 Found 861 hits with Last Name = 'kumar' and Initial = 'b'
Found 861 hits with Last Name = 'kumar' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315548
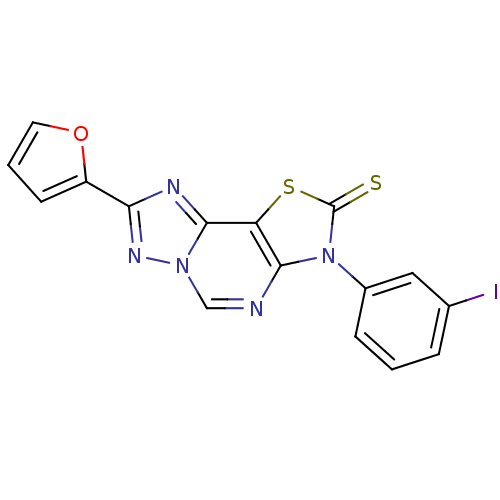
(8-(2-Thioxo-7(3-m-iodophenyl)-2-(2-furyl)thiazolo[...)Show InChI InChI=1S/C16H8IN5OS2/c17-9-3-1-4-10(7-9)22-14-12(25-16(22)24)15-19-13(11-5-2-6-23-11)20-21(15)8-18-14/h1-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315541

(8-(2-Thioxo-7(3-allyl)-2-(2-furyl)thiazole[4,3-e]1...)Show InChI InChI=1S/C13H9N5OS2/c1-2-5-17-11-9(21-13(17)20)12-15-10(8-4-3-6-19-8)16-18(12)7-14-11/h2-4,6-7H,1,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50315544
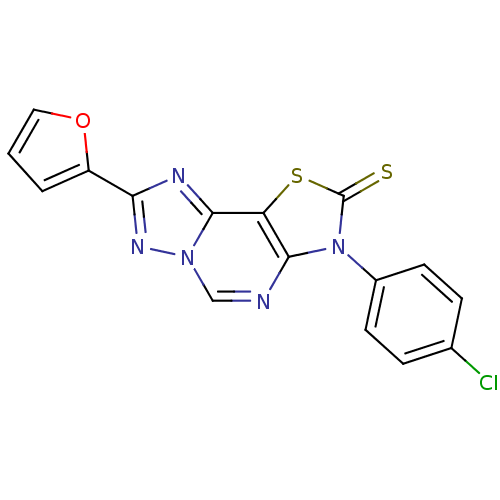
(8-(2-Thioxo-7(3-p-chlorophenyl.)-2-(2-furyl)thiazo...)Show SMILES Clc1ccc(cc1)-n1c2ncn3nc(nc3c2sc1=S)-c1ccco1 Show InChI InChI=1S/C16H8ClN5OS2/c17-9-3-5-10(6-4-9)22-14-12(25-16(22)24)15-19-13(11-2-1-7-23-11)20-21(15)8-18-14/h1-8H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315545
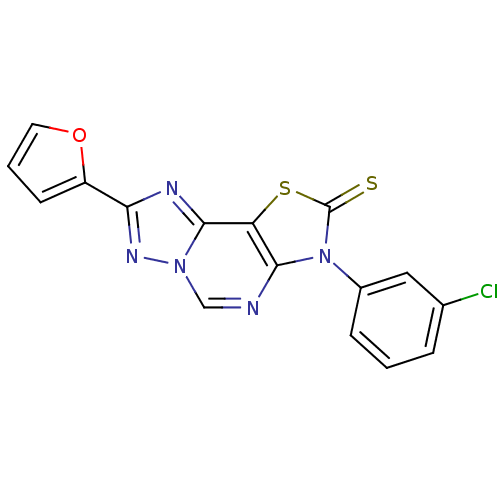
(8-(2-Thioxo-7(3-m-chlorophenyl)-2-(2-furyl)thiazol...)Show SMILES Clc1cccc(c1)-n1c2ncn3nc(nc3c2sc1=S)-c1ccco1 Show InChI InChI=1S/C16H8ClN5OS2/c17-9-3-1-4-10(7-9)22-14-12(25-16(22)24)15-19-13(11-5-2-6-23-11)20-21(15)8-18-14/h1-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50315538
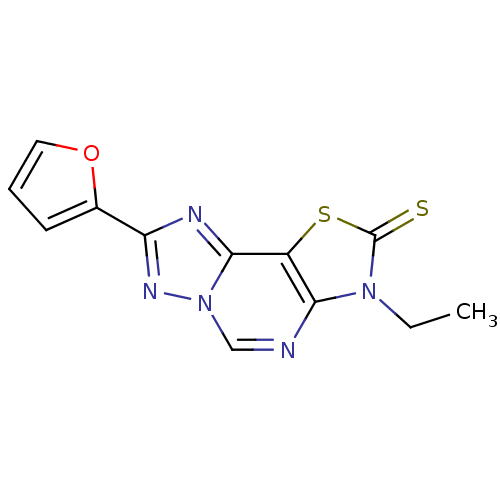
(8-(2-Thioxo-7(3-ethyl)-2-(2-furyl)thiazolo[4,3-e]1...)Show InChI InChI=1S/C12H9N5OS2/c1-2-16-10-8(20-12(16)19)11-14-9(7-4-3-5-18-7)15-17(11)6-13-10/h3-6H,2H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615747
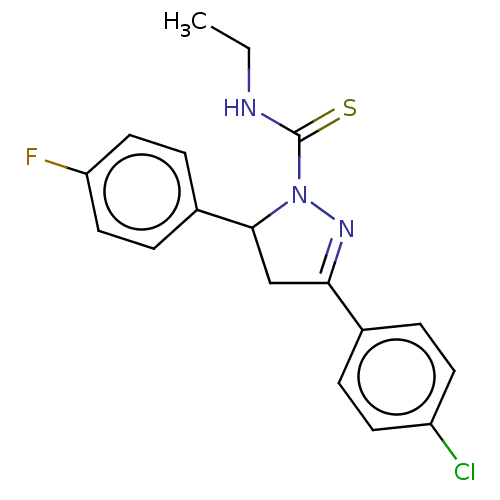
(CHEMBL5280524)Show SMILES CCNC(=S)N1N=C(CC1c1ccc(F)cc1)c1ccc(Cl)cc1 |c:6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615748

