 Found 211 hits with Last Name = 'kuntz' and Initial = 'id'
Found 211 hits with Last Name = 'kuntz' and Initial = 'id' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin D
(Homo sapiens (Human)) | BDBM50076285
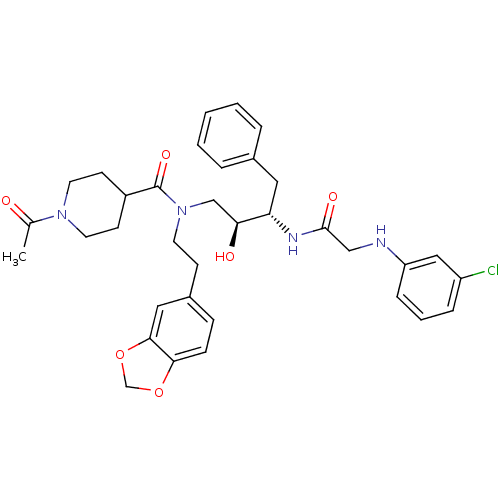
(1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N(CCc1ccc2OCOc2c1)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)CNc1cccc(Cl)c1 Show InChI InChI=1S/C35H41ClN4O6/c1-24(41)39-16-13-27(14-17-39)35(44)40(15-12-26-10-11-32-33(19-26)46-23-45-32)22-31(42)30(18-25-6-3-2-4-7-25)38-34(43)21-37-29-9-5-8-28(36)20-29/h2-11,19-20,27,30-31,37,42H,12-18,21-23H2,1H3,(H,38,43)/t30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50076294
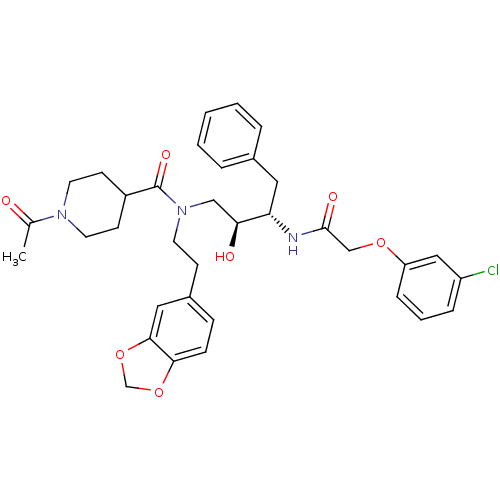
(1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N(CCc1ccc2OCOc2c1)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(Cl)c1 Show InChI InChI=1S/C35H40ClN3O7/c1-24(40)38-16-13-27(14-17-38)35(43)39(15-12-26-10-11-32-33(19-26)46-23-45-32)21-31(41)30(18-25-6-3-2-4-7-25)37-34(42)22-44-29-9-5-8-28(36)20-29/h2-11,19-20,27,30-31,41H,12-18,21-23H2,1H3,(H,37,42)/t30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076291
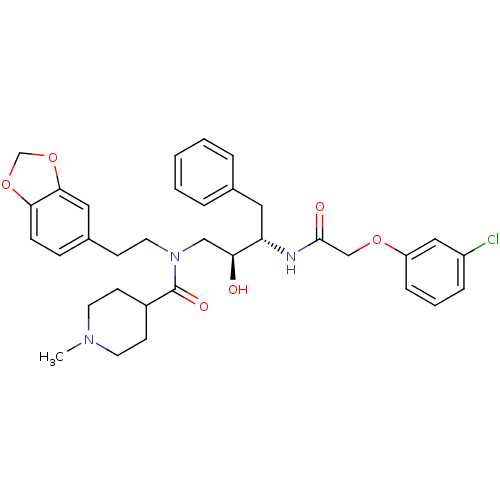
(1-Methyl-piperidine-4-carboxylic acid (2-benzo[1,3...)Show SMILES CN1CCC(CC1)C(=O)N(CCc1ccc2OCOc2c1)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(Cl)c1 Show InChI InChI=1S/C34H40ClN3O6/c1-37-15-13-26(14-16-37)34(41)38(17-12-25-10-11-31-32(19-25)44-23-43-31)21-30(39)29(18-24-6-3-2-4-7-24)36-33(40)22-42-28-9-5-8-27(35)20-28/h2-11,19-20,26,29-30,39H,12-18,21-23H2,1H3,(H,36,40)/t29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076292
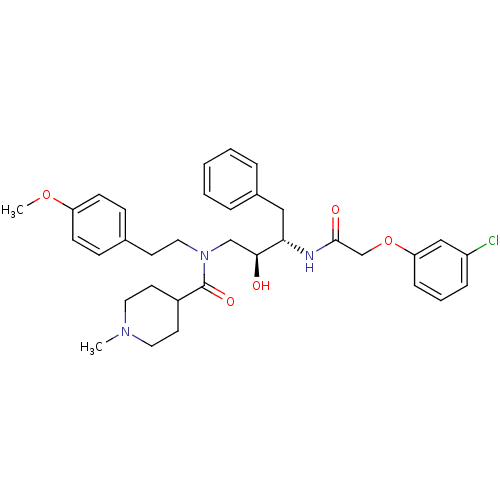
(1-Methyl-piperidine-4-carboxylic acid {(2S,3S)-3-[...)Show SMILES COc1ccc(CCN(C[C@H](O)[C@H](Cc2ccccc2)NC(=O)COc2cccc(Cl)c2)C(=O)C2CCN(C)CC2)cc1 Show InChI InChI=1S/C34H42ClN3O5/c1-37-18-16-27(17-19-37)34(41)38(20-15-25-11-13-29(42-2)14-12-25)23-32(39)31(21-26-7-4-3-5-8-26)36-33(40)24-43-30-10-6-9-28(35)22-30/h3-14,22,27,31-32,39H,15-21,23-24H2,1-2H3,(H,36,40)/t31-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50084626
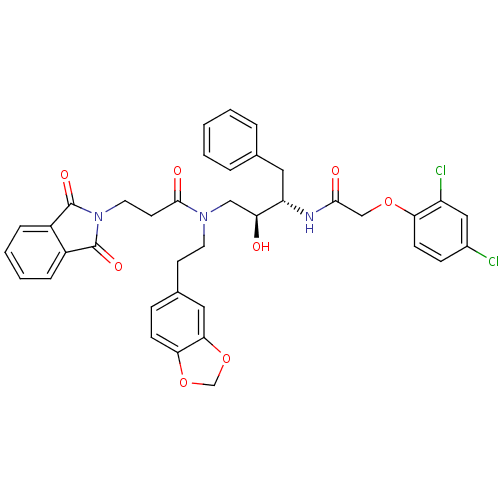
(CHEMBL284440 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N...)Show SMILES O[C@@H](CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@H](Cc1ccccc1)NC(=O)COc1ccc(Cl)cc1Cl Show InChI InChI=1S/C38H35Cl2N3O8/c39-26-11-13-32(29(40)20-26)49-22-35(45)41-30(18-24-6-2-1-3-7-24)31(44)21-42(16-14-25-10-12-33-34(19-25)51-23-50-33)36(46)15-17-43-37(47)27-8-4-5-9-28(27)38(43)48/h1-13,19-20,30-31,44H,14-18,21-23H2,(H,41,45)/t30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human Cathepsin D |
J Med Chem 45: 1412-9 (2002)
BindingDB Entry DOI: 10.7270/Q25T3JSH |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076285

