 Found 857 hits with Last Name = 'kun' and Initial = 's'
Found 857 hits with Last Name = 'kun' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398667

(CHEMBL2178135)Show SMILES COc1cc(C(=O)N2CCOCC2)c(F)cc1Nc1ncc(c(NC2CC2)n1)C(F)(F)F Show InChI InChI=1S/C20H21F4N5O3/c1-31-16-8-12(18(30)29-4-6-32-7-5-29)14(21)9-15(16)27-19-25-10-13(20(22,23)24)17(28-19)26-11-2-3-11/h8-11H,2-7H2,1H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398676
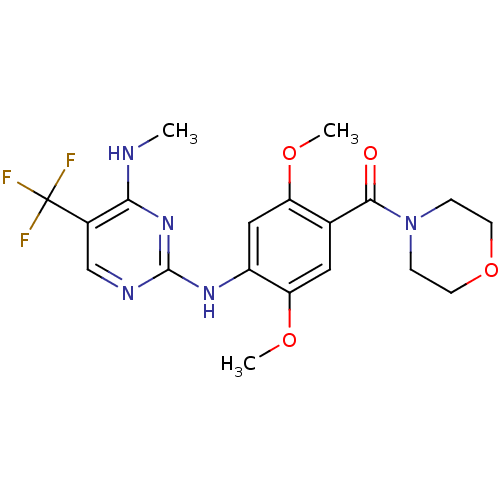
(CHEMBL2178125)Show SMILES CNc1nc(Nc2cc(OC)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O4/c1-23-16-12(19(20,21)22)10-24-18(26-16)25-13-9-14(29-2)11(8-15(13)30-3)17(28)27-4-6-31-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398662
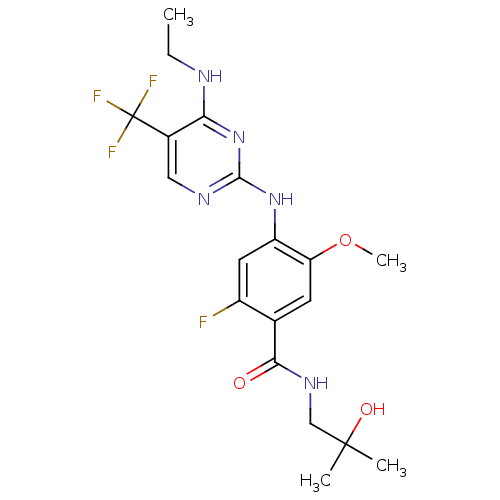
(CHEMBL2178140)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)NCC(C)(C)O)ncc1C(F)(F)F Show InChI InChI=1S/C19H23F4N5O3/c1-5-24-15-11(19(21,22)23)8-25-17(28-15)27-13-7-12(20)10(6-14(13)31-4)16(29)26-9-18(2,3)30/h6-8,30H,5,9H2,1-4H3,(H,26,29)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668
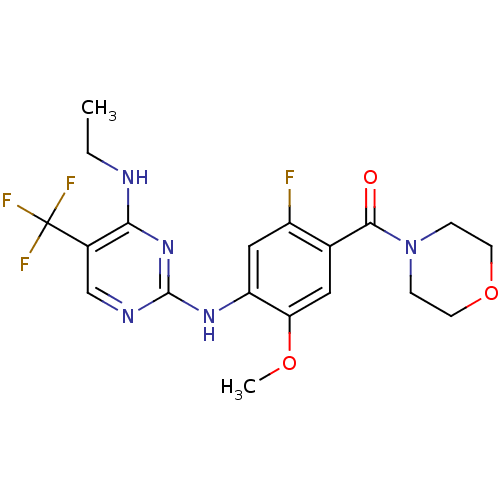
(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398677
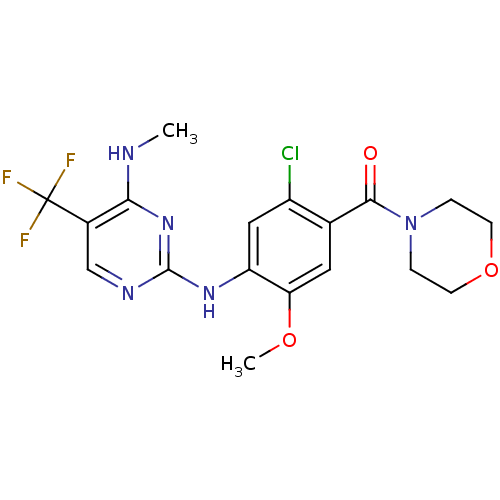
(CHEMBL2178124 | US8802674, 292)Show SMILES CNc1nc(Nc2cc(Cl)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-12(19)10(7-14(13)29-2)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398672
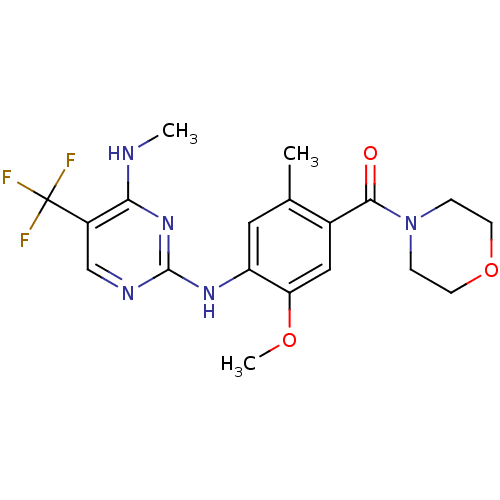
(CHEMBL2178130)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O3/c1-11-8-14(25-18-24-10-13(19(20,21)22)16(23-2)26-18)15(29-3)9-12(11)17(28)27-4-6-30-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396150
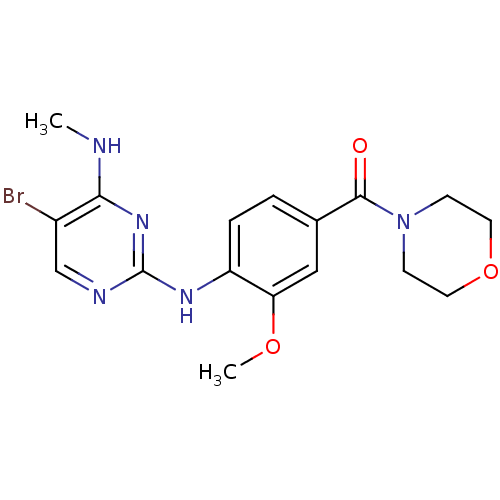
(CHEMBL2171743)Show InChI InChI=1S/C17H20BrN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398665
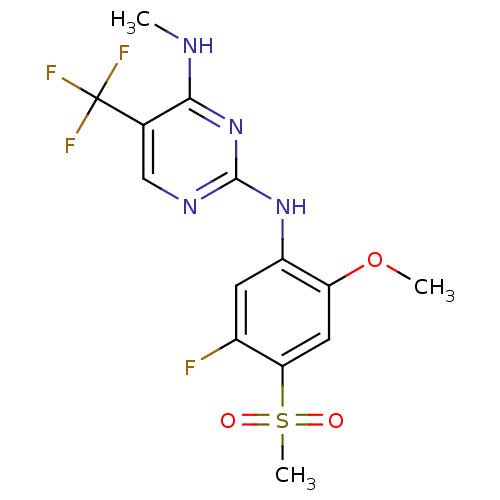
(CHEMBL2178137 | US9145402, 15)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C14H14F4N4O3S/c1-19-12-7(14(16,17)18)6-20-13(22-12)21-9-4-8(15)11(26(3,23)24)5-10(9)25-2/h4-6H,1-3H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM482160
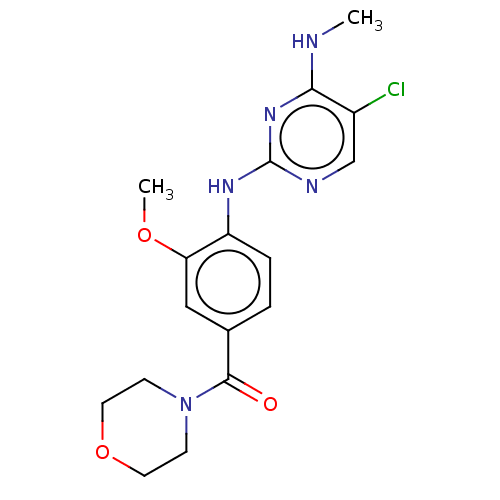
(BDBM50396041 | HG-10-102-01)Show InChI InChI=1S/C17H20ClN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398674
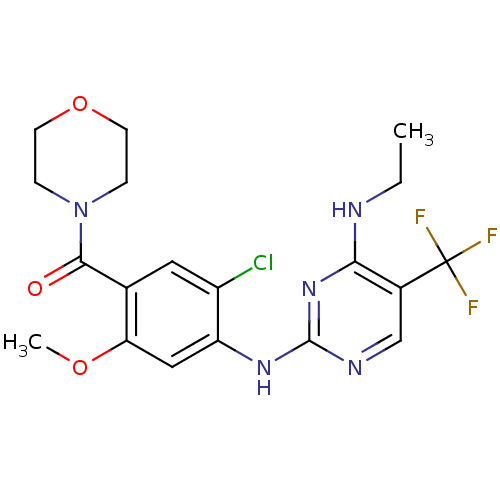
(CHEMBL2178127 | US8802674, 147)Show SMILES CCNc1nc(Nc2cc(OC)c(cc2Cl)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21ClF3N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-15(30-2)11(8-13(14)20)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398664
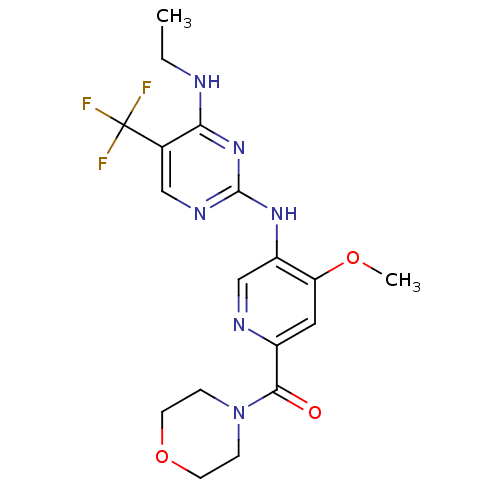
(CHEMBL2178138)Show SMILES CCNc1nc(Nc2cnc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H21F3N6O3/c1-3-22-15-11(18(19,20)21)9-24-17(26-15)25-13-10-23-12(8-14(13)29-2)16(28)27-4-6-30-7-5-27/h8-10H,3-7H2,1-2H3,(H2,22,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398661
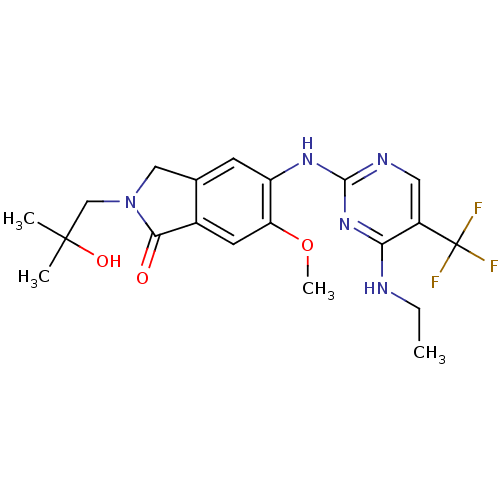
(CHEMBL2178141 | US8796296, 24)Show SMILES CCNc1nc(Nc2cc3CN(CC(C)(C)O)C(=O)c3cc2OC)ncc1C(F)(F)F Show InChI InChI=1S/C20H24F3N5O3/c1-5-24-16-13(20(21,22)23)8-25-18(27-16)26-14-6-11-9-28(10-19(2,3)30)17(29)12(11)7-15(14)31-4/h6-8,30H,5,9-10H2,1-4H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398675
