 Found 522 hits with Last Name = 'lazo' and Initial = 'js'
Found 522 hits with Last Name = 'lazo' and Initial = 'js' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50084504
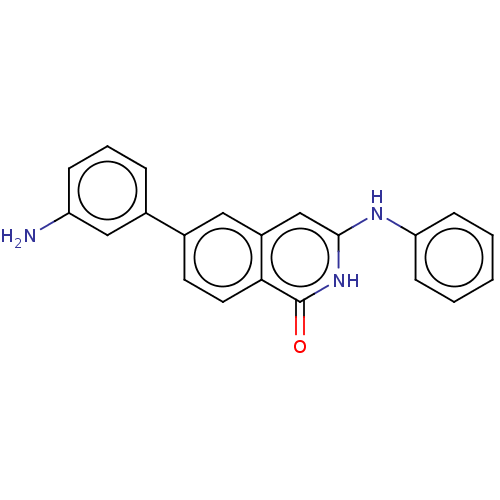
(CHEMBL3426991)Show SMILES Nc1cccc(c1)-c1ccc2c(cc(Nc3ccccc3)[nH]c2=O)c1 Show InChI InChI=1S/C21H17N3O/c22-17-6-4-5-14(12-17)15-9-10-19-16(11-15)13-20(24-21(19)25)23-18-7-2-1-3-8-18/h1-13H,22H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged human recombinant Cdc25B catalytic domain (350 to 566 residues) expressed in Escherichia coli BL21 (D3) after 2... |
Bioorg Med Chem 23: 2810-8 (2015)
Article DOI: 10.1016/j.bmc.2015.01.043
BindingDB Entry DOI: 10.7270/Q2CJ8G62 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50096680

((E)-2-(2-Chloro-phenyl)-ethenesulfonic acid [4-(4-...)Show SMILES CCCc1sc(NS(=O)(=O)\C=C\c2ccccc2Cl)nc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H18Cl2N2O2S2/c1-2-5-18-19(15-8-10-16(21)11-9-15)23-20(27-18)24-28(25,26)13-12-14-6-3-4-7-17(14)22/h3-4,6-13H,2,5H2,1H3,(H,23,24)/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant Cdc25B phosphatase enzyme |
Bioorg Med Chem Lett 11: 313-7 (2001)
BindingDB Entry DOI: 10.7270/Q2DR2TRG |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50096693
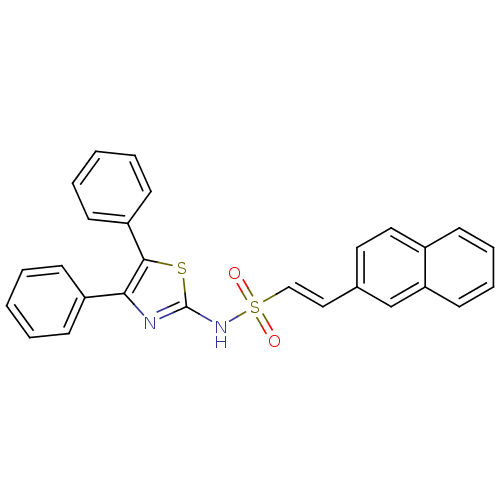
((E)-2-Naphthalen-2-yl-ethenesulfonic acid (4,5-dip...)Show SMILES O=S(=O)(Nc1nc(c(s1)-c1ccccc1)-c1ccccc1)\C=C\c1ccc2ccccc2c1 Show InChI InChI=1S/C27H20N2O2S2/c30-33(31,18-17-20-15-16-21-9-7-8-14-24(21)19-20)29-27-28-25(22-10-3-1-4-11-22)26(32-27)23-12-5-2-6-13-23/h1-19H,(H,28,29)/b18-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Cdc25B phosphatase enzyme |
Bioorg Med Chem Lett 11: 313-7 (2001)
BindingDB Entry DOI: 10.7270/Q2DR2TRG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50479538