(1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N(CCc1ccc2OCOc2c1)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)CNc1cccc(Cl)c1 Show InChI InChI=1S/C35H41ClN4O6/c1-24(41)39-16-13-27(14-17-39)35(44)40(15-12-26-10-11-32-33(19-26)46-23-45-32)22-31(42)30(18-25-6-3-2-4-7-25)38-34(43)21-37-29-9-5-8-28(36)20-29/h2-11,19-20,27,30-31,37,42H,12-18,21-23H2,1H3,(H,38,43)/t30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076294

(1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N(CCc1ccc2OCOc2c1)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(Cl)c1 Show InChI InChI=1S/C35H40ClN3O7/c1-24(40)38-16-13-27(14-17-38)35(43)39(15-12-26-10-11-32-33(19-26)46-23-45-32)21-31(41)30(18-25-6-3-2-4-7-25)37-34(42)22-44-29-9-5-8-28(36)20-29/h2-11,19-20,27,30-31,41H,12-18,21-23H2,1H3,(H,37,42)/t30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076287
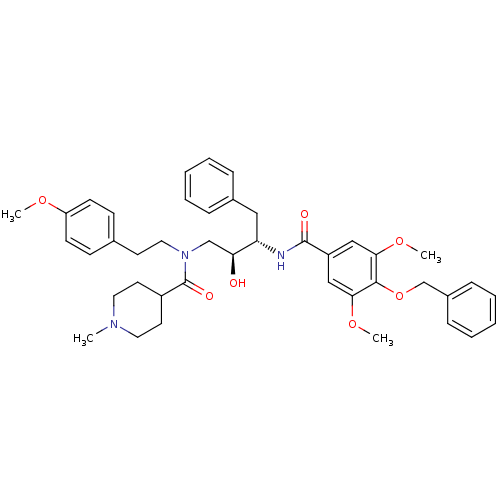
(1-Methyl-piperidine-4-carboxylic acid [(2S,3S)-3-(...)Show SMILES COc1ccc(CCN(C[C@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(OC)c(OCc3ccccc3)c(OC)c2)C(=O)C2CCN(C)CC2)cc1 Show InChI InChI=1S/C42H51N3O7/c1-44-22-20-33(21-23-44)42(48)45(24-19-30-15-17-35(49-2)18-16-30)28-37(46)36(25-31-11-7-5-8-12-31)43-41(47)34-26-38(50-3)40(39(27-34)51-4)52-29-32-13-9-6-10-14-32/h5-18,26-27,33,36-37,46H,19-25,28-29H2,1-4H3,(H,43,47)/t36-,37-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50076293
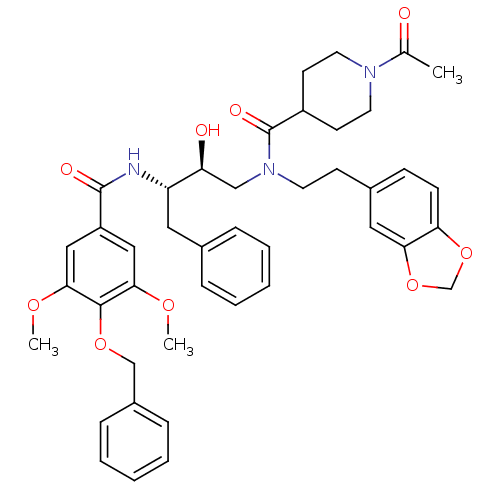
(1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...)Show SMILES COc1cc(cc(OC)c1OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(CCc1ccc2OCOc2c1)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C43H49N3O9/c1-29(47)45-20-17-33(18-21-45)43(50)46(19-16-31-14-15-37-38(23-31)55-28-54-37)26-36(48)35(22-30-10-6-4-7-11-30)44-42(49)34-24-39(51-2)41(40(25-34)52-3)53-27-32-12-8-5-9-13-32/h4-15,23-25,33,35-36,48H,16-22,26-28H2,1-3H3,(H,44,49)/t35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM8019

(2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...)Show SMILES COc1ccc(CCN(C[C@H](O)[C@H](Cc2ccccc2)NC(=O)COc2cccc(Cl)c2)C(=O)C2CCNCC2)cc1 |r| Show InChI InChI=1S/C33H40ClN3O5/c1-41-28-12-10-24(11-13-28)16-19-37(33(40)26-14-17-35-18-15-26)22-31(38)30(20-25-6-3-2-4-7-25)36-32(39)23-42-29-9-5-8-27(34)21-29/h2-13,21,26,30-31,35,38H,14-20,22-23H2,1H3,(H,36,39)/t30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076293

(1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...)Show SMILES COc1cc(cc(OC)c1OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(CCc1ccc2OCOc2c1)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C43H49N3O9/c1-29(47)45-20-17-33(18-21-45)43(50)46(19-16-31-14-15-37-38(23-31)55-28-54-37)26-36(48)35(22-30-10-6-4-7-11-30)44-42(49)34-24-39(51-2)41(40(25-34)52-3)53-27-32-12-8-5-9-13-32/h4-15,23-25,33,35-36,48H,16-22,26-28H2,1-3H3,(H,44,49)/t35-,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50110934

(CHEMBL30483 | N-((1S,3S)-1-Benzyl-3-{[2-(2,4-dichl...)Show SMILES COc1cc(Br)c(cc1OC)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(CCc1ccc(Cl)cc1Cl)C(=O)CCN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C38H36BrCl2N3O7/c1-50-33-20-28(29(39)21-34(33)51-2)36(47)42-31(18-23-8-4-3-5-9-23)32(45)22-43(16-14-24-12-13-25(40)19-30(24)41)35(46)15-17-44-37(48)26-10-6-7-11-27(26)38(44)49/h3-13,19-21,31-32,45H,14-18,22H2,1-2H3,(H,42,47)/t31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human Cathepsin D |
J Med Chem 45: 1412-9 (2002)
BindingDB Entry DOI: 10.7270/Q25T3JSH |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50076291