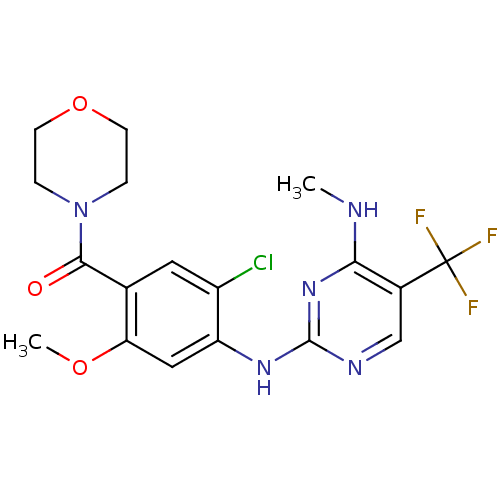
(CHEMBL2178126 | US8802674, 296)Show SMILES CNc1nc(Nc2cc(OC)c(cc2Cl)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-14(29-2)10(7-12(13)19)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398659
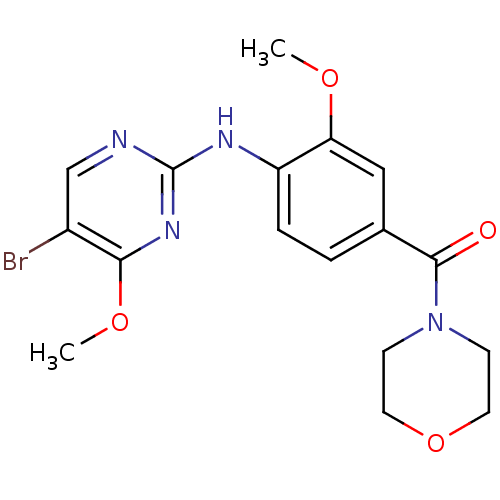
(CHEMBL2178123)Show InChI InChI=1S/C17H19BrN4O4/c1-24-14-9-11(16(23)22-5-7-26-8-6-22)3-4-13(14)20-17-19-10-12(18)15(21-17)25-2/h3-4,9-10H,5-8H2,1-2H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398669
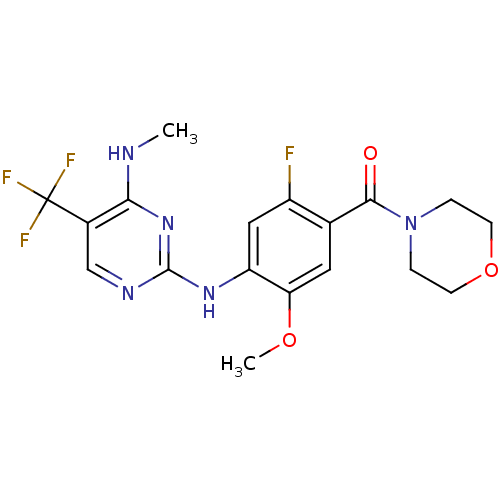
(CHEMBL2178133 | US8802674, 239)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19F4N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-12(19)10(7-14(13)29-2)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398673
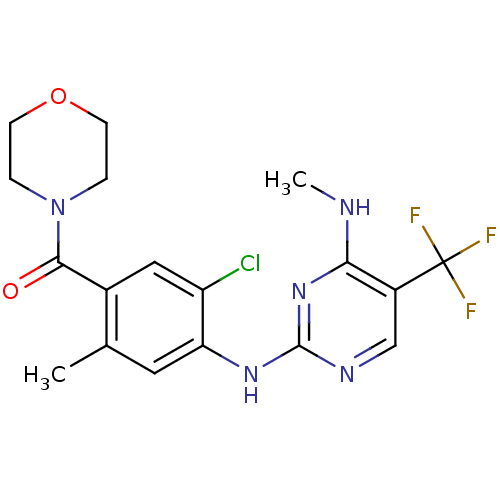
(CHEMBL2178129)Show SMILES CNc1nc(Nc2cc(C)c(cc2Cl)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O2/c1-10-7-14(13(19)8-11(10)16(28)27-3-5-29-6-4-27)25-17-24-9-12(18(20,21)22)15(23-2)26-17/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398671
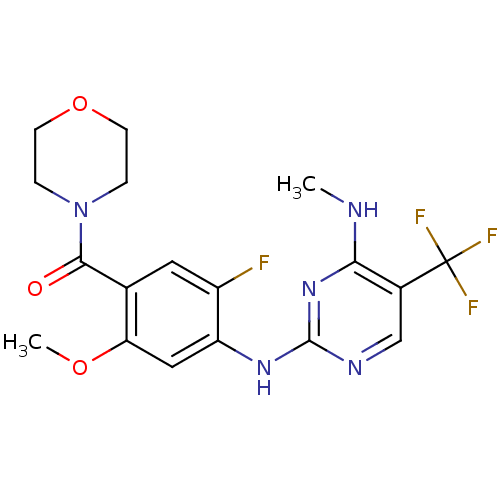
(CHEMBL2178131 | US8802674, 237)Show SMILES CNc1nc(Nc2cc(OC)c(cc2F)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19F4N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-14(29-2)10(7-12(13)19)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM86674
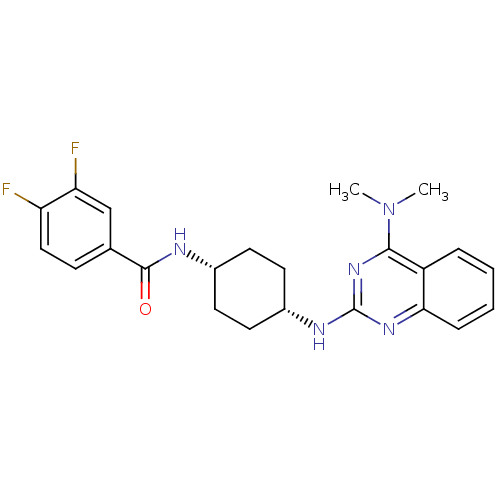
(ATC0175)Show SMILES CN(C)c1nc(N[C@@H]2CC[C@@H](CC2)NC(=O)c2ccc(F)c(F)c2)nc2ccccc12 |r,wU:10.13,7.6,(-7.34,-2.98,;-7.34,-1.44,;-8.67,-.67,;-6,-.67,;-4.67,-1.44,;-3.33,-.67,;-2,-1.44,;-.67,-.67,;.67,-1.44,;2,-.67,;2,.87,;.67,1.64,;-.67,.87,;3.33,1.64,;4.67,.87,;4.67,-.67,;6,1.64,;7.34,.87,;8.67,1.64,;8.67,3.18,;10,3.95,;7.34,3.95,;7.34,5.49,;6,3.18,;-3.33,.87,;-4.67,1.64,;-4.67,3.18,;-6,3.95,;-7.34,3.18,;-7.34,1.64,;-6,.87,)| Show InChI InChI=1S/C23H25F2N5O/c1-30(2)21-17-5-3-4-6-20(17)28-23(29-21)27-16-10-8-15(9-11-16)26-22(31)14-7-12-18(24)19(25)13-14/h3-7,12-13,15-16H,8-11H2,1-2H3,(H,26,31)(H,27,28,29)/t15-,16+ | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 831-9 (2005)
Article DOI: 10.1124/jpet.104.081711
BindingDB Entry DOI: 10.7270/Q2DB80D6 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398666
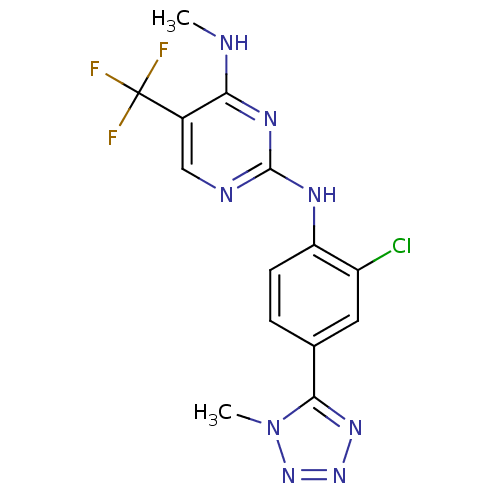
(CHEMBL2178136 | US8791130, 2)Show InChI InChI=1S/C14H12ClF3N8/c1-19-11-8(14(16,17)18)6-20-13(22-11)21-10-4-3-7(5-9(10)15)12-23-24-25-26(12)2/h3-6H,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398660
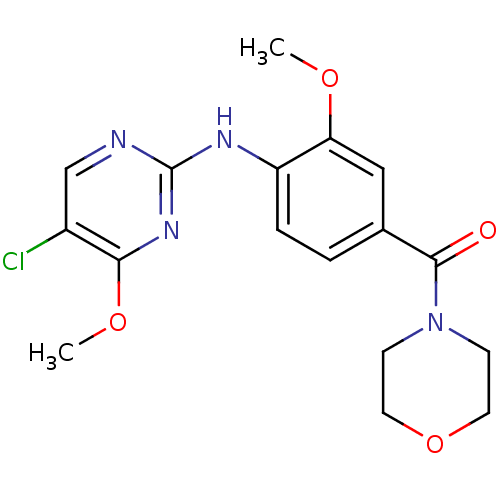
(CHEMBL2178142 | US8802674, 61)Show InChI InChI=1S/C17H19ClN4O4/c1-24-14-9-11(16(23)22-5-7-26-8-6-22)3-4-13(14)20-17-19-10-12(18)15(21-17)25-2/h3-4,9-10H,5-8H2,1-2H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398663
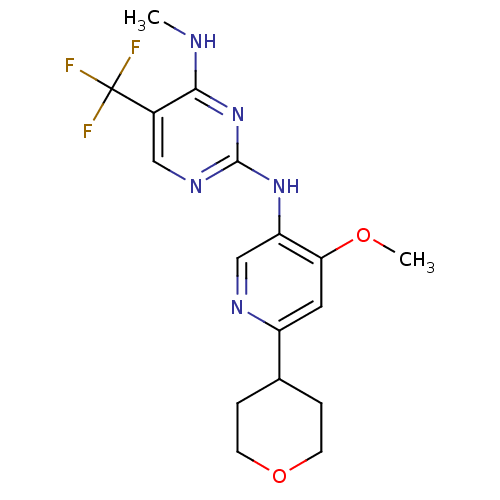
(CHEMBL2178139)Show InChI InChI=1S/C17H20F3N5O2/c1-21-15-11(17(18,19)20)8-23-16(25-15)24-13-9-22-12(7-14(13)26-2)10-3-5-27-6-4-10/h7-10H,3-6H2,1-2H3,(H2,21,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM86674