(CAS 381679-98-5 | CHEMBL490678)Show InChI InChI=1S/C17H21NO2S2/c1-3-14-4-6-15(7-5-14)12-13-18-22(19,20)17-10-8-16(21-2)9-11-17/h4-11,18H,3,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 1247-50 (2009)
Article DOI: 10.1021/jm801278g
BindingDB Entry DOI: 10.7270/Q23T9M14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50479539
(CAS 307545-04-6 | CHEMBL491275)Show InChI InChI=1S/C18H18N2O2S/c1-12(2)13-7-9-14(10-8-13)22-11-17(21)20-18-19-15-5-3-4-6-16(15)23-18/h3-10,12H,11H2,1-2H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| <5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 1247-50 (2009)
Article DOI: 10.1021/jm801278g
BindingDB Entry DOI: 10.7270/Q23T9M14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50479540
(CAS 329920-60-7 | CHEMBL452613)Show InChI InChI=1S/C21H21NO2/c1-2-16-10-12-19(13-11-16)24-15-21(23)22-14-18-8-5-7-17-6-3-4-9-20(17)18/h3-13H,2,14-15H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| <5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 1247-50 (2009)
Article DOI: 10.1021/jm801278g
BindingDB Entry DOI: 10.7270/Q23T9M14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50479541
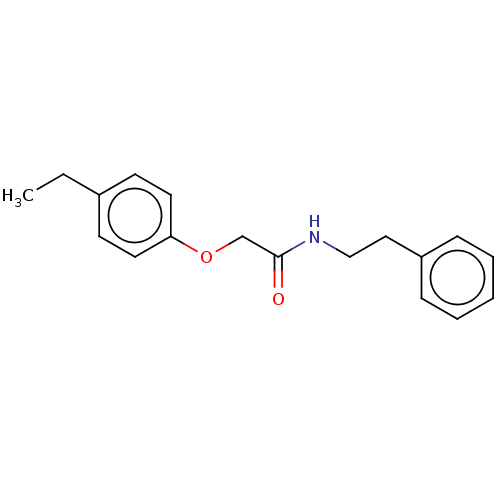
(CAS 303796-42-1 | CHEMBL446965)Show InChI InChI=1S/C18H21NO2/c1-2-15-8-10-17(11-9-15)21-14-18(20)19-13-12-16-6-4-3-5-7-16/h3-11H,2,12-14H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| <5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 1247-50 (2009)
Article DOI: 10.1021/jm801278g
BindingDB Entry DOI: 10.7270/Q23T9M14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50479542
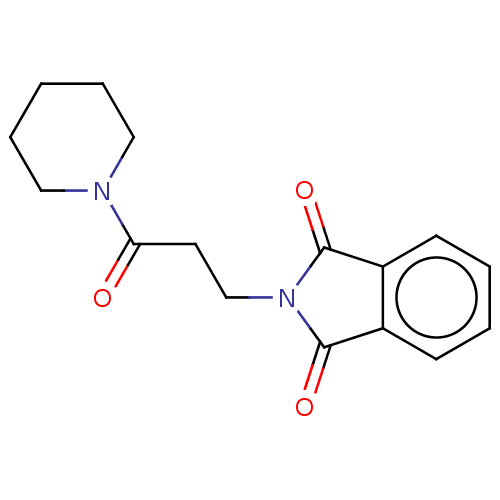
(CAS 31122-64-2 | CHEMBL491273)Show InChI InChI=1S/C16H18N2O3/c19-14(17-9-4-1-5-10-17)8-11-18-15(20)12-6-2-3-7-13(12)16(18)21/h2-3,6-7H,1,4-5,8-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| <5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 1247-50 (2009)
Article DOI: 10.1021/jm801278g
BindingDB Entry DOI: 10.7270/Q23T9M14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50479536
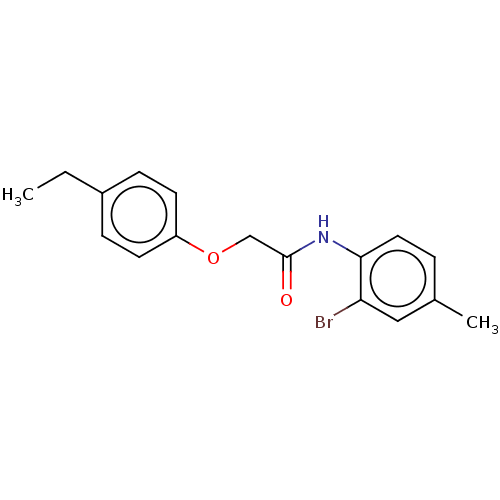
(CHEMBL489096)Show InChI InChI=1S/C17H18BrNO2/c1-3-13-5-7-14(8-6-13)21-11-17(20)19-16-9-4-12(2)10-15(16)18/h4-10H,3,11H2,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| <5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 1247-50 (2009)
Article DOI: 10.1021/jm801278g
BindingDB Entry DOI: 10.7270/Q23T9M14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50479535
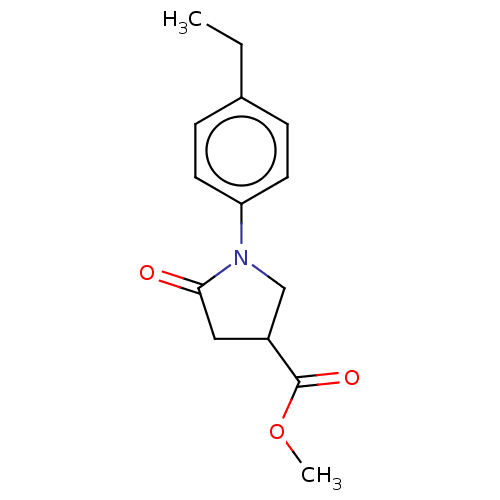
(CAS 160693-53-8 | CHEMBL491274)Show InChI InChI=1S/C14H17NO3/c1-3-10-4-6-12(7-5-10)15-9-11(8-13(15)16)14(17)18-2/h4-7,11H,3,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| <5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 1247-50 (2009)
Article DOI: 10.1021/jm801278g
BindingDB Entry DOI: 10.7270/Q23T9M14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50479537
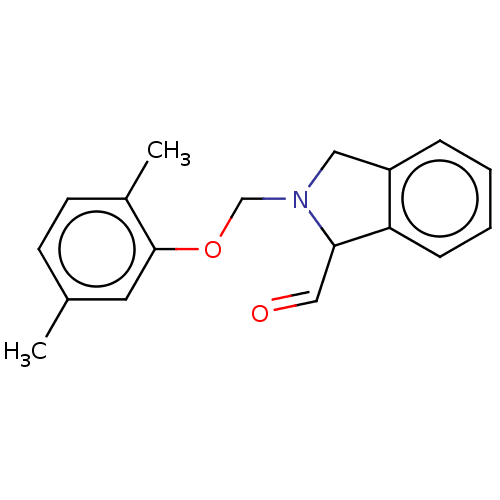
(CHEMBL504747)Show InChI InChI=1S/C18H19NO2/c1-13-7-8-14(2)18(9-13)21-12-19-10-15-5-3-4-6-16(15)17(19)11-20/h3-9,11,17H,10,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <5.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 1247-50 (2009)
Article DOI: 10.1021/jm801278g
BindingDB Entry DOI: 10.7270/Q23T9M14 |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM396097