(1-Methyl-piperidine-4-carboxylic acid (2-benzo[1,3...)Show SMILES CN1CCC(CC1)C(=O)N(CCc1ccc2OCOc2c1)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(Cl)c1 Show InChI InChI=1S/C34H40ClN3O6/c1-37-15-13-26(14-16-37)34(41)38(17-12-25-10-11-31-32(19-25)44-23-43-31)21-30(39)29(18-24-6-3-2-4-7-24)36-33(40)22-42-28-9-5-8-27(35)20-28/h2-11,19-20,26,29-30,39H,12-18,21-23H2,1H3,(H,36,40)/t29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36570
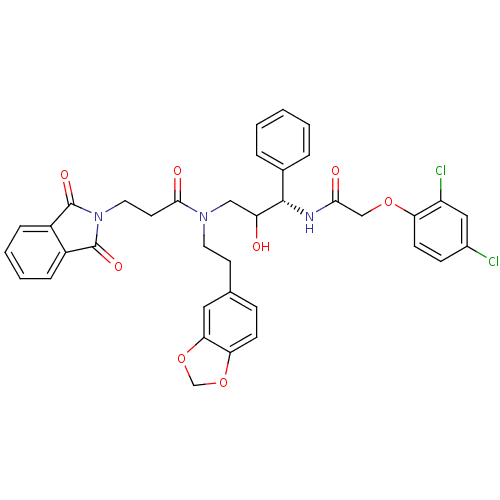
(EHM)Show SMILES OC(CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@@H](NC(=O)COc1ccc(Cl)cc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C37H33Cl2N3O8/c38-25-11-13-30(28(39)19-25)48-21-33(44)40-35(24-6-2-1-3-7-24)29(43)20-41(16-14-23-10-12-31-32(18-23)50-22-49-31)34(45)15-17-42-36(46)26-8-4-5-9-27(26)37(42)47/h1-13,18-19,29,35,43H,14-17,20-22H2,(H,40,44)/t29?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | 14 | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076288
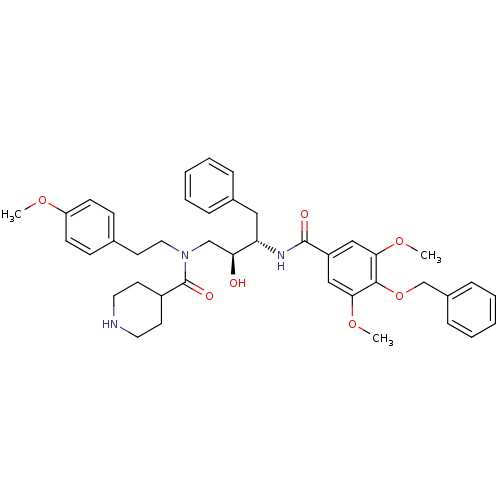
(CHEMBL284955 | Piperidine-4-carboxylic acid [(2S,3...)Show SMILES COc1ccc(CCN(C[C@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(OC)c(OCc3ccccc3)c(OC)c2)C(=O)C2CCNCC2)cc1 Show InChI InChI=1S/C41H49N3O7/c1-48-34-16-14-29(15-17-34)20-23-44(41(47)32-18-21-42-22-19-32)27-36(45)35(24-30-10-6-4-7-11-30)43-40(46)33-25-37(49-2)39(38(26-33)50-3)51-28-31-12-8-5-9-13-31/h4-17,25-26,32,35-36,42,45H,18-24,27-28H2,1-3H3,(H,43,46)/t35-,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50076292

(1-Methyl-piperidine-4-carboxylic acid {(2S,3S)-3-[...)Show SMILES COc1ccc(CCN(C[C@H](O)[C@H](Cc2ccccc2)NC(=O)COc2cccc(Cl)c2)C(=O)C2CCN(C)CC2)cc1 Show InChI InChI=1S/C34H42ClN3O5/c1-37-18-16-27(17-19-37)34(41)38(20-15-25-11-13-29(42-2)14-12-25)23-32(39)31(21-26-7-4-3-5-8-26)36-33(40)24-43-30-10-6-9-28(35)22-30/h3-14,22,27,31-32,39H,15-21,23-24H2,1-2H3,(H,36,40)/t31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36569
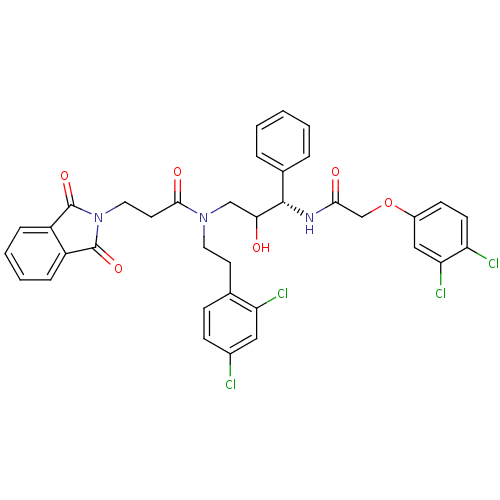
(FHO)Show SMILES OC(CN(CCc1ccc(Cl)cc1Cl)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@@H](NC(=O)COc1ccc(Cl)c(Cl)c1)c1ccccc1 |r| Show InChI InChI=1S/C36H31Cl4N3O6/c37-24-11-10-22(29(39)18-24)14-16-42(33(46)15-17-43-35(47)26-8-4-5-9-27(26)36(43)48)20-31(44)34(23-6-2-1-3-7-23)41-32(45)21-49-25-12-13-28(38)30(40)19-25/h1-13,18-19,31,34,44H,14-17,20-21H2,(H,41,45)/t31?,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | 18 | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36567