(ATC0175)Show SMILES CN(C)c1nc(N[C@@H]2CC[C@@H](CC2)NC(=O)c2ccc(F)c(F)c2)nc2ccccc12 |r,wU:10.13,7.6,(-7.34,-2.98,;-7.34,-1.44,;-8.67,-.67,;-6,-.67,;-4.67,-1.44,;-3.33,-.67,;-2,-1.44,;-.67,-.67,;.67,-1.44,;2,-.67,;2,.87,;.67,1.64,;-.67,.87,;3.33,1.64,;4.67,.87,;4.67,-.67,;6,1.64,;7.34,.87,;8.67,1.64,;8.67,3.18,;10,3.95,;7.34,3.95,;7.34,5.49,;6,3.18,;-3.33,.87,;-4.67,1.64,;-4.67,3.18,;-6,3.95,;-7.34,3.18,;-7.34,1.64,;-6,.87,)| Show InChI InChI=1S/C23H25F2N5O/c1-30(2)21-17-5-3-4-6-20(17)28-23(29-21)27-16-10-8-15(9-11-16)26-22(31)14-7-12-18(24)19(25)13-14/h3-7,12-13,15-16H,8-11H2,1-2H3,(H,26,31)(H,27,28,29)/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 831-9 (2005)
Article DOI: 10.1124/jpet.104.081711
BindingDB Entry DOI: 10.7270/Q2DB80D6 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396147
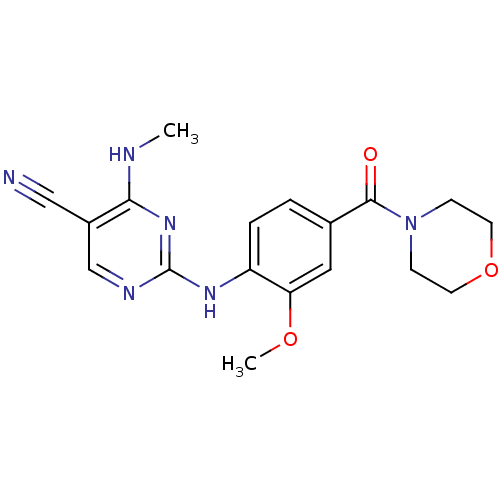
(CHEMBL2171746 | US8802674, 314)Show InChI InChI=1S/C18H20N6O3/c1-20-16-13(10-19)11-21-18(23-16)22-14-4-3-12(9-15(14)26-2)17(25)24-5-7-27-8-6-24/h3-4,9,11H,5-8H2,1-2H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398670
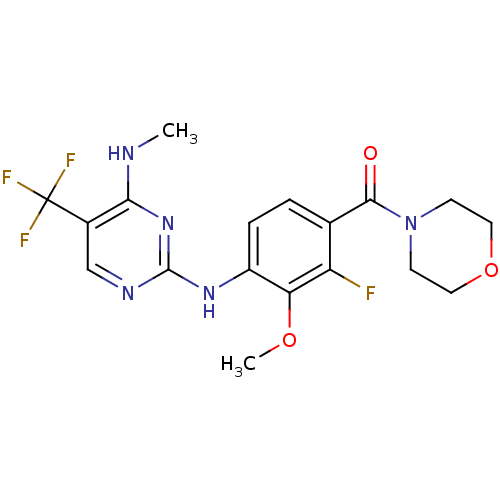
(CHEMBL2178132 | US8802674, 297)Show SMILES CNc1nc(Nc2ccc(C(=O)N3CCOCC3)c(F)c2OC)ncc1C(F)(F)F Show InChI InChI=1S/C18H19F4N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-12-4-3-10(13(19)14(12)29-2)16(28)27-5-7-30-8-6-27/h3-4,9H,5-8H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM86673
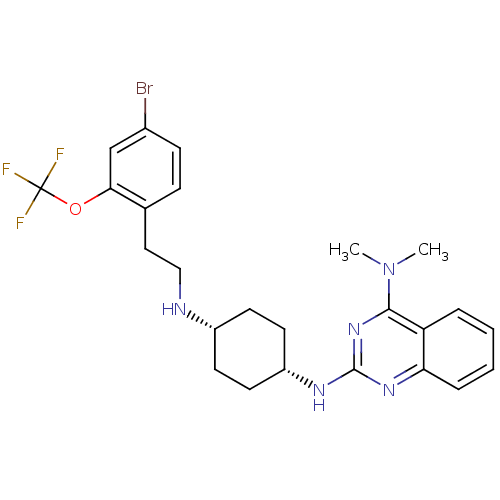
(ATC 0065 | ATC0065 | CAS_510732-84-0)Show SMILES CN(C)c1nc(N[C@@H]2CC[C@@H](CC2)NCCc2ccc(Br)cc2OC(F)(F)F)nc2ccccc12 |r,wU:10.13,7.6,(-7.34,-2.98,;-7.34,-1.44,;-8.67,-.67,;-6,-.67,;-4.67,-1.44,;-3.33,-.67,;-2,-1.44,;-.67,-.67,;.67,-1.44,;2,-.67,;2,.87,;.67,1.64,;-.67,.87,;3.33,1.64,;4.67,.87,;6,1.64,;7.34,.87,;7.34,-.67,;8.67,-1.44,;10,-.67,;11.34,-1.44,;10,.87,;8.67,1.64,;8.67,3.18,;10,3.95,;10.77,2.62,;9.23,5.29,;11.34,4.72,;-3.33,.87,;-4.67,1.64,;-4.67,3.18,;-6,3.95,;-7.34,3.18,;-7.34,1.64,;-6,.87,)| Show InChI InChI=1S/C25H29BrF3N5O/c1-34(2)23-20-5-3-4-6-21(20)32-24(33-23)31-19-11-9-18(10-12-19)30-14-13-16-7-8-17(26)15-22(16)35-25(27,28)29/h3-8,15,18-19,30H,9-14H2,1-2H3,(H,31,32,33)/t18-,19+ | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 831-9 (2005)
Article DOI: 10.1124/jpet.104.081711
BindingDB Entry DOI: 10.7270/Q2DB80D6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86674