(US10308663, JMS-631-053)Show InChI InChI=1S/C13H8N2O2S/c14-10-11-8(12(16)15-13(10)17)6-9(18-11)7-4-2-1-3-5-7/h1-6,14H,(H,15,16,17) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The in vitro biochemical evaluation of all compounds was carried out using recombinant human PTP4A3, overexpressed as a His6-tag fusion protein in E.... |
Bioorg Med Chem Lett 17: 4664-9 (2007)
BindingDB Entry DOI: 10.7270/Q20867N2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336960
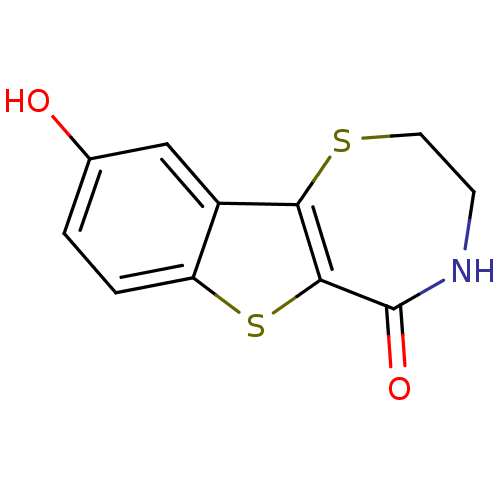
(3,4-Dihydro-9-hydroxy-[1]benzothieno[2,3-f]-1,4-th...)Show InChI InChI=1S/C11H9NO2S2/c13-6-1-2-8-7(5-6)9-10(16-8)11(14)12-3-4-15-9/h1-2,5,13H,3-4H2,(H,12,14) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD1 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336960