(EHO)Show SMILES OC(CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@@H](NC(=O)COc1ccc(Cl)c(Cl)c1)c1ccccc1 |r| Show InChI InChI=1S/C37H33Cl2N3O8/c38-28-12-11-25(19-29(28)39)48-21-33(44)40-35(24-6-2-1-3-7-24)30(43)20-41(16-14-23-10-13-31-32(18-23)50-22-49-31)34(45)15-17-42-36(46)26-8-4-5-9-27(26)37(42)47/h1-13,18-19,30,35,43H,14-17,20-22H2,(H,40,44)/t30?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | 19 | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36571
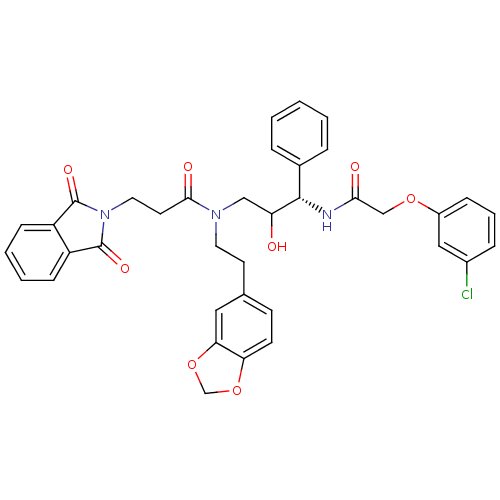
(EHR)Show SMILES OC(CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@@H](NC(=O)COc1cccc(Cl)c1)c1ccccc1 |r| Show InChI InChI=1S/C37H34ClN3O8/c38-26-9-6-10-27(20-26)47-22-33(43)39-35(25-7-2-1-3-8-25)30(42)21-40(17-15-24-13-14-31-32(19-24)49-23-48-31)34(44)16-18-41-36(45)28-11-4-5-12-29(28)37(41)46/h1-14,19-20,30,35,42H,15-18,21-23H2,(H,39,43)/t30?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | 20 | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50076286
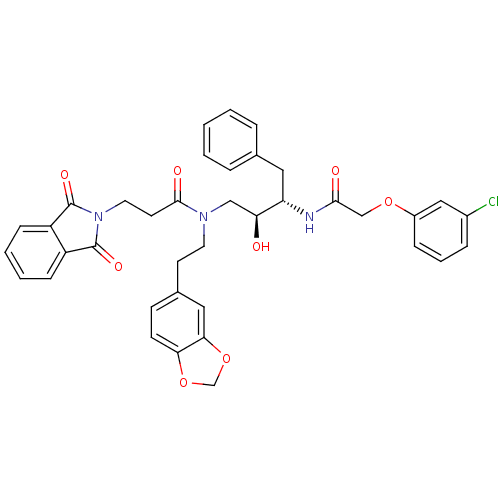
(CHEMBL34051 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N-...)Show SMILES O[C@@H](CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(Cl)c1 Show InChI InChI=1S/C38H36ClN3O8/c39-27-9-6-10-28(21-27)48-23-35(44)40-31(19-25-7-2-1-3-8-25)32(43)22-41(17-15-26-13-14-33-34(20-26)50-24-49-33)36(45)16-18-42-37(46)29-11-4-5-12-30(29)38(42)47/h1-14,20-21,31-32,43H,15-19,22-24H2,(H,40,44)/t31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076341

((S)-N-Methyl-3-pentafluorophenyl-2-[3-(5-thioxo-4,...)Show SMILES CNC(=O)[C@H](Cc1c(F)c(F)c(F)c(F)c1F)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C13H10F5N5O2S2/c1-19-10(24)4(20-11(25)21-12-22-23-13(26)27-12)2-3-5(14)7(16)9(18)8(17)6(3)15/h4H,2H2,1H3,(H,19,24)(H,23,26)(H2,20,21,22,25)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (MMP-3) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50076287

(1-Methyl-piperidine-4-carboxylic acid [(2S,3S)-3-(...)Show SMILES COc1ccc(CCN(C[C@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(OC)c(OCc3ccccc3)c(OC)c2)C(=O)C2CCN(C)CC2)cc1 Show InChI InChI=1S/C42H51N3O7/c1-44-22-20-33(21-23-44)42(48)45(24-19-30-15-17-35(49-2)18-16-30)28-37(46)36(25-31-11-7-5-8-12-31)43-41(47)34-26-38(50-3)40(39(27-34)51-4)52-29-32-13-9-6-10-14-32/h5-18,26-27,33,36-37,46H,19-25,28-29H2,1-4H3,(H,43,47)/t36-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36572
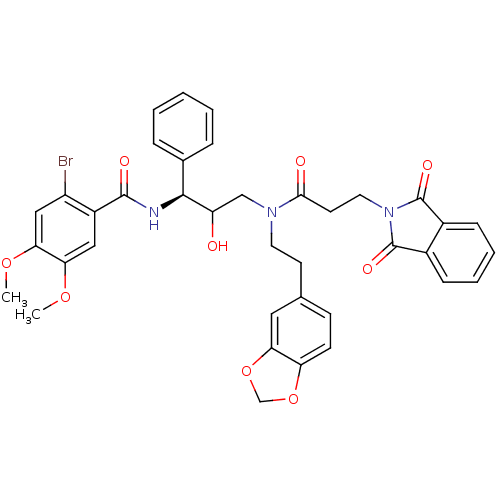
(EHS)Show SMILES COc1cc(Br)c(cc1OC)C(=O)N[C@H](C(O)CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)c1ccccc1 |r| Show InChI InChI=1S/C38H36BrN3O9/c1-48-31-19-27(28(39)20-32(31)49-2)36(45)40-35(24-8-4-3-5-9-24)29(43)21-41(16-14-23-12-13-30-33(18-23)51-22-50-30)34(44)15-17-42-37(46)25-10-6-7-11-26(25)38(42)47/h3-13,18-20,29,35,43H,14-17,21-22H2,1-2H3,(H,40,45)/t29?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | 64 | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM8019

(2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...)Show SMILES COc1ccc(CCN(C[C@H](O)[C@H](Cc2ccccc2)NC(=O)COc2cccc(Cl)c2)C(=O)C2CCNCC2)cc1 |r| Show InChI InChI=1S/C33H40ClN3O5/c1-41-28-12-10-24(11-13-28)16-19-37(33(40)26-14-17-35-18-15-26)22-31(38)30(20-25-6-3-2-4-7-25)36-32(39)23-42-29-9-5-8-27(34)21-29/h2-13,21,26,30-31,35,38H,14-20,22-23H2,1H3,(H,36,39)/t30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50076288