(ATC0175)Show SMILES CN(C)c1nc(N[C@@H]2CC[C@@H](CC2)NC(=O)c2ccc(F)c(F)c2)nc2ccccc12 |r,wU:10.13,7.6,(-7.34,-2.98,;-7.34,-1.44,;-8.67,-.67,;-6,-.67,;-4.67,-1.44,;-3.33,-.67,;-2,-1.44,;-.67,-.67,;.67,-1.44,;2,-.67,;2,.87,;.67,1.64,;-.67,.87,;3.33,1.64,;4.67,.87,;4.67,-.67,;6,1.64,;7.34,.87,;8.67,1.64,;8.67,3.18,;10,3.95,;7.34,3.95,;7.34,5.49,;6,3.18,;-3.33,.87,;-4.67,1.64,;-4.67,3.18,;-6,3.95,;-7.34,3.18,;-7.34,1.64,;-6,.87,)| Show InChI InChI=1S/C23H25F2N5O/c1-30(2)21-17-5-3-4-6-20(17)28-23(29-21)27-16-10-8-15(9-11-16)26-22(31)14-7-12-18(24)19(25)13-14/h3-7,12-13,15-16H,8-11H2,1-2H3,(H,26,31)(H,27,28,29)/t15-,16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 831-9 (2005)
Article DOI: 10.1124/jpet.104.081711
BindingDB Entry DOI: 10.7270/Q2DB80D6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TTK |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50148913
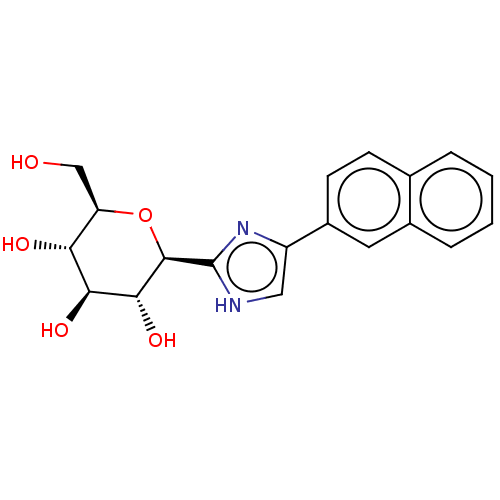
(CHEMBL3770455)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H20N2O5/c22-9-14-15(23)16(24)17(25)18(26-14)19-20-8-13(21-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,22-25H,9H2,(H,20,21)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorg... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50148913