(3,4-Dihydro-9-hydroxy-[1]benzothieno[2,3-f]-1,4-th...)Show InChI InChI=1S/C11H9NO2S2/c13-6-1-2-8-7(5-6)9-10(16-8)11(14)12-3-4-15-9/h1-2,5,13H,3-4H2,(H,12,14) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28.3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM50535759
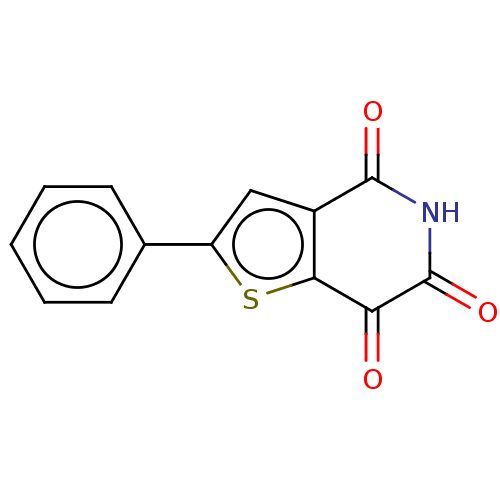
(CHEMBL4471843)Show InChI InChI=1S/C13H7NO3S/c15-10-11-8(12(16)14-13(10)17)6-9(18-11)7-4-2-1-3-5-7/h1-6H,(H,14,16,17) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-6-tagged PTP4A3 expressed in Escherichia coli assessed as reduction in hydrolysis using DIFMUP as substrate incub... |
Bioorg Med Chem Lett 29: 2008-2015 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.048
BindingDB Entry DOI: 10.7270/Q2VH5SBF |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM396097

(US10308663, JMS-631-053)Show InChI InChI=1S/C13H8N2O2S/c14-10-11-8(12(16)15-13(10)17)6-9(18-11)7-4-2-1-3-5-7/h1-6,14H,(H,15,16,17) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-6-tagged PTP4A3 expressed in Escherichia coli assessed as reduction in hydrolysis using DIFMUP as substrate incub... |
Bioorg Med Chem Lett 29: 2008-2015 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.048
BindingDB Entry DOI: 10.7270/Q2VH5SBF |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50336958

(2-Methoxy-7H,8H,9H-1,4-thiazepino[7',6'-5,4]thioph...)Show InChI InChI=1S/C10H9N3O2S2/c1-15-10-12-4-5-6(13-10)7-8(17-5)9(14)11-2-3-16-7/h4H,2-3H2,1H3,(H,11,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD2 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 1
(Homo sapiens (Human)) | BDBM396097