(CHEMBL284955 | Piperidine-4-carboxylic acid [(2S,3...)Show SMILES COc1ccc(CCN(C[C@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(OC)c(OCc3ccccc3)c(OC)c2)C(=O)C2CCNCC2)cc1 Show InChI InChI=1S/C41H49N3O7/c1-48-34-16-14-29(15-17-34)20-23-44(41(47)32-18-21-42-22-19-32)27-36(45)35(24-30-10-6-4-7-11-30)43-40(46)33-25-37(49-2)39(38(26-33)50-3)51-28-31-12-8-5-9-13-31/h4-17,25-26,32,35-36,42,45H,18-24,27-28H2,1-3H3,(H,43,46)/t35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against human liver cathepsin D |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36559
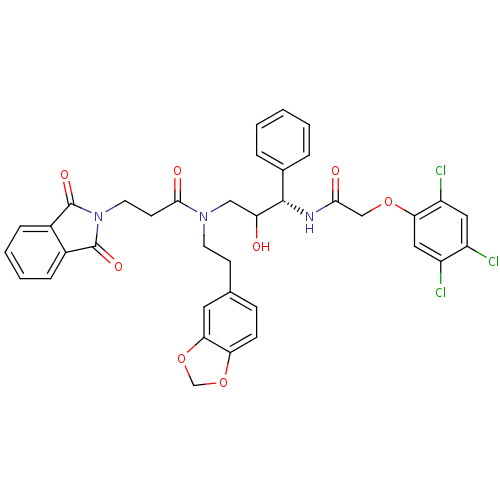
(EHD)Show SMILES OC(CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@@H](NC(=O)COc1cc(Cl)c(Cl)cc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C37H32Cl3N3O8/c38-26-17-28(40)31(18-27(26)39)49-20-33(45)41-35(23-6-2-1-3-7-23)29(44)19-42(14-12-22-10-11-30-32(16-22)51-21-50-30)34(46)13-15-43-36(47)24-8-4-5-9-25(24)37(43)48/h1-11,16-18,29,35,44H,12-15,19-21H2,(H,41,45)/t29?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50110932
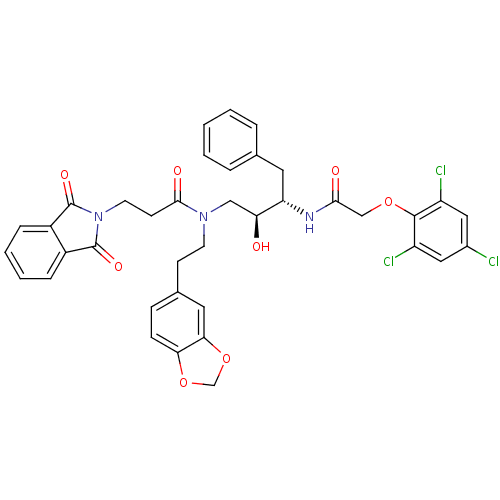
(CHEMBL283863 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-3...)Show SMILES O[C@@H](CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@H](Cc1ccccc1)NC(=O)COc1c(Cl)cc(Cl)cc1Cl Show InChI InChI=1S/C38H34Cl3N3O8/c39-25-18-28(40)36(29(41)19-25)50-21-34(46)42-30(16-23-6-2-1-3-7-23)31(45)20-43(14-12-24-10-11-32-33(17-24)52-22-51-32)35(47)13-15-44-37(48)26-8-4-5-9-27(26)38(44)49/h1-11,17-19,30-31,45H,12-16,20-22H2,(H,42,46)/t30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human Cathepsin D |
J Med Chem 45: 1412-9 (2002)
BindingDB Entry DOI: 10.7270/Q25T3JSH |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076289

(CHEMBL32997 | N-((1S,2S)-3-{(2-Benzo[1,3]dioxol-5-...)Show SMILES COc1cc(cc(OC)c1OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C46H45N3O10/c1-55-40-25-33(26-41(56-2)43(40)57-28-32-13-7-4-8-14-32)44(52)47-36(23-30-11-5-3-6-12-30)37(50)27-48(21-19-31-17-18-38-39(24-31)59-29-58-38)42(51)20-22-49-45(53)34-15-9-10-16-35(34)46(49)54/h3-18,24-26,36-37,50H,19-23,27-29H2,1-2H3,(H,47,52)/t36-,37-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin D
(Homo sapiens (Human)) | BDBM36561

(EHJ)Show SMILES COc1ccc(C(=O)N[C@H](C(O)CN(CCc2ccc3OCOc3c2)C(=O)CCN2C(=O)c3ccccc3C2=O)c2ccccc2)c(Cl)c1OC |r| Show InChI InChI=1S/C38H36ClN3O9/c1-48-30-15-13-27(33(39)35(30)49-2)36(45)40-34(24-8-4-3-5-9-24)28(43)21-41(18-16-23-12-14-29-31(20-23)51-22-50-29)32(44)17-19-42-37(46)25-10-6-7-11-26(25)38(42)47/h3-15,20,28,34,43H,16-19,21-22H2,1-2H3,(H,40,45)/t28?,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36562
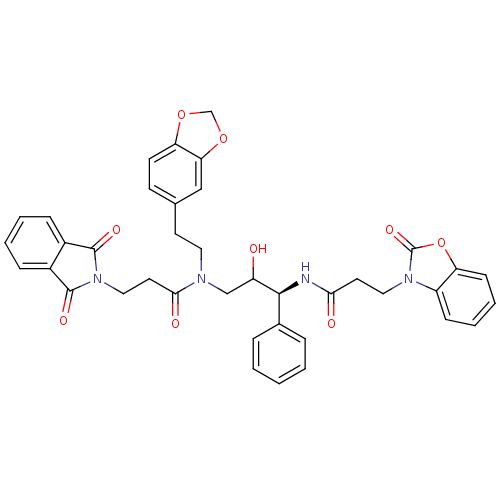
(EHA)Show SMILES OC(CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@@H](NC(=O)CCn1c2ccccc2oc1=O)c1ccccc1 |r| Show InChI InChI=1S/C39H36N4O9/c44-30(36(26-8-2-1-3-9-26)40-34(45)17-20-42-29-12-6-7-13-31(29)52-39(42)49)23-41(19-16-25-14-15-32-33(22-25)51-24-50-32)35(46)18-21-43-37(47)27-10-4-5-11-28(27)38(43)48/h1-15,22,30,36,44H,16-21,23-24H2,(H,40,45)/t30?,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36563
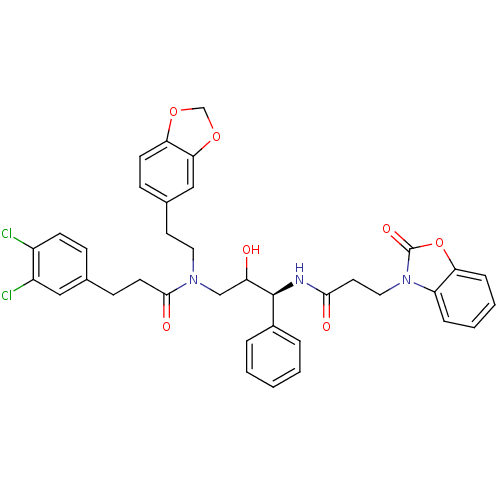
(EFA)Show SMILES OC(CN(CCc1ccc2OCOc2c1)C(=O)CCc1ccc(Cl)c(Cl)c1)[C@@H](NC(=O)CCn1c2ccccc2oc1=O)c1ccccc1 |r| Show InChI InChI=1S/C37H35Cl2N3O7/c38-27-13-10-24(20-28(27)39)12-15-35(45)41(18-16-25-11-14-32-33(21-25)48-23-47-32)22-30(43)36(26-6-2-1-3-7-26)40-34(44)17-19-42-29-8-4-5-9-31(29)49-37(42)46/h1-11,13-14,20-21,30,36,43H,12,15-19,22-23H2,(H,40,44)/t30?,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 171 | n/a | n/a | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076286

