

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor expressed in CHO cells | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289804 (Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468037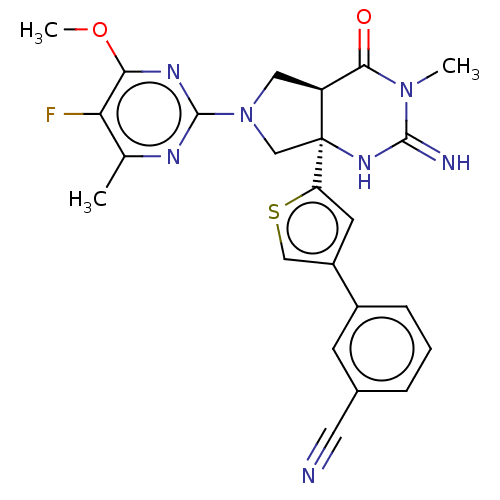 (CHEMBL4293298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289851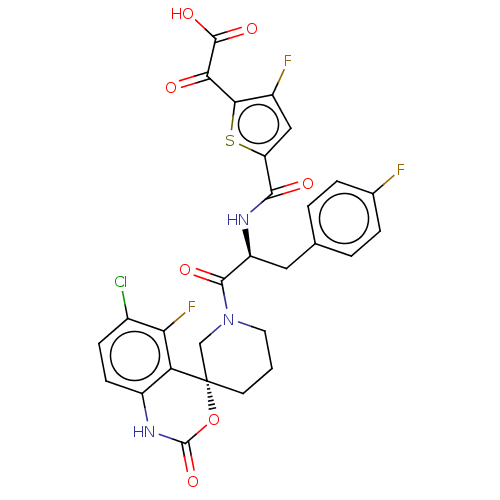 (2-(5-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468040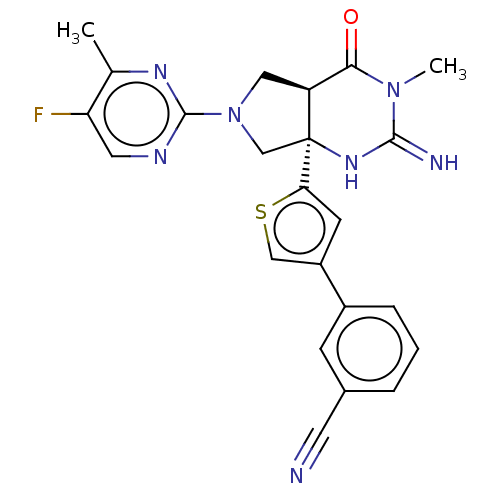 (CHEMBL4289763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807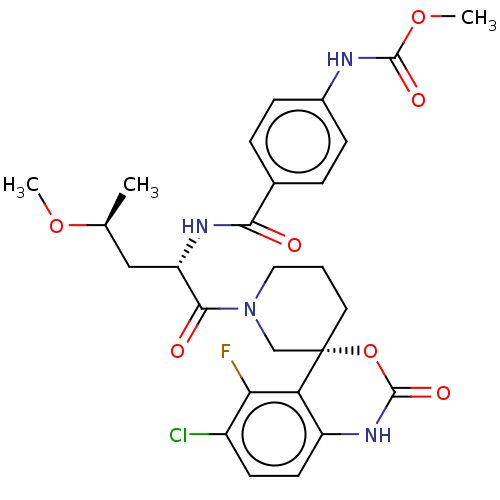 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468044 (CHEMBL4279084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164512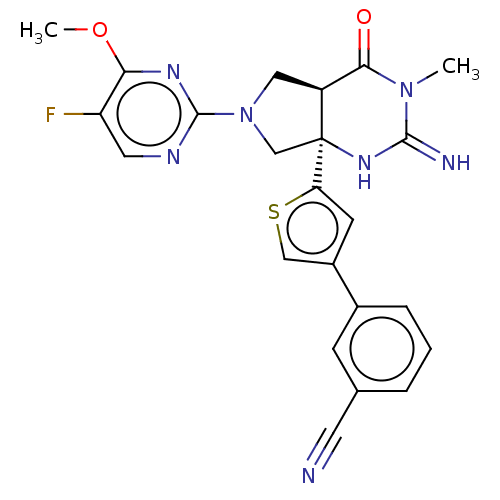 (CHEMBL3800286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468043 (CHEMBL4288838) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468039 (CHEMBL4278329) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468048 (CHEMBL4280271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468038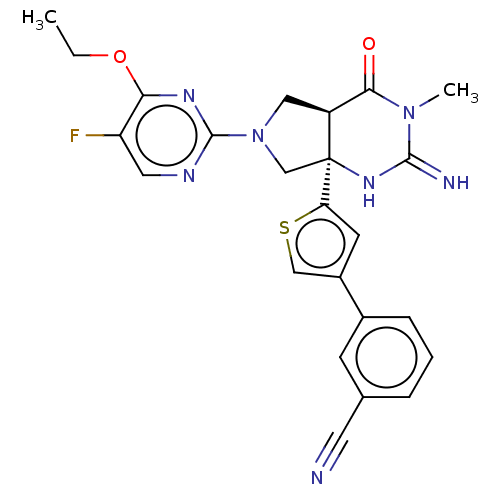 (CHEMBL4294236) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468046 (CHEMBL4278154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468047 (CHEMBL4278011) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468045 (CHEMBL4288644) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289844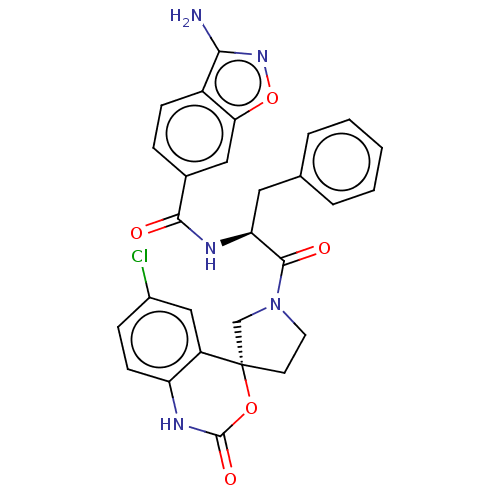 (3-amino-N—((S)-1-(R)-6-chloro-2-oxo-1,2-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.52 