(CHEMBL3770455)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H20N2O5/c22-9-14-15(23)16(24)17(25)18(26-14)19-20-8-13(21-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,22-25H,9H2,(H,20,21)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-b using alpha-D-glucose-1-phosphate as substrate by Dixon plot analysis |
ACS Med Chem Lett 6: 1215-9 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00361
BindingDB Entry DOI: 10.7270/Q2W37Z56 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396149
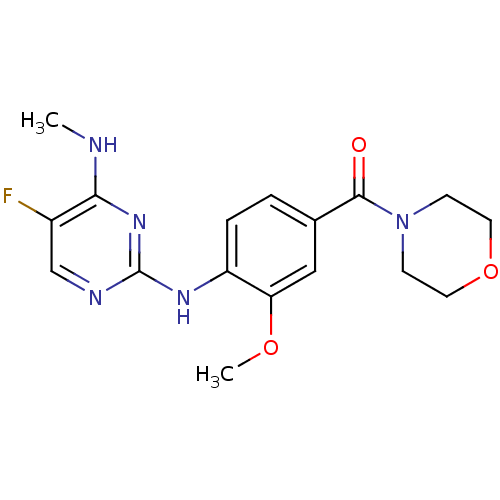
(CHEMBL2171744)Show InChI InChI=1S/C17H20FN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50148913