(CHEMBL5272772)Show SMILES CCNC(=S)N1N=C(CC1c1ccc(F)cc1)c1ccc(C)cc1 |c:6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615749
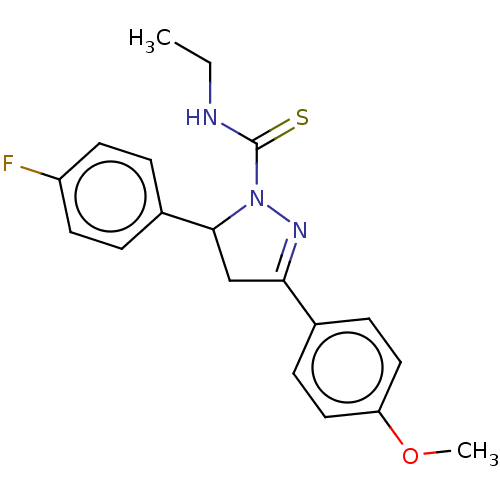
(CHEMBL5271570)Show SMILES CCNC(=S)N1N=C(CC1c1ccc(F)cc1)c1ccc(OC)cc1 |c:6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615750
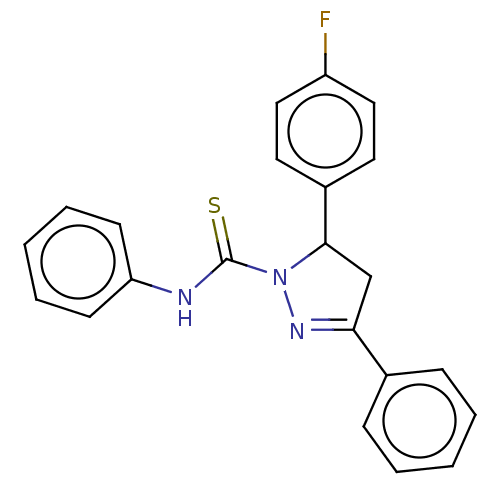
(CHEMBL5268306)Show SMILES Fc1ccc(cc1)C1CC(=NN1C(=S)Nc1ccccc1)c1ccccc1 |c:10| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615751

(CHEMBL5273835)Show SMILES COc1ccc(cc1)C1=NN(C(C1)c1ccc(F)cc1)C(=S)Nc1ccccc1 |t:9| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615752
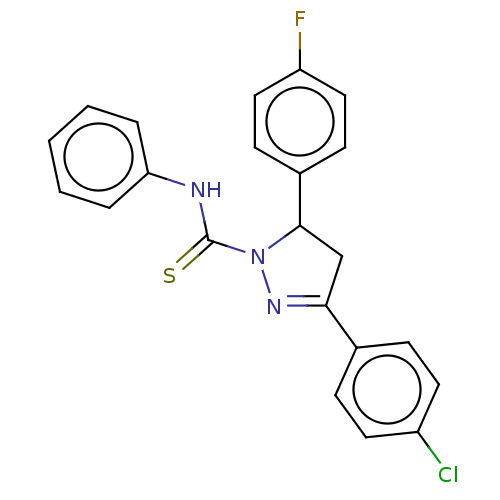
(CHEMBL5284012)Show SMILES Fc1ccc(cc1)C1CC(=NN1C(=S)Nc1ccccc1)c1ccc(Cl)cc1 |c:10| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615753

(CHEMBL5275680)Show SMILES Fc1ccc(cc1)C1CC(=NN1C(=S)NCC=C)c1ccccc1 |c:10| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615754

(CHEMBL5290358)Show SMILES Fc1ccc(cc1)C1CC(=NN1C(=S)NCC=C)c1ccc(Cl)cc1 |c:10| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615755