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289795 (Methyl 2-amino-3-(5-fluoropyridin-2-yl)propanoate ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.82 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468035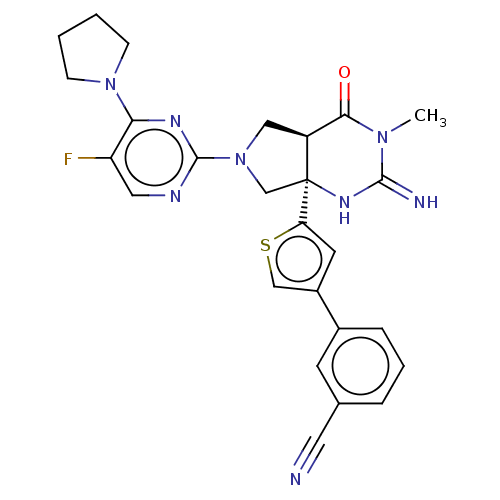 (CHEMBL4285940) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289853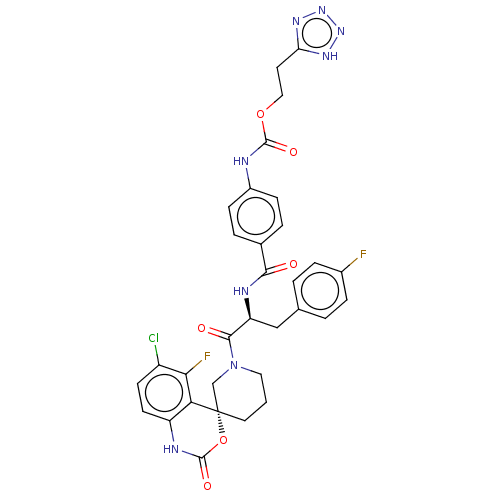 (2-(1H-tetrazol-5-yl)ethyl (4-(((S)-1-((R)-6-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.22 | -48.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468036 (CHEMBL4286732) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398693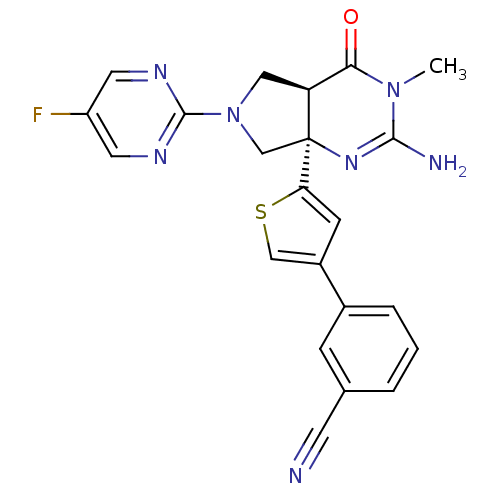 (CHEMBL2178718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289805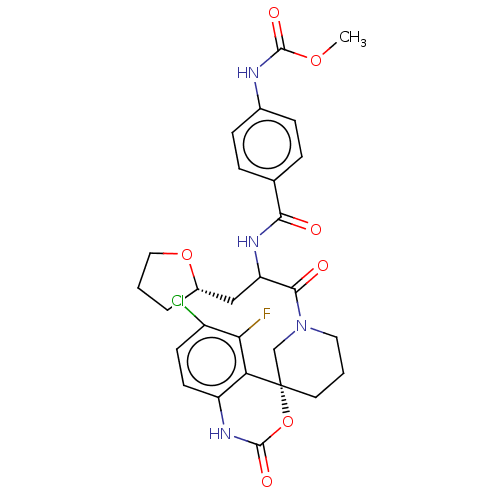 (US10093683, Example 116A | US10093683, Example 116...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.21 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50591808 (CHEMBL5207397) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114269 BindingDB Entry DOI: 10.7270/Q2JM2FM0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398687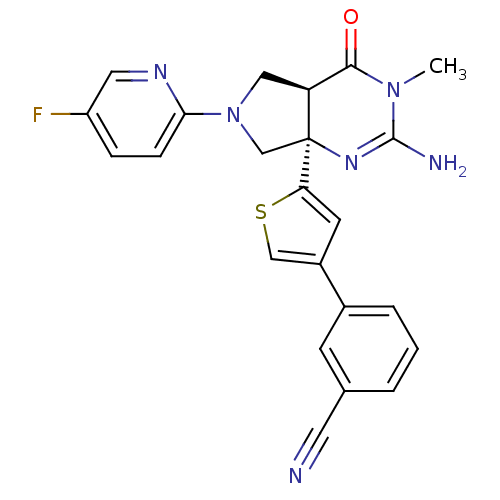 (CHEMBL2178714) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50514305 (CHEMBL4560429) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to recombinant human His/SUMO-tagged STAT3 (127 to 688 residues) expressed in Escherichia coli Rosetta (DE3) incubated for 1 hr