(CHEMBL3770455)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H20N2O5/c22-9-14-15(23)16(24)17(25)18(26-14)19-20-8-13(21-19)12-6-5-10-3-1-2-4-11(10)7-12/h1-8,14-18,22-25H,9H2,(H,20,21)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using varying levels of glucose-1-phospha... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50148915
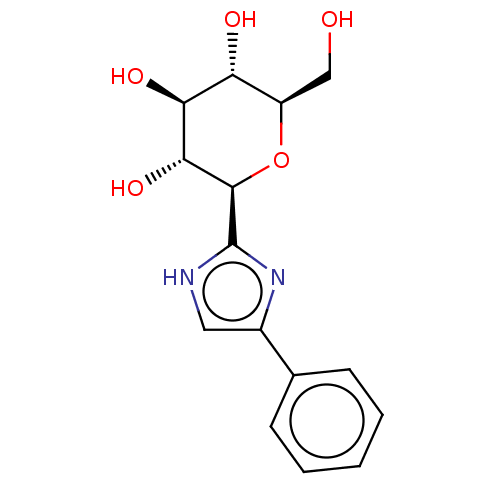
(CHEMBL3770514)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H18N2O5/c18-7-10-11(19)12(20)13(21)14(22-10)15-16-6-9(17-15)8-4-2-1-3-5-8/h1-6,10-14,18-21H,7H2,(H,16,17)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorg... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437354

(CHEMBL2408225)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H19N3O5/c22-8-12-13(23)14(24)15(25)16(26-12)18-19-17(20-21-18)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-16,22-25H,8H2,(H,19,20,21)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50148915

(CHEMBL3770514)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H18N2O5/c18-7-10-11(19)12(20)13(21)14(22-10)15-16-6-9(17-15)8-4-2-1-3-5-8/h1-6,10-14,18-21H,7H2,(H,16,17)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using varying levels of glucose-1-phospha... |
Eur J Med Chem 123: 737-745 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.049
BindingDB Entry DOI: 10.7270/Q2TT4SXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM86673

