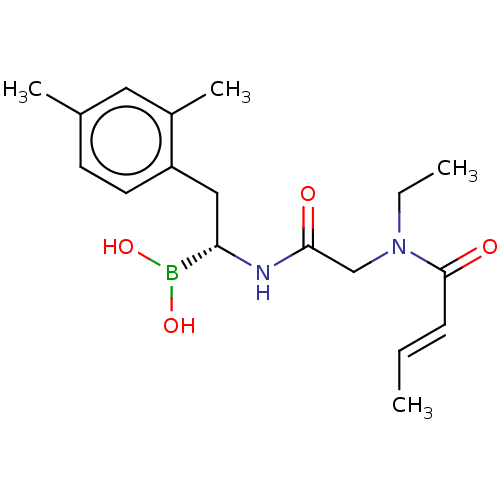
(US10669289, Compound 23)Show SMILES CCN(CC(=O)N[C@@H](Cc1ccc(C)cc1C)B(O)O)C(=O)\C=C\C |r| Show InChI InChI=1S/C18H27BN2O4/c1-5-7-18(23)21(6-2)12-17(22)20-16(19(24)25)11-15-9-8-13(3)10-14(15)4/h5,7-10,16,24-25H,6,11-12H2,1-4H3,(H,20,22)/b7-5+/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446619
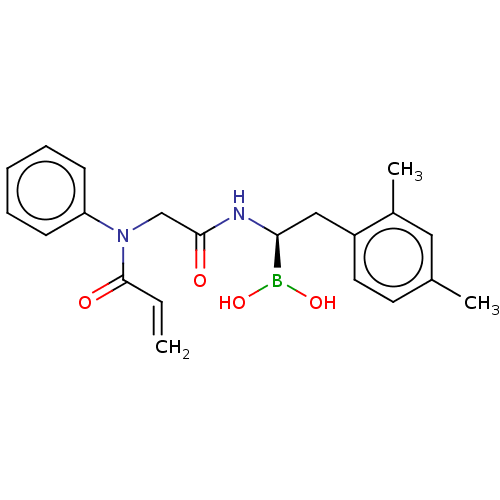
(US10669289, Compound 26)Show SMILES Cc1ccc(C[C@H](NC(=O)CN(C(=O)C=C)c2ccccc2)B(O)O)c(C)c1 |r| Show InChI InChI=1S/C21H25BN2O4/c1-4-21(26)24(18-8-6-5-7-9-18)14-20(25)23-19(22(27)28)13-17-11-10-15(2)12-16(17)3/h4-12,19,27-28H,1,13-14H2,2-3H3,(H,23,25)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446622
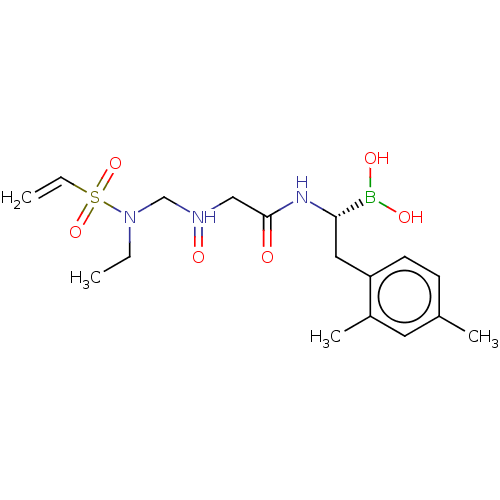
(US10669289, Compound 29)Show SMILES CCN(CN(=O)CC(=O)N[C@@H](Cc1ccc(C)cc1C)B(O)O)S(=O)(=O)C=C |r| Show InChI InChI=1S/C17H27BN3O6S/c1-5-21(28(26,27)6-2)12-20(25)11-17(22)19-16(18(23)24)10-15-8-7-13(3)9-14(15)4/h6-9,16,23-24H,2,5,10-12H2,1,3-4H3,(H,19,22)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446688
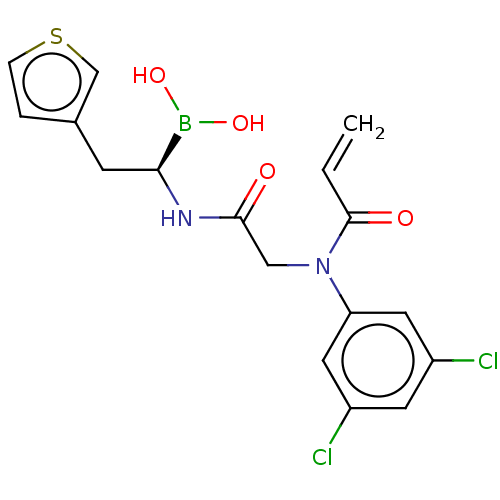
(US10669289, Compound 95 | [(1R)-1-[[2-(3,5-dichlor...)Show SMILES OB(O)[C@H](Cc1ccsc1)NC(=O)CN(C(=O)C=C)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C17H17BCl2N2O4S/c1-2-17(24)22(14-7-12(19)6-13(20)8-14)9-16(23)21-15(18(25)26)5-11-3-4-27-10-11/h2-4,6-8,10,15,25-26H,1,5,9H2,(H,21,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446689
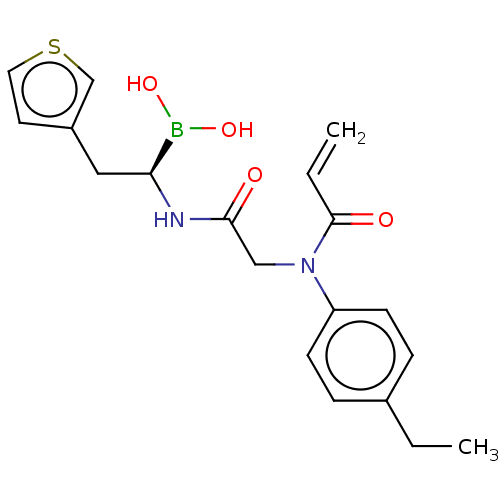
(US10669289, Compound 96)Show SMILES CCc1ccc(cc1)N(CC(=O)N[C@@H](Cc1ccsc1)B(O)O)C(=O)C=C |r| Show InChI InChI=1S/C19H23BN2O4S/c1-3-14-5-7-16(8-6-14)22(19(24)4-2)12-18(23)21-17(20(25)26)11-15-9-10-27-13-15/h4-10,13,17,25-26H,2-3,11-12H2,1H3,(H,21,23)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446690