by f... | J Med Chem 62: 11280-11300 (2019) Article DOI: 10.1021/acs.jmedchem.9b01530 BindingDB Entry DOI: 10.7270/Q2FF3WQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289805 (US10093683, Example 116A | US10093683, Example 116...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.51 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50591807 (CHEMBL5178760) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114269 BindingDB Entry DOI: 10.7270/Q2JM2FM0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289849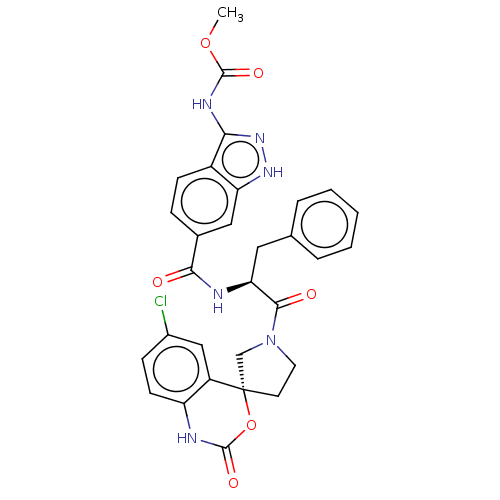 (Methyl 3-amino-6-(((S)-1-((R)-6-chloro-2-oxo-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289787 (US10093683, Example 14b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.5 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468042 (CHEMBL4294969) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468049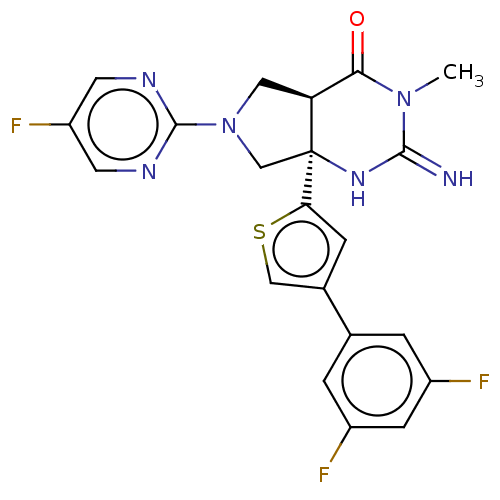 (CHEMBL4291347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289811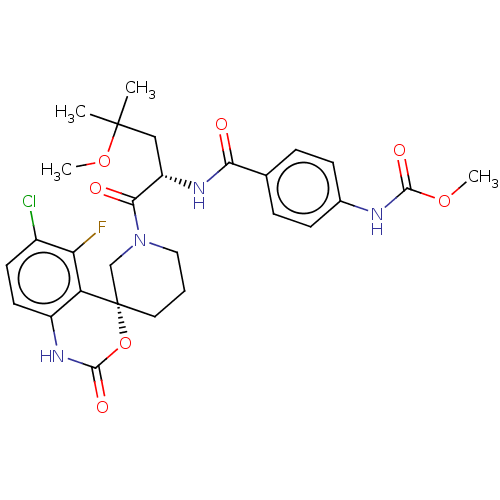 (Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.2 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398696 (CHEMBL2178717) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50514306 (CHEMBL4471887) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to recombinant human His/SUMO-tagged STAT3 (127 to 688 residues) expressed in Escherichia coli Rosetta (DE3) incubated for 1 hr by f... | J Med Chem 62: 11280-11300 (2019) Article