(ATC 0065 | ATC0065 | CAS_510732-84-0)Show SMILES CN(C)c1nc(N[C@@H]2CC[C@@H](CC2)NCCc2ccc(Br)cc2OC(F)(F)F)nc2ccccc12 |r,wU:10.13,7.6,(-7.34,-2.98,;-7.34,-1.44,;-8.67,-.67,;-6,-.67,;-4.67,-1.44,;-3.33,-.67,;-2,-1.44,;-.67,-.67,;.67,-1.44,;2,-.67,;2,.87,;.67,1.64,;-.67,.87,;3.33,1.64,;4.67,.87,;6,1.64,;7.34,.87,;7.34,-.67,;8.67,-1.44,;10,-.67,;11.34,-1.44,;10,.87,;8.67,1.64,;8.67,3.18,;10,3.95,;10.77,2.62,;9.23,5.29,;11.34,4.72,;-3.33,.87,;-4.67,1.64,;-4.67,3.18,;-6,3.95,;-7.34,3.18,;-7.34,1.64,;-6,.87,)| Show InChI InChI=1S/C25H29BrF3N5O/c1-34(2)23-20-5-3-4-6-21(20)32-24(33-23)31-19-11-9-18(10-12-19)30-14-13-16-7-8-17(26)15-22(16)35-25(27,28)29/h3-8,15,18-19,30H,9-14H2,1-2H3,(H,31,32,33)/t18-,19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 831-9 (2005)
Article DOI: 10.1124/jpet.104.081711
BindingDB Entry DOI: 10.7270/Q2DB80D6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50148915

(CHEMBL3770514)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C15H18N2O5/c18-7-10-11(19)12(20)13(21)14(22-10)15-16-6-9(17-15)8-4-2-1-3-5-8/h1-6,10-14,18-21H,7H2,(H,16,17)/t10-,11-,12+,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase-b using alpha-D-glucose-1-phosphate as substrate by Dixon plot analysis |
ACS Med Chem Lett 6: 1215-9 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00361
BindingDB Entry DOI: 10.7270/Q2W37Z56 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50263773

(1-(2-naphthoyl)-3-((2R,3R,4S,5S,6R)-3,4,5-trihydro...)Show SMILES OC[C@H]1O[C@@H](NC(=O)NC(=O)c2ccc3ccccc3c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C18H20N2O7/c21-8-12-13(22)14(23)15(24)17(27-12)20-18(26)19-16(25)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-15,17,21-24H,8H2,(H2,19,20,25,26)/t12-,13-,14+,15-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b |
Bioorg Med Chem 17: 4773-85 (2009)
Article DOI: 10.1016/j.bmc.2009.04.036
BindingDB Entry DOI: 10.7270/Q2Q52PPV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50437354

(CHEMBL2408225)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H19N3O5/c22-8-12-13(23)14(24)15(25)16(26-12)18-19-17(20-21-18)11-6-5-9-3-1-2-4-10(9)7-11/h1-7,12-16,22-25H,8H2,(H,19,20,21)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b |
Eur J Med Chem 76: 567-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.02.041
BindingDB Entry DOI: 10.7270/Q2ZP47N4 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50009082
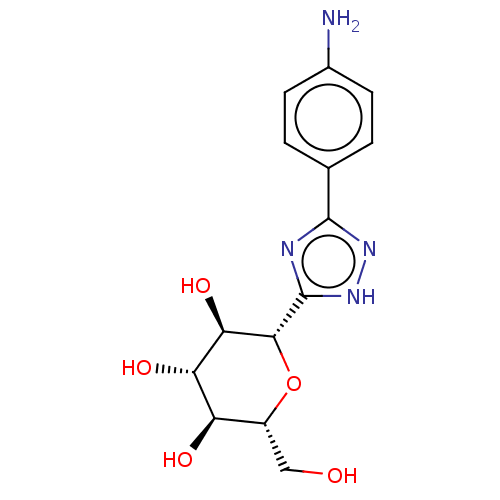
(CHEMBL3237972)Show SMILES Nc1ccc(cc1)-c1n[nH]c(n1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H18N4O5/c15-7-3-1-6(2-4-7)13-16-14(18-17-13)12-11(22)10(21)9(20)8(5-19)23-12/h1-4,8-12,19-22H,5,15H2,(H,16,17,18)/t8-,9-,10+,11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b |
Eur J Med Chem 76: 567-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.02.041
BindingDB Entry DOI: 10.7270/Q2ZP47N4 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312225
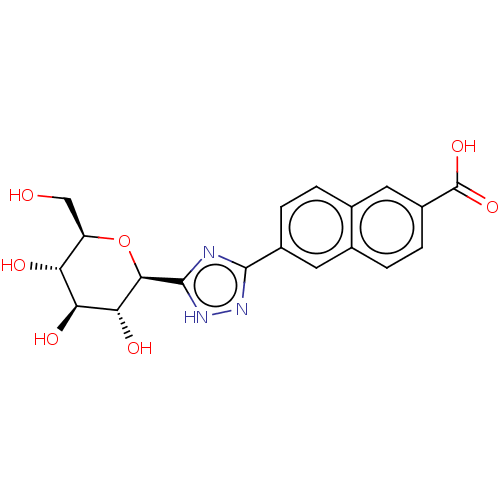
(CHEMBL4161353)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc2cc(ccc2c1)C(O)=O |r| Show InChI InChI=1S/C19H19N3O7/c23-7-12-13(24)14(25)15(26)16(29-12)18-20-17(21-22-18)10-3-1-9-6-11(19(27)28)4-2-8(9)5-10/h1-6,12-16,23-26H,7H2,(H,27,28)(H,20,21,22)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312361

(CHEMBL4169281)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc-2c(Cc3ccccc-23)c1 |r| Show InChI InChI=1S/C21H21N3O5/c25-9-15-16(26)17(27)18(28)19(29-15)21-22-20(23-24-21)11-5-6-14-12(8-11)7-10-3-1-2-4-13(10)14/h1-6,8,15-19,25-28H,7,9H2,(H,22,23,24)/t15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50313014

(CHEMBL4172670)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C21H21N3O7/c25-9-14-15(26)16(27)17(28)18(31-14)20-22-19(23-24-20)12-5-1-10(2-6-12)11-3-7-13(8-4-11)21(29)30/h1-8,14-18,25-28H,9H2,(H,29,30)(H,22,23,24)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312361