(CHEMBL5284128)Show SMILES COc1ccc(cc1)C1=NN(C(C1)c1ccc(F)cc1)C(=S)NCC=C |t:9| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615756
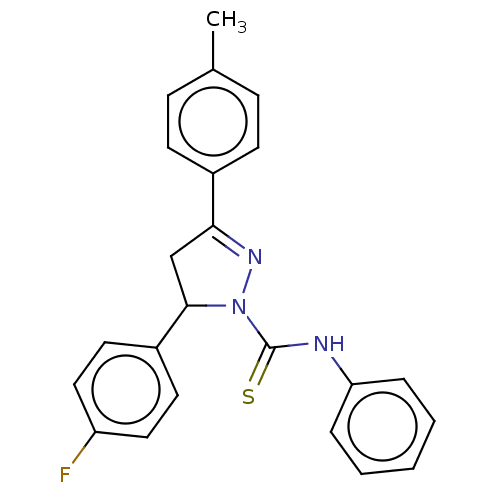
(CHEMBL5282135)Show SMILES Cc1ccc(cc1)C1=NN(C(C1)c1ccc(F)cc1)C(=S)Nc1ccccc1 |t:8| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615757

(CHEMBL5270226)Show SMILES Cc1ccc(cc1)C1=NN(C(C1)c1ccc(F)cc1)C(=S)NCC=C |t:8| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615746

(CHEMBL5291452)Show SMILES CCNC(=S)N1N=C(CC1c1ccc(F)cc1)c1ccccc1 |c:6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615745

(CHEMBL5272518)Show SMILES CNC(=S)N1N=C(CC1c1ccc(F)cc1)c1ccc(OC)cc1 |c:5| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615744
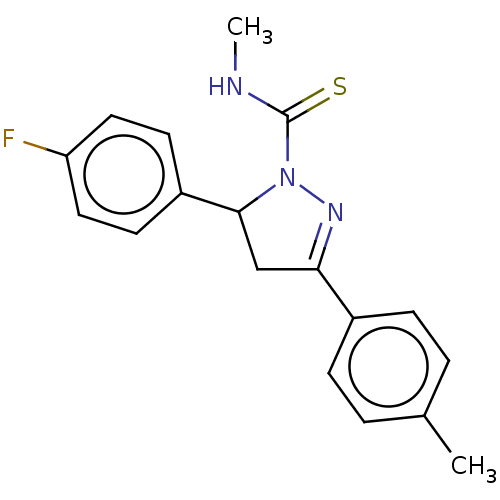
(CHEMBL5276967)Show SMILES CNC(=S)N1N=C(CC1c1ccc(F)cc1)c1ccc(C)cc1 |c:5| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615743
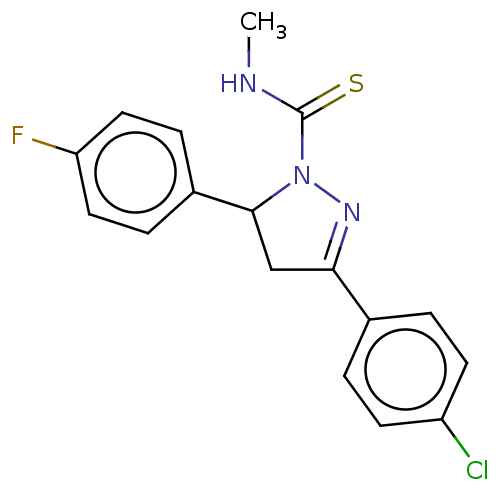
(CHEMBL5283319)Show SMILES CNC(=S)N1N=C(CC1c1ccc(F)cc1)c1ccc(Cl)cc1 |c:5| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50615742
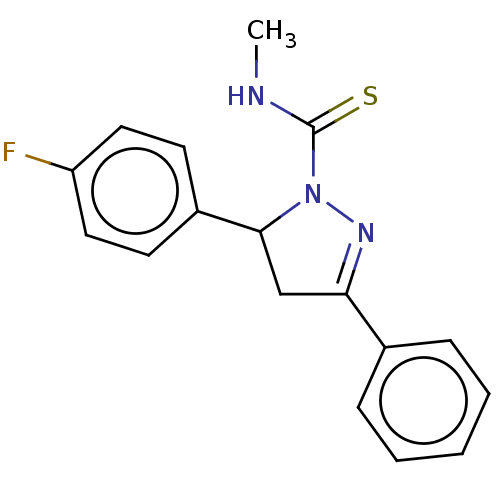
(CHEMBL5269241) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315540

(8-(2-Thioxo-7(3-butyl)-2-(2-furyl),thiazolo,[4,3-e...)Show InChI InChI=1S/C14H13N5OS2/c1-2-3-6-18-12-10(22-14(18)21)13-16-11(9-5-4-7-20-9)17-19(13)8-15-12/h4-5,7-8H,2-3,6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315544

(8-(2-Thioxo-7(3-p-chlorophenyl.)-2-(2-furyl)thiazo...)Show SMILES Clc1ccc(cc1)-n1c2ncn3nc(nc3c2sc1=S)-c1ccco1 Show InChI InChI=1S/C16H8ClN5OS2/c17-9-3-5-10(6-4-9)22-14-12(25-16(22)24)15-19-13(11-2-1-7-23-11)20-21(15)8-18-14/h1-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50315545