DOI: 10.1021/acs.jmedchem.9b01530 BindingDB Entry DOI: 10.7270/Q2FF3WQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289801 (Methyl (4-(((S)-1-((R)-6-chloro-2-oxo-1,2-dihydros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50591806 (CHEMBL5205864) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114269 BindingDB Entry DOI: 10.7270/Q2JM2FM0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50514299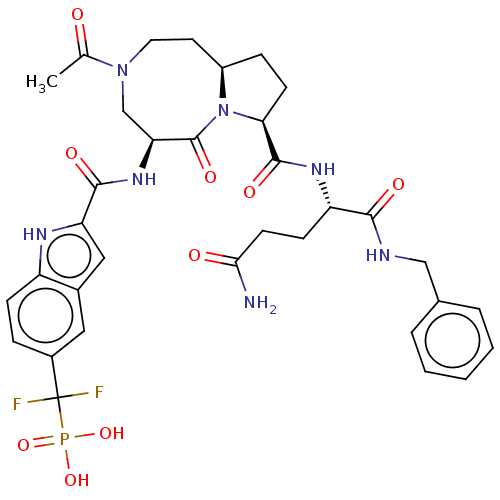 (CHEMBL4515406) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to recombinant human His/SUMO-tagged STAT3 (127 to 688 residues) expressed in Escherichia coli Rosetta (DE3) incubated for 1 hr by f... | J Med Chem 62: 11280-11300 (2019) Article DOI: 10.1021/acs.jmedchem.9b01530 BindingDB Entry DOI: 10.7270/Q2FF3WQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50514303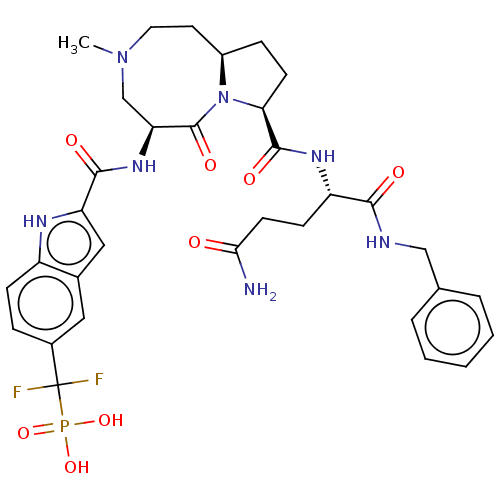 (CHEMBL4443339) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to recombinant human His/SUMO-tagged STAT3 (127 to 688 residues) expressed in Escherichia coli Rosetta (DE3) incubated for 1 hr by f... | J Med Chem 62: 11280-11300 (2019) Article DOI: 10.1021/acs.jmedchem.9b01530 BindingDB Entry DOI: 10.7270/Q2FF3WQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50514297 (CHEMBL4575382) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to recombinant human His/SUMO-tagged STAT3 (127 to 688 residues) expressed in Escherichia coli Rosetta (DE3) incubated for 1 hr by f... | J Med Chem 