(CHEMBL4169281)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc-2c(Cc3ccccc-23)c1 |r| Show InChI InChI=1S/C21H21N3O5/c25-9-15-16(26)17(27)18(28)19(29-15)21-22-20(23-24-21)11-5-6-14-12(8-11)7-10-3-1-2-4-13(10)14/h1-6,8,15-19,25-28H,7,9H2,(H,22,23,24)/t15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-b assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50437355
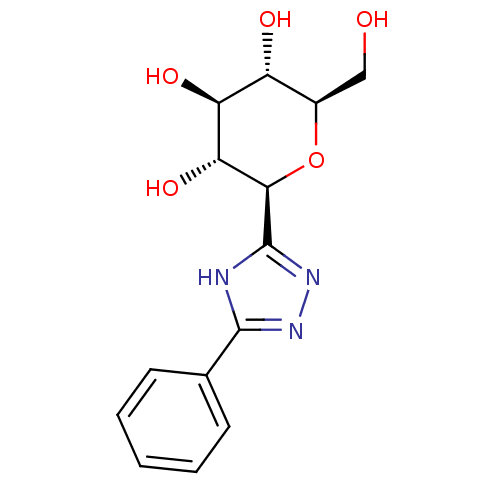
(CHEMBL2408224)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nnc([nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C14H17N3O5/c18-6-8-9(19)10(20)11(21)12(22-8)14-15-13(16-17-14)7-4-2-1-3-5-7/h1-5,8-12,18-21H,6H2,(H,15,16,17)/t8-,9-,10+,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa using Glc-1-P as substrate incubated for 15 mins |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115196
BindingDB Entry DOI: 10.7270/Q25X2DGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50009074

(CHEMBL3237966)Show SMILES Cc1ccc(cc1)-c1n[nH]c(n1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H19N3O5/c1-7-2-4-8(5-3-7)14-16-15(18-17-14)13-12(22)11(21)10(20)9(6-19)23-13/h2-5,9-13,19-22H,6H2,1H3,(H,16,17,18)/t9-,10-,11+,12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b |
Eur J Med Chem 76: 567-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.02.041
BindingDB Entry DOI: 10.7270/Q2ZP47N4 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50009080

(CHEMBL3237970)Show SMILES COc1ccc(cc1)-c1n[nH]c(n1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H19N3O6/c1-23-8-4-2-7(3-5-8)14-16-15(18-17-14)13-12(22)11(21)10(20)9(6-19)24-13/h2-5,9-13,19-22H,6H2,1H3,(H,16,17,18)/t9-,10-,11+,12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b |
Eur J Med Chem 76: 567-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.02.041
BindingDB Entry DOI: 10.7270/Q2ZP47N4 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50312361

(CHEMBL4169281)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc-2c(Cc3ccccc-23)c1 |r| Show InChI InChI=1S/C21H21N3O5/c25-9-15-16(26)17(27)18(28)19(29-15)21-22-20(23-24-21)11-5-6-14-12(8-11)7-10-3-1-2-4-13(10)14/h1-6,8,15-19,25-28H,7,9H2,(H,22,23,24)/t15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of g... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50312362
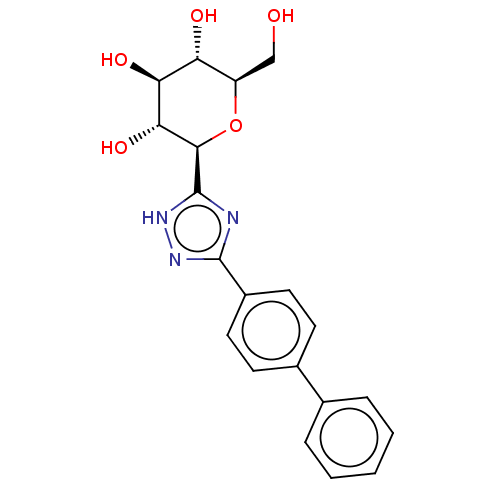
(CHEMBL4176460)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc(n[nH]1)-c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C20H21N3O5/c24-10-14-15(25)16(26)17(27)18(28-14)20-21-19(22-23-20)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-18,24-27H,10H2,(H,21,22,23)/t14-,15-,16+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of... |
Eur J Med Chem 147: 266-278 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.095
BindingDB Entry DOI: 10.7270/Q2J67KF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50009066
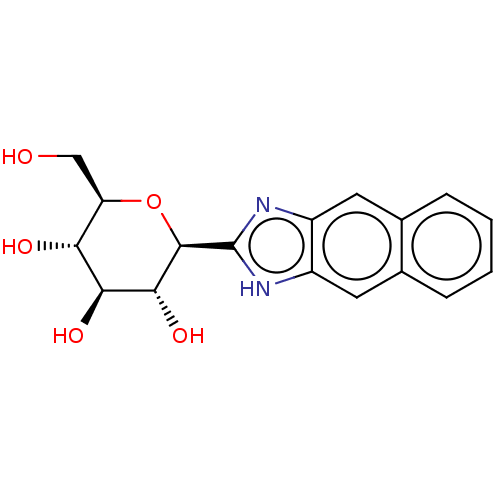
(CHEMBL3237963)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1nc2cc3ccccc3cc2[nH]1 |r| Show InChI InChI=1S/C17H18N2O5/c20-7-12-13(21)14(22)15(23)16(24-12)17-18-10-5-8-3-1-2-4-9(8)6-11(10)19-17/h1-6,12-16,20-23H,7H2,(H,18,19)/t12-,13-,14+,15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase b |
Eur J Med Chem 76: 567-79 (2014)
Article DOI: 10.1016/j.ejmech.2014.02.041
BindingDB Entry DOI: 10.7270/Q2ZP47N4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data