(US10669289, Compound 97)Show SMILES OB(O)[C@H](Cc1ccsc1)NC(=O)CN(C(=O)C=C)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H17BCl2N2O4S/c1-2-17(24)22(12-3-4-13(19)14(20)8-12)9-16(23)21-15(18(25)26)7-11-5-6-27-10-11/h2-6,8,10,15,25-26H,1,7,9H2,(H,21,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446691

(US10669289, Compound 98)Show SMILES OB(O)[C@H](Cc1cccc(F)c1)NC(=O)CN(C(=O)C=C)c1ccccc1F |r| Show InChI InChI=1S/C19H19BF2N2O4/c1-2-19(26)24(16-9-4-3-8-15(16)22)12-18(25)23-17(20(27)28)11-13-6-5-7-14(21)10-13/h2-10,17,27-28H,1,11-12H2,(H,23,25)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446692
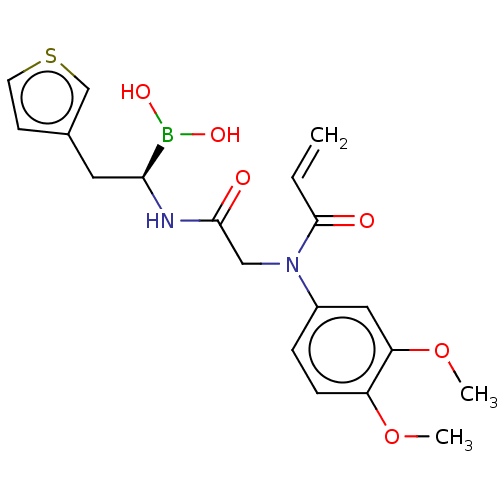
(US10669289, Compound 99)Show SMILES COc1ccc(cc1OC)N(CC(=O)N[C@@H](Cc1ccsc1)B(O)O)C(=O)C=C |r| Show InChI InChI=1S/C19H23BN2O6S/c1-4-19(24)22(14-5-6-15(27-2)16(10-14)28-3)11-18(23)21-17(20(25)26)9-13-7-8-29-12-13/h4-8,10,12,17,25-26H,1,9,11H2,2-3H3,(H,21,23)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446693
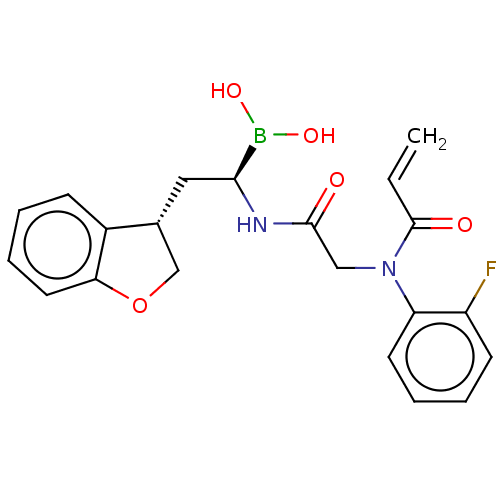
(US10669289, Compound 100)Show SMILES OB(O)[C@H](C[C@@H]1COc2ccccc12)NC(=O)CN(C(=O)C=C)c1ccccc1F |r| Show InChI InChI=1S/C21H22BFN2O5/c1-2-21(27)25(17-9-5-4-8-16(17)23)12-20(26)24-19(22(28)29)11-14-13-30-18-10-6-3-7-15(14)18/h2-10,14,19,28-29H,1,11-13H2,(H,24,26)/t14-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM446694

(US10669289, Compound 101)Show SMILES OB(O)[C@H](CCc1ccccc1)NC(=O)CN(C(=O)C=C)c1ccccc1F |r| Show InChI InChI=1S/C20H22BFN2O4/c1-2-20(26)24(17-11-7-6-10-16(17)22)14-19(25)23-18(21(27)28)13-12-15-8-4-3-5-9-15/h2-11,18,27-28H,1,12-14H2,(H,23,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK PATENT GMBH
US Patent
| Assay Description
Measurement of LMP7 inhibition is performed in 384 well format based on fluorescence intensity assay.Purified human immuno proteasome (0.25 nM) and s... |
US Patent US10669289 (2020)
BindingDB Entry DOI: 10.7270/Q2ZS30MF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data