62: 11280-11300 (2019) Article DOI: 10.1021/acs.jmedchem.9b01530 BindingDB Entry DOI: 10.7270/Q2FF3WQF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50493249 (CHEMBL2420249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289803 (N—((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.6 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center Curated by ChEMBL | Assay Description Inhibition of PTP1B | Eur J Med Chem 44: 3147-57 (2009) Article DOI: 10.1016/j.ejmech.2009.03.009 BindingDB Entry DOI: 10.7270/Q2FJ2GTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289848 (Methyl 3-amino-6-(((S)-1-((R)-6-chloro-2-oxo-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.1 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50514298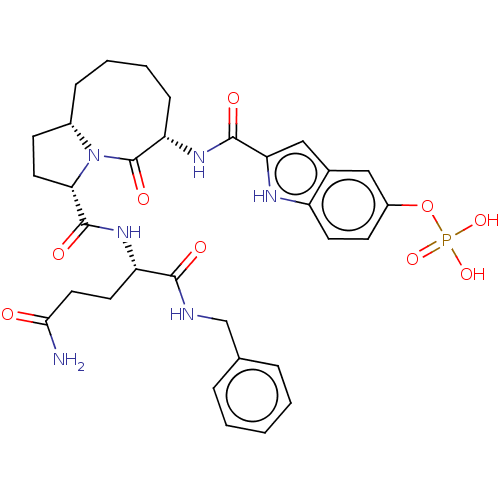 (CHEMBL4571181) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to recombinant human His/SUMO-tagged STAT3 (127 to 688 residues) expressed in Escherichia coli Rosetta (DE3) incubated for 1 hr by f... | J Med Chem 62: 11280-11300 (2019) Article DOI: 10.1021/acs.jmedchem.9b01530 BindingDB Entry DOI: 10.7270/Q2FF3WQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398680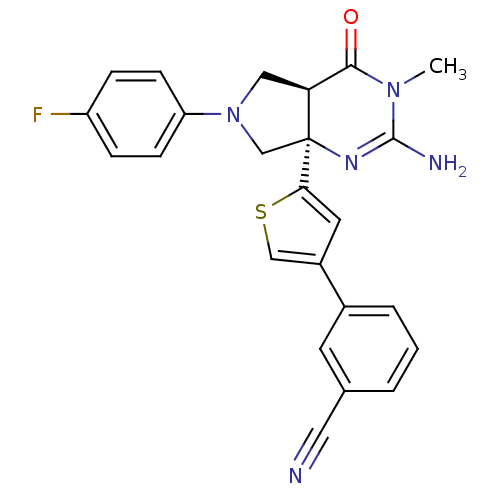 (CHEMBL2178150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289796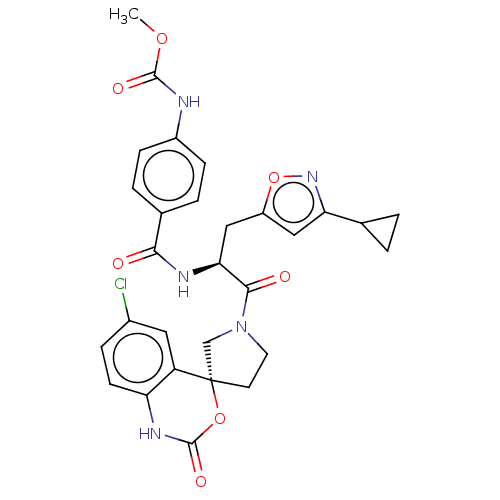 (Methyl(4-(((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28.1 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2256 total ) | Next | Last >> |