(US10308663, JMS-631-053)Show InChI InChI=1S/C13H8N2O2S/c14-10-11-8(12(16)15-13(10)17)6-9(18-11)7-4-2-1-3-5-7/h1-6,14H,(H,15,16,17) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of PTP4A1 (unknown origin) expressed in Escherichia coli assessed as reduction in hydrolysis using DIFMUP as substrate incubated for 30 mi... |
Bioorg Med Chem Lett 29: 2008-2015 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.048
BindingDB Entry DOI: 10.7270/Q2VH5SBF |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 2
(Homo sapiens (Human)) | BDBM396097

(US10308663, JMS-631-053)Show InChI InChI=1S/C13H8N2O2S/c14-10-11-8(12(16)15-13(10)17)6-9(18-11)7-4-2-1-3-5-7/h1-6,14H,(H,15,16,17) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of PTP4A2 (unknown origin) expressed in Escherichia coli assessed as reduction in hydrolysis using DIFMUP as substrate incubated for 30 mi... |
Bioorg Med Chem Lett 29: 2008-2015 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.048
BindingDB Entry DOI: 10.7270/Q2VH5SBF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM50336960

(3,4-Dihydro-9-hydroxy-[1]benzothieno[2,3-f]-1,4-th...)Show InChI InChI=1S/C11H9NO2S2/c13-6-1-2-8-7(5-6)9-10(16-8)11(14)12-3-4-15-9/h1-2,5,13H,3-4H2,(H,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53.2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D2
(Homo sapiens (Human)) | BDBM50336960

(3,4-Dihydro-9-hydroxy-[1]benzothieno[2,3-f]-1,4-th...)Show InChI InChI=1S/C11H9NO2S2/c13-6-1-2-8-7(5-6)9-10(16-8)11(14)12-3-4-15-9/h1-2,5,13H,3-4H2,(H,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58.7 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM50336962

(2,3,4,5-Tetrahydro-10-hydroxybenzo[b]thieno[2,3-f]...)Show InChI InChI=1S/C12H11NO2S2/c14-7-2-3-9-8(6-7)10-11(17-9)12(15)13-4-1-5-16-10/h2-3,6,14H,1,4-5H2,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58.8 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336961
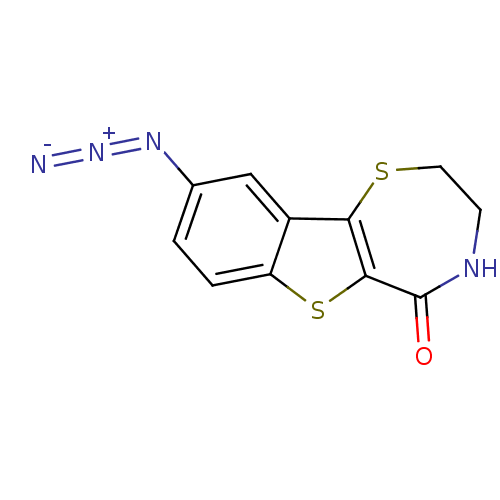
(3,4-Dihydro-9-azido-[1]benzothieno[2,3-f]-1,4-thia...)Show InChI InChI=1S/C11H8N4OS2/c12-15-14-6-1-2-8-7(5-6)9-10(18-8)11(16)13-3-4-17-9/h1-2,5H,3-4H2,(H,13,16) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD1 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50267086

(Adociaquinone B | CHEMBL476648)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C4=NCCS(=O)(=O)C4C(=O)c3cc21 |r,t:19| Show InChI InChI=1S/C22H17NO6S/c1-22-4-2-3-10-9-29-20(15(10)22)18(25)13-7-11-12(8-14(13)22)19(26)21-16(17(11)24)23-5-6-30(21,27)28/h7-9,21H,2-6H2,1H3/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged full length human Cdc25B expressed in Escherichia coli |
Bioorg Med Chem 17: 2276-81 (2009)
Article DOI: 10.1016/j.bmc.2008.10.090
BindingDB Entry DOI: 10.7270/Q21J99MN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50119286

(3-Methoxy-7,8-dihydro-6H-5,10