(8-(2-Thioxo-7(3-m-chlorophenyl)-2-(2-furyl)thiazol...)Show SMILES Clc1cccc(c1)-n1c2ncn3nc(nc3c2sc1=S)-c1ccco1 Show InChI InChI=1S/C16H8ClN5OS2/c17-9-3-1-4-10(7-9)22-14-12(25-16(22)24)15-19-13(11-5-2-6-23-11)20-21(15)8-18-14/h1-8H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315539
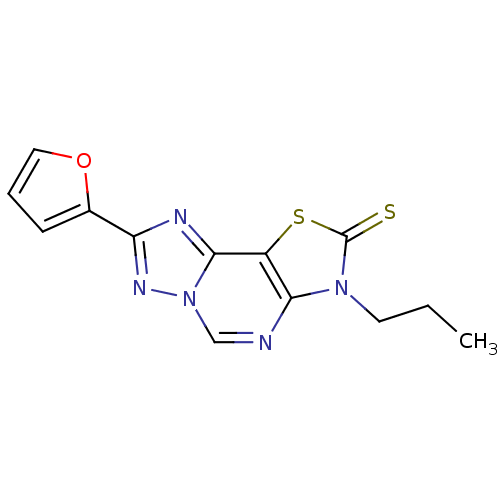
(8-(2-Thioxo-7(3-propyl)-2-(2-furyl)thiazolo[4,3-e]...)Show InChI InChI=1S/C13H11N5OS2/c1-2-5-17-11-9(21-13(17)20)12-15-10(8-4-3-6-19-8)16-18(12)7-14-11/h3-4,6-7H,2,5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515811
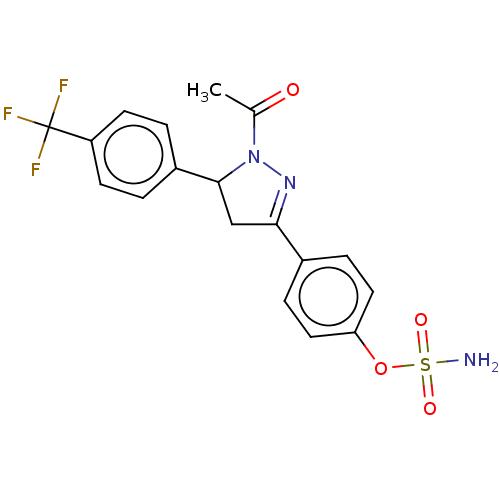
(CHEMBL4580659)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)C(F)(F)F)c1ccc(OS(N)(=O)=O)cc1 |c:4| Show InChI InChI=1S/C18H16F3N3O4S/c1-11(25)24-17(13-2-6-14(7-3-13)18(19,20)21)10-16(23-24)12-4-8-15(9-5-12)28-29(22,26)27/h2-9,17H,10H2,1H3,(H2,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515806

(CHEMBL4533252)Show SMILES COc1ccc(cc1)C1=NN(C(C1)c1ccc(OS(N)(=O)=O)cc1)C(C)=O |t:9| Show InChI InChI=1S/C18H19N3O5S/c1-12(22)21-18(14-5-9-16(10-6-14)26-27(19,23)24)11-17(20-21)13-3-7-15(25-2)8-4-13/h3-10,18H,11H2,1-2H3,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515838
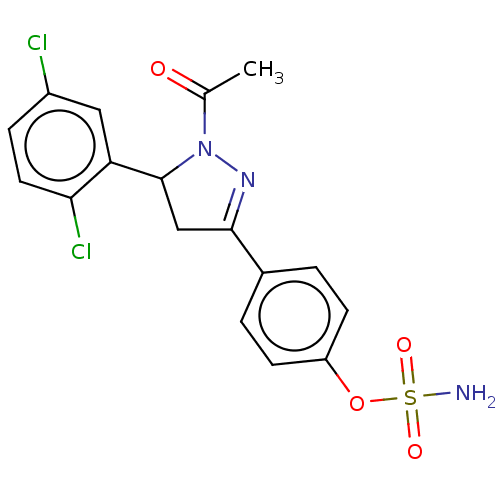
(CHEMBL4459675)Show SMILES CC(=O)N1N=C(CC1c1cc(Cl)ccc1Cl)c1ccc(OS(N)(=O)=O)cc1 |c:4| Show InChI InChI=1S/C17H15Cl2N3O4S/c1-10(23)22-17(14-8-12(18)4-7-15(14)19)9-16(21-22)11-2-5-13(6-3-11)26-27(20,24)25/h2-8,17H,9H2,1H3,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515807