(CHEMBL34051 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N-...)Show SMILES O[C@@H](CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(Cl)c1 Show InChI InChI=1S/C38H36ClN3O8/c39-27-9-6-10-28(21-27)48-23-35(44)40-31(19-25-7-2-1-3-8-25)32(43)22-41(17-15-26-13-14-33-34(20-26)50-24-49-33)36(45)16-18-42-37(46)29-11-4-5-12-30(29)38(42)47/h1-14,20-21,31-32,43H,15-19,22-24H2,(H,40,44)/t31-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin D
(Homo sapiens (Human)) | BDBM36573
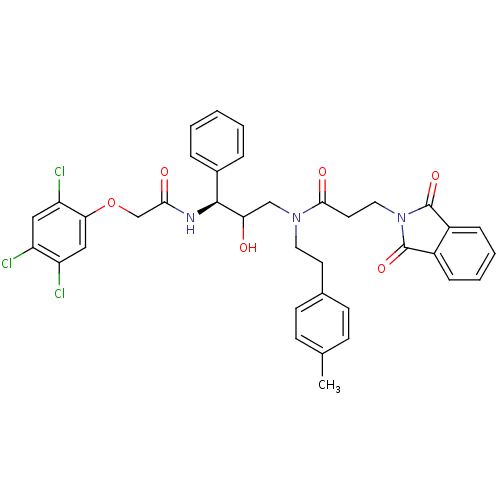
(UHD)Show SMILES Cc1ccc(CCN(CC(O)[C@@H](NC(=O)COc2cc(Cl)c(Cl)cc2Cl)c2ccccc2)C(=O)CCN2C(=O)c3ccccc3C2=O)cc1 |r| Show InChI InChI=1S/C37H34Cl3N3O6/c1-23-11-13-24(14-12-23)15-17-42(34(46)16-18-43-36(47)26-9-5-6-10-27(26)37(43)48)21-31(44)35(25-7-3-2-4-8-25)41-33(45)22-49-32-20-29(39)28(38)19-30(32)40/h2-14,19-20,31,35,44H,15-18,21-22H2,1H3,(H,41,45)/t31?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | 229 | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36564
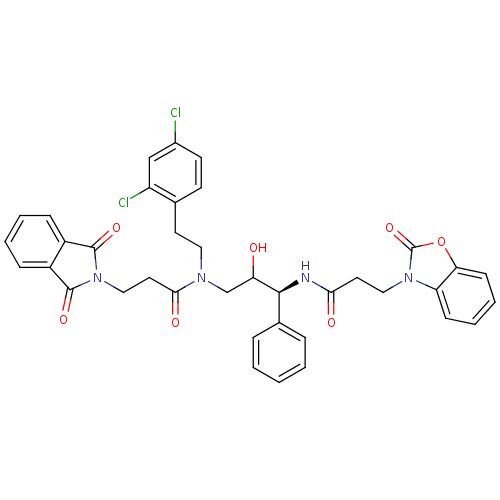
(FHA)Show SMILES OC(CN(CCc1ccc(Cl)cc1Cl)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@@H](NC(=O)CCn1c2ccccc2oc1=O)c1ccccc1 |r| Show InChI InChI=1S/C38H34Cl2N4O7/c39-26-15-14-24(29(40)22-26)16-19-42(34(47)18-21-44-36(48)27-10-4-5-11-28(27)37(44)49)23-31(45)35(25-8-2-1-3-9-25)41-33(46)17-20-43-30-12-6-7-13-32(30)51-38(43)50/h1-15,22,31,35,45H,16-21,23H2,(H,41,46)/t31?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50110931
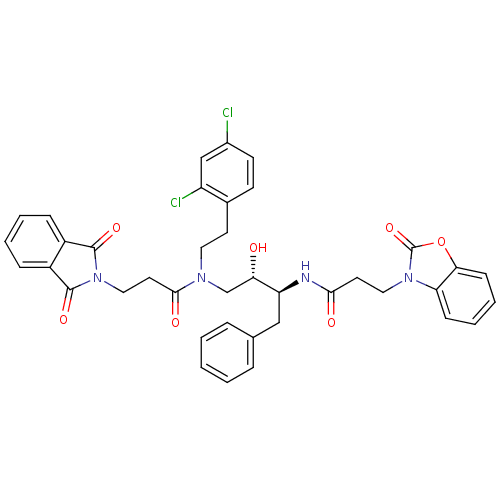
(CHEMBL284441 | N-((1S,3S)-1-Benzyl-3-{[2-(2,4-dich...)Show SMILES O[C@@H](CN(CCc1ccc(Cl)cc1Cl)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@H](Cc1ccccc1)NC(=O)CCn1c2ccccc2oc1=O Show InChI InChI=1S/C39H36Cl2N4O7/c40-27-15-14-26(30(41)23-27)16-19-43(36(48)18-21-45-37(49)28-10-4-5-11-29(28)38(45)50)24-33(46)31(22-25-8-2-1-3-9-25)42-35(47)17-20-44-32-12-6-7-13-34(32)52-39(44)51/h1-15,23,31,33,46H,16-22,24H2,(H,42,47)/t31-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human Cathepsin D |
J Med Chem 45: 1412-9 (2002)
BindingDB Entry DOI: 10.7270/Q25T3JSH |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50076290

(CHEMBL284151 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N...)Show SMILES Cc1ccc(C)c(OCC(=O)N[C@@H](Cc2ccccc2)[C@@H](O)CN(CCc2ccc3OCOc3c2)C(=O)CCN2C(=O)c3ccccc3C2=O)c1 Show InChI InChI=1S/C40H41N3O8/c1-26-12-13-27(2)35(20-26)49-24-37(45)41-32(21-28-8-4-3-5-9-28)33(44)23-42(18-16-29-14-15-34-36(22-29)51-25-50-34)38(46)17-19-43-39(47)30-10-6-7-11-31(30)40(43)48/h3-15,20,22,32-33,44H,16-19,21,23-25H2,1-2H3,(H,41,45)/t32-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmepsin 2 |
J Med Chem 42: 1428-40 (1999)
Article DOI: 10.1021/jm980641t
BindingDB Entry DOI: 10.7270/Q2MS3RZX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin D
(Homo sapiens (Human)) | BDBM36565
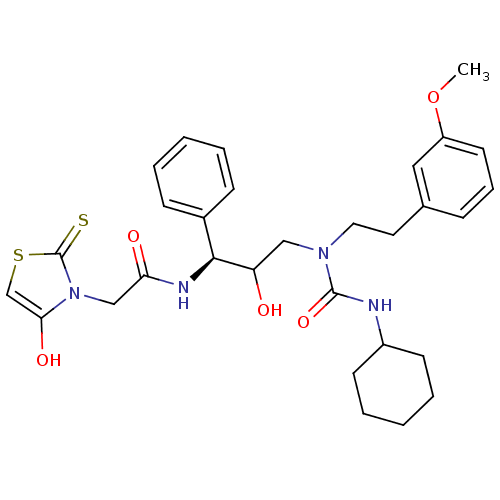
(FBB)Show SMILES COc1cccc(CCN(CC(O)[C@@H](NC(=O)Cn2c(O)csc2=S)c2ccccc2)C(=O)NC2CCCCC2)c1 |r| Show InChI InChI=1S/C30H38N4O5S2/c1-39-24-14-8-9-21(17-24)15-16-33(29(38)31-23-12-6-3-7-13-23)18-25(35)28(22-10-4-2-5-11-22)32-26(36)19-34-27(37)20-41-30(34)40/h2,4-5,8-11,14,17,20,23,25,28,35,37H,3,6-7,12-13,15-16,18-19H2,1H3,(H,31,38)(H,32,36)/t25?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 356 | n/a | n/a | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM36566