(CHEMBL4561870)Show SMILES CC(=O)N1N=C(CC1c1cccc(OS(N)(=O)=O)c1)c1ccc(F)cc1 |c:4| Show InChI InChI=1S/C17H16FN3O4S/c1-11(22)21-17(10-16(20-21)12-5-7-14(18)8-6-12)13-3-2-4-15(9-13)25-26(19,23)24/h2-9,17H,10H2,1H3,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50315546
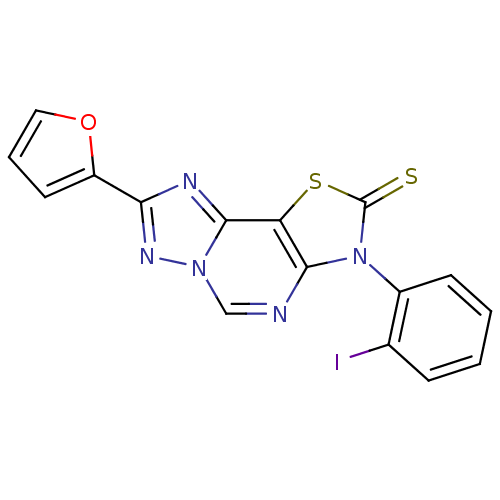
(8-(2-Thioxo-7(3-o-iodophenyl)-2-(2-furyl)thiazolo[...)Show SMILES Ic1ccccc1-n1c2ncn3nc(nc3c2sc1=S)-c1ccco1 |(-4.9,-21.48,;-6.44,-21.47,;-7.21,-20.13,;-8.75,-20.12,;-9.52,-21.46,;-8.75,-22.79,;-7.22,-22.79,;-6.46,-24.12,;-4.99,-24.61,;-3.66,-23.84,;-2.32,-24.6,;-2.32,-26.15,;-1.17,-27.19,;-1.8,-28.6,;-3.34,-28.43,;-3.66,-26.92,;-4.99,-26.15,;-6.46,-26.63,;-7.37,-25.38,;-8.91,-25.37,;-.91,-29.85,;.63,-29.86,;1.09,-31.33,;-.17,-32.22,;-1.41,-31.31,)| Show InChI InChI=1S/C16H8IN5OS2/c17-9-4-1-2-5-10(9)22-14-12(25-16(22)24)15-19-13(11-6-3-7-23-11)20-21(15)8-18-14/h1-8H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515814
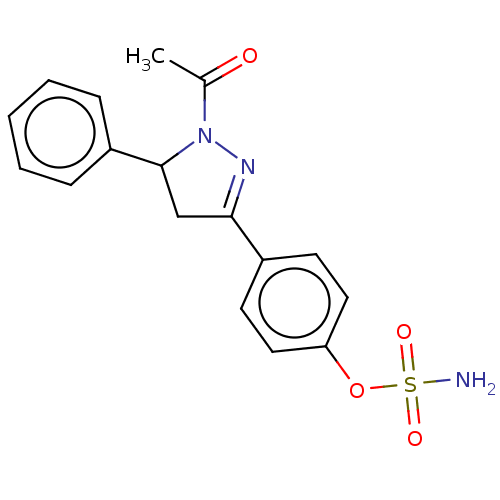
(CHEMBL4540711)Show SMILES CC(=O)N1N=C(CC1c1ccccc1)c1ccc(OS(N)(=O)=O)cc1 |c:4| Show InChI InChI=1S/C17H17N3O4S/c1-12(21)20-17(14-5-3-2-4-6-14)11-16(19-20)13-7-9-15(10-8-13)24-25(18,22)23/h2-10,17H,11H2,1H3,(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515839
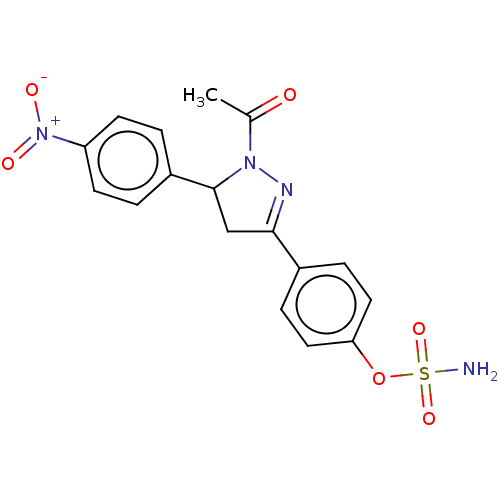
(CHEMBL4590447)Show SMILES CC(=O)N1N=C(CC1c1ccc(cc1)[N+]([O-])=O)c1ccc(OS(N)(=O)=O)cc1 |c:4| Show InChI InChI=1S/C17H16N4O6S/c1-11(22)20-17(13-2-6-14(7-3-13)21(23)24)10-16(19-20)12-4-8-15(9-5-12)27-28(18,25)26/h2-9,17H,10H2,1H3,(H2,18,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515815
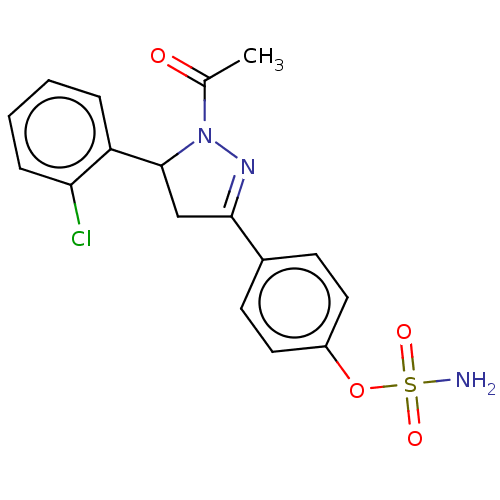
(CHEMBL4559197)Show SMILES CC(=O)N1N=C(CC1c1ccccc1Cl)c1ccc(OS(N)(=O)=O)cc1 |c:4| Show InChI InChI=1S/C17H16ClN3O4S/c1-11(22)21-17(14-4-2-3-5-15(14)18)10-16(20-21)12-6-8-13(9-7-12)25-26(19,23)24/h2-9,17H,10H2,1H3,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15613
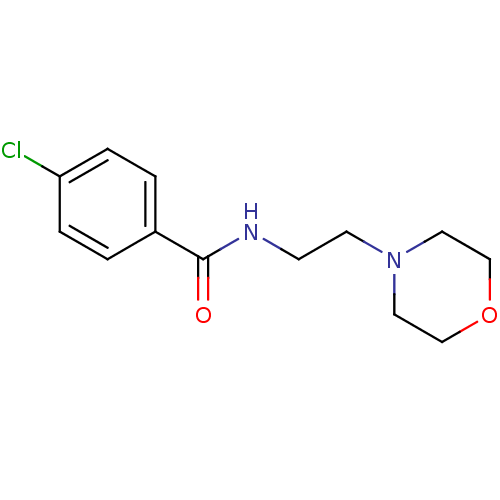
(4-chloro-N-(2-morpholin-4-ylethyl)benzamide | 4-ch...)Show InChI InChI=1S/C13H17ClN2O2/c14-12-3-1-11(2-4-12)13(17)15-5-6-16-7-9-18-10-8-16/h1-4H,5-10H2,(H,15,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315547
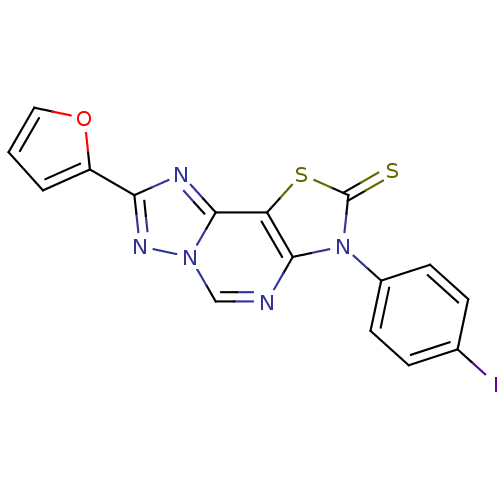
(8-(2-Thioxo-7(3-p-iodophenyl)-2-(2-furyl)thiazolo[...)Show InChI InChI=1S/C16H8IN5OS2/c17-9-3-5-10(6-4-9)22-14-12(25-16(22)24)15-19-13(11-2-1-7-23-11)20-21(15)8-18-14/h1-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315546