(FDB)Show SMILES CCCCCCC(=O)N(CCc1cccc(OC)c1)CC(O)[C@@H](NC(=O)Cn1c(O)csc1=S)c1ccccc1 |r| Show InChI InChI=1S/C30H39N3O5S2/c1-3-4-5-9-15-27(36)32(17-16-22-11-10-14-24(18-22)38-2)19-25(34)29(23-12-7-6-8-13-23)31-26(35)20-33-28(37)21-40-30(33)39/h6-8,10-14,18,21,25,29,34,37H,3-5,9,15-17,19-20H2,1-2H3,(H,31,35)/t25?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 595 | n/a | n/a | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241372
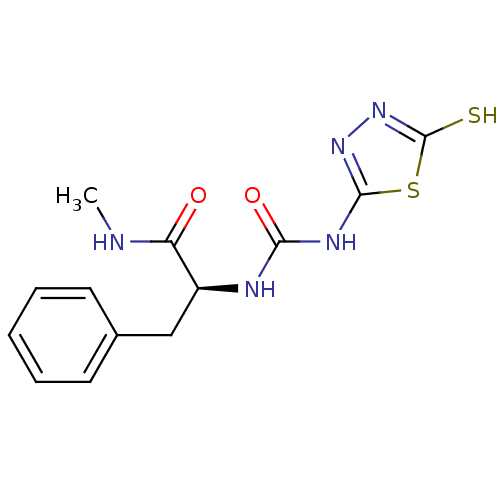
((S)-1-(1-(methylamino)-1-oxo-3-phenylpropan-2-yl)-...)Show InChI InChI=1S/C13H15N5O2S2/c1-14-10(19)9(7-8-5-3-2-4-6-8)15-11(20)16-12-17-18-13(21)22-12/h2-6,9H,7H2,1H3,(H,14,19)(H,18,21)(H2,15,16,17,20)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (MMP-3) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076370
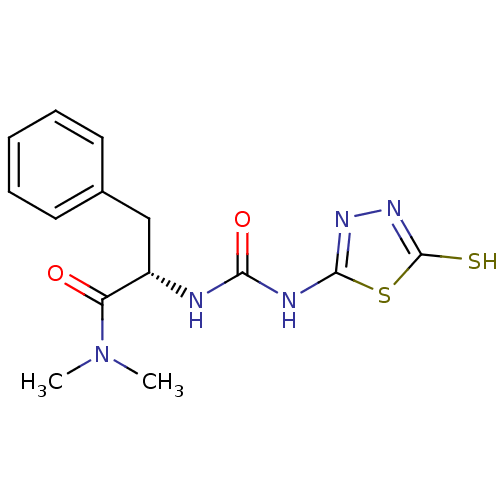
((S)-N,N-Dimethyl-3-phenyl-2-[3-(5-thioxo-4,5-dihyd...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C14H17N5O2S2/c1-19(2)11(20)10(8-9-6-4-3-5-7-9)15-12(21)16-13-17-18-14(22)23-13/h3-7,10H,8H2,1-2H3,(H,18,22)(H2,15,16,17,21)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (MMP-3) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50241372

((S)-1-(1-(methylamino)-1-oxo-3-phenylpropan-2-yl)-...)Show InChI InChI=1S/C13H15N5O2S2/c1-14-10(19)9(7-8-5-3-2-4-6-8)15-11(20)16-12-17-18-13(21)22-12/h2-6,9H,7H2,1H3,(H,14,19)(H,18,21)(H2,15,16,17,20)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-2 (gelatinase-A) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50076341