(8-(2-Thioxo-7(3-o-iodophenyl)-2-(2-furyl)thiazolo[...)Show SMILES Ic1ccccc1-n1c2ncn3nc(nc3c2sc1=S)-c1ccco1 |(-4.9,-21.48,;-6.44,-21.47,;-7.21,-20.13,;-8.75,-20.12,;-9.52,-21.46,;-8.75,-22.79,;-7.22,-22.79,;-6.46,-24.12,;-4.99,-24.61,;-3.66,-23.84,;-2.32,-24.6,;-2.32,-26.15,;-1.17,-27.19,;-1.8,-28.6,;-3.34,-28.43,;-3.66,-26.92,;-4.99,-26.15,;-6.46,-26.63,;-7.37,-25.38,;-8.91,-25.37,;-.91,-29.85,;.63,-29.86,;1.09,-31.33,;-.17,-32.22,;-1.41,-31.31,)| Show InChI InChI=1S/C16H8IN5OS2/c17-9-4-1-2-5-10(9)22-14-12(25-16(22)24)15-19-13(11-6-3-7-23-11)20-21(15)8-18-14/h1-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315542

(8-(2-Thioxo-7(3-phenyl)-2-(2-furyl)thiazolo[4,3-e]...)Show InChI InChI=1S/C16H9N5OS2/c23-16-21(10-5-2-1-3-6-10)14-12(24-16)15-18-13(11-7-4-8-22-11)19-20(15)9-17-14/h1-9H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50048466
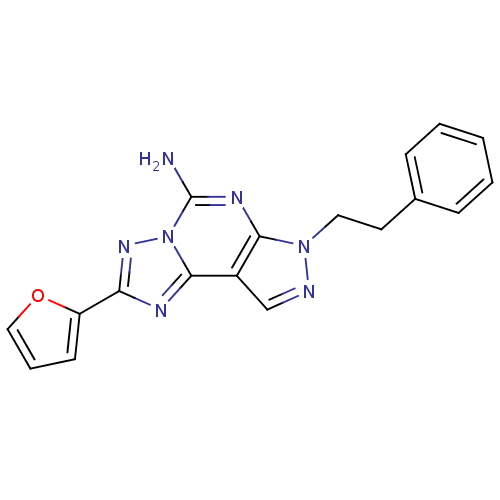
(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
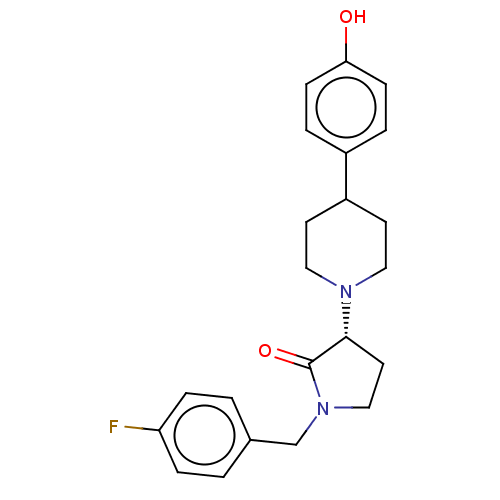
(Rattus norvegicus (Rat)) | BDBM198665
(US9221796, 2b)Show SMILES Oc1ccc(cc1)C1CCN(CC1)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H25FN2O2/c23-19-5-1-16(2-6-19)15-25-14-11-21(22(25)27)24-12-9-18(10-13-24)17-3-7-20(26)8-4-17/h1-8,18,21,26H,9-15H2/t21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
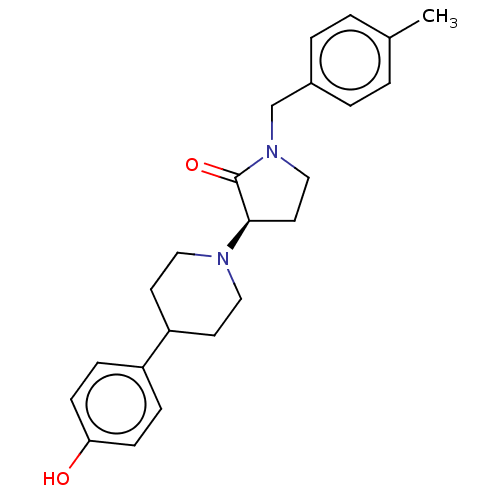
(Rattus norvegicus (Rat)) | BDBM198694
(US9221796, 23b)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(CC3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c1-17-2-4-18(5-3-17)16-25-15-12-22(23(25)27)24-13-10-20(11-14-24)19-6-8-21(26)9-7-19/h2-9,20,22,26H,10-16H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50315543
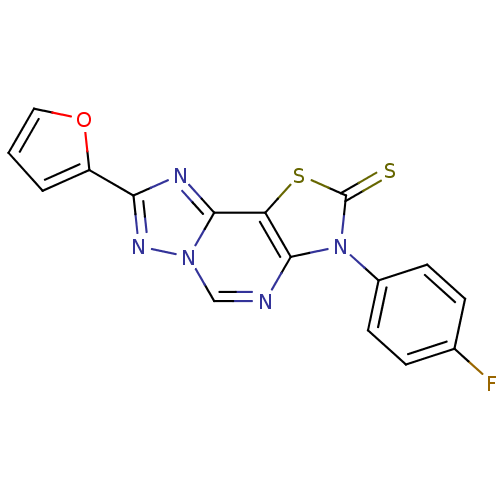
(8-(2-Thioxo-7(3-p fluorophenyl)-2-(2-furyl)thiazol...)Show InChI InChI=1S/C16H8FN5OS2/c17-9-3-5-10(6-4-9)22-14-12(25-16(22)24)15-19-13(11-2-1-7-23-11)20-21(15)8-18-14/h1-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50315548

(8-(2-Thioxo-7(3-m-iodophenyl)-2-(2-furyl)thiazolo[...)Show InChI InChI=1S/C16H8IN5OS2/c17-9-3-1-4-10(7-9)22-14-12(25-16(22)24)15-19-13(11-5-2-6-23-11)20-21(15)8-18-14/h1-8H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50315543