((S)-N-Methyl-3-pentafluorophenyl-2-[3-(5-thioxo-4,...)Show SMILES CNC(=O)[C@H](Cc1c(F)c(F)c(F)c(F)c1F)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C13H10F5N5O2S2/c1-19-10(24)4(20-11(25)21-12-22-23-13(26)27-12)2-3-5(14)7(16)9(18)8(17)6(3)15/h4H,2H2,1H3,(H,19,24)(H,23,26)(H2,20,21,22,25)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-2 (gelatinase-A) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076343
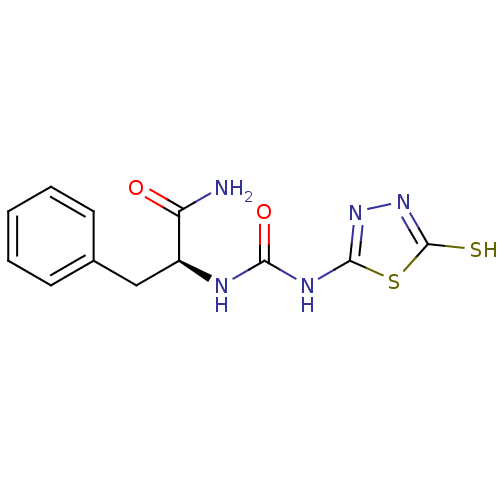
((S)-3-Phenyl-2-[3-(5-thioxo-4,5-dihydro-[1,3,4]thi...)Show InChI InChI=1S/C12H13N5O2S2/c13-9(18)8(6-7-4-2-1-3-5-7)14-10(19)15-11-16-17-12(20)21-11/h1-5,8H,6H2,(H2,13,18)(H,17,20)(H2,14,15,16,19)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (MMP-3) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50110933
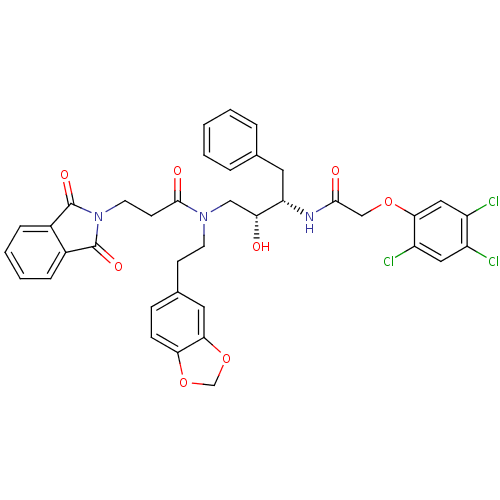
(CHEMBL30571 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-3-...)Show SMILES O[C@H](CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@H](Cc1ccccc1)NC(=O)COc1cc(Cl)c(Cl)cc1Cl Show InChI InChI=1S/C38H34Cl3N3O8/c39-27-18-29(41)33(19-28(27)40)50-21-35(46)42-30(16-23-6-2-1-3-7-23)31(45)20-43(14-12-24-10-11-32-34(17-24)52-22-51-32)36(47)13-15-44-37(48)25-8-4-5-9-26(25)38(44)49/h1-11,17-19,30-31,45H,12-16,20-22H2,(H,42,46)/t30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human Cathepsin D |
J Med Chem 45: 1412-9 (2002)
BindingDB Entry DOI: 10.7270/Q25T3JSH |
More data for this
Ligand-Target Pair | |
Cathepsin D
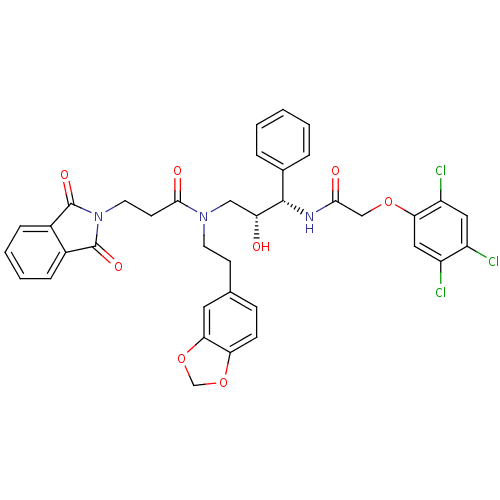
(Homo sapiens (Human)) | BDBM36560
((R)-EHD)Show SMILES O[C@H](CN(CCc1ccc2OCOc2c1)C(=O)CCN1C(=O)c2ccccc2C1=O)[C@@H](NC(=O)COc1cc(Cl)c(Cl)cc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C37H32Cl3N3O8/c38-26-17-28(40)31(18-27(26)39)49-20-33(45)41-35(23-6-2-1-3-7-23)29(44)19-42(14-12-22-10-11-30-32(16-22)51-21-50-30)34(46)13-15-43-36(47)24-8-4-5-9-25(24)37(43)48/h1-11,16-18,29,35,44H,12-15,19-21H2,(H,41,45)/t29-,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 3.7 | n/a |
University of California Berkeley
| Assay Description
A fluorometric high through-put assay for inhibitor towards human liver cathepsin D from Calbiochem. |
Chem Biol 4: 297-307 (1997)
Article DOI: 10.1016/s1074-5521(97)90073-9
BindingDB Entry DOI: 10.7270/Q21R6NW9 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241377
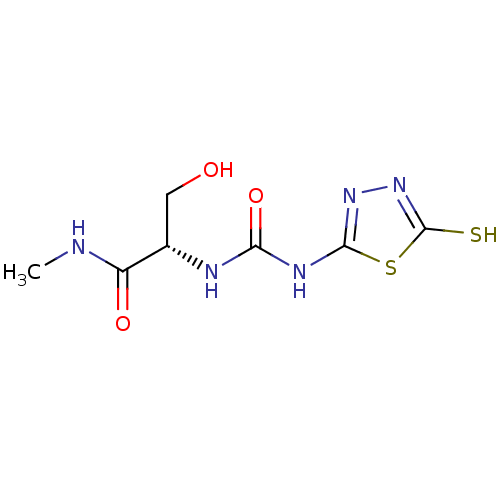
((S)-1-(3-hydroxy-1-(methylamino)-1-oxopropan-2-yl)...)Show InChI InChI=1S/C7H11N5O3S2/c1-8-4(14)3(2-13)9-5(15)10-6-11-12-7(16)17-6/h3,13H,2H2,1H3,(H,8,14)(H,12,16)(H2,9,10,11,15)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (MMP-3) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50241371

(1-(2-(methylamino)-2-oxoethyl)-3-(5-thioxo-4,5-dih...)Show InChI InChI=1S/C6H9N5O2S2/c1-7-3(12)2-8-4(13)9-5-10-11-6(14)15-5/h2H2,1H3,(H,7,12)(H,11,14)(H2,8,9,10,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-2 (gelatinase-A) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50110935
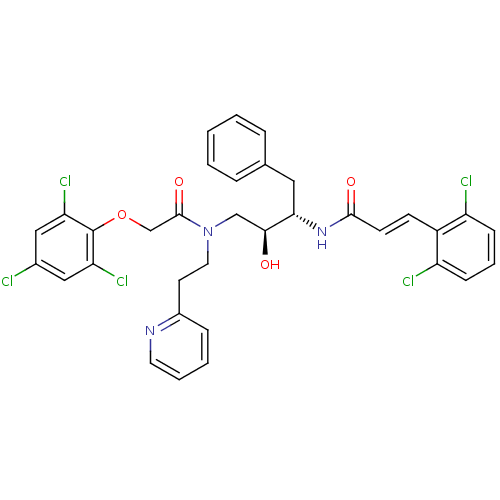
((E)-N-((1S,3S)-1-Benzyl-2-hydroxy-3-{(2-pyridin-2-...)Show SMILES O[C@@H](CN(CCc1ccccn1)C(=O)COc1c(Cl)cc(Cl)cc1Cl)[C@H](Cc1ccccc1)NC(=O)\C=C\c1c(Cl)cccc1Cl Show InChI InChI=1S/C34H30Cl5N3O4/c35-23-18-28(38)34(29(39)19-23)46-21-33(45)42(16-14-24-9-4-5-15-40-24)20-31(43)30(17-22-7-2-1-3-8-22)41-32(44)13-12-25-26(36)10-6-11-27(25)37/h1-13,15,18-19,30-31,43H,14,16-17,20-21H2,(H,41,44)/b13-12+/t30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human Cathepsin D |
J Med Chem 45: 1412-9 (2002)
BindingDB Entry DOI: 10.7270/Q25T3JSH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241371

(1-(2-(methylamino)-2-oxoethyl)-3-(5-thioxo-4,5-dih...)Show InChI InChI=1S/C6H9N5O2S2/c1-7-3(12)2-8-4(13)9-5-10-11-6(14)15-5/h2H2,1H3,(H,7,12)(H,11,14)(H2,8,9,10,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (MMP-3) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50241377

((S)-1-(3-hydroxy-1-(methylamino)-1-oxopropan-2-yl)...)Show InChI InChI=1S/C7H11N5O3S2/c1-8-4(14)3(2-13)9-5(15)10-6-11-12-7(16)17-6/h3,13H,2H2,1H3,(H,8,14)(H,12,16)(H2,9,10,11,15)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-2 (gelatinase-A) |
J Med Chem 47: 3065-74 (2004)
Article DOI: 10.1021/jm030570k
BindingDB Entry DOI: 10.7270/Q26M368W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data