(8-(2-Thioxo-7(3-p fluorophenyl)-2-(2-furyl)thiazol...)Show InChI InChI=1S/C16H8FN5OS2/c17-9-3-5-10(6-4-9)22-14-12(25-16(22)24)15-19-13(11-2-1-7-23-11)20-21(15)8-18-14/h1-8H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem 18: 2491-500 (2010)
Article DOI: 10.1016/j.bmc.2010.02.048
BindingDB Entry DOI: 10.7270/Q27W6D57 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50570985
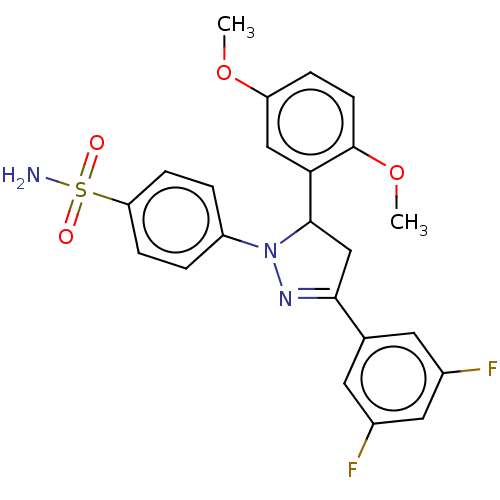
(CHEMBL4872387)Show SMILES COc1ccc(OC)c(c1)C1CC(=NN1c1ccc(cc1)S(N)(=O)=O)c1cc(F)cc(F)c1 |c:13| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50570979
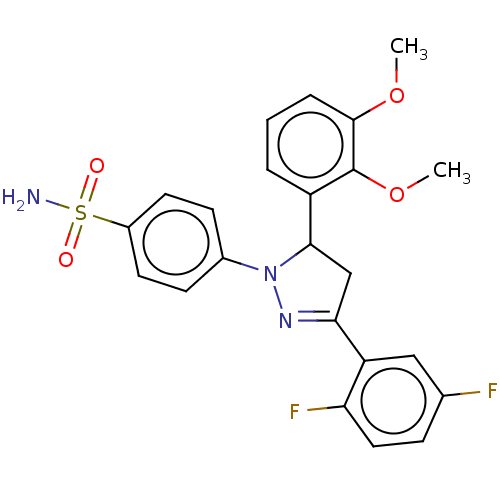
(CHEMBL4876038)Show SMILES COc1cccc(C2CC(=NN2c2ccc(cc2)S(N)(=O)=O)c2cc(F)ccc2F)c1OC |c:9| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50570980
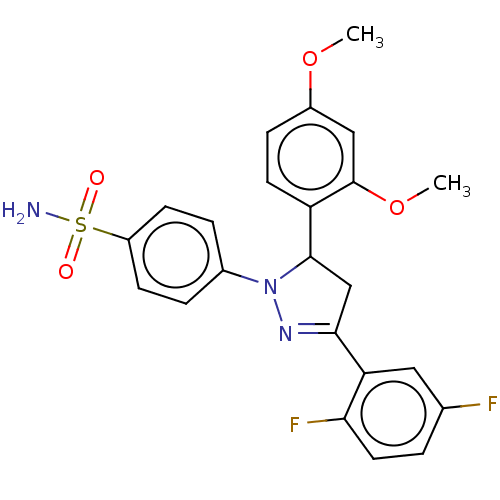
(CHEMBL4847624)Show SMILES COc1ccc(C2CC(=NN2c2ccc(cc2)S(N)(=O)=O)c2cc(F)ccc2F)c(OC)c1 |c:8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50570981
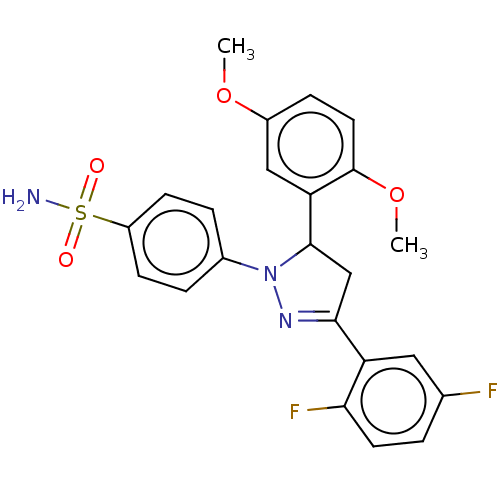
(CHEMBL4869398)Show SMILES COc1ccc(OC)c(c1)C1CC(=NN1c1ccc(cc1)S(N)(=O)=O)c1cc(F)ccc1F |c:13| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50570982

(CHEMBL4849196)Show SMILES COc1ccc(cc1OC)C1CC(=NN1c1ccc(cc1)S(N)(=O)=O)c1cc(F)ccc1F |c:13| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50570983
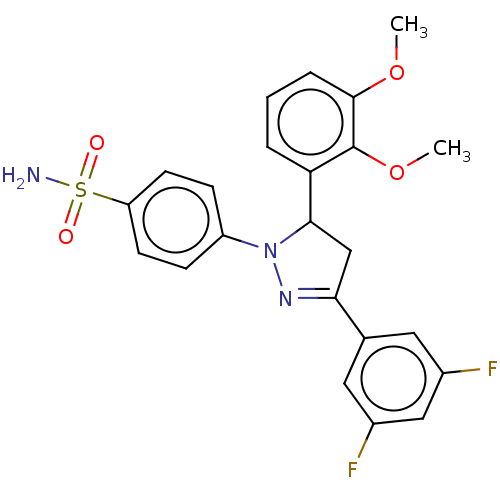
(CHEMBL4857592)Show SMILES COc1cccc(C2CC(=NN2c2ccc(cc2)S(N)(=O)=O)c2cc(F)cc(F)c2)c1OC |c:9| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50570984

(CHEMBL4847613)Show SMILES COc1ccc(C2CC(=NN2c2ccc(cc2)S(N)(=O)=O)c2cc(F)cc(F)c2)c(OC)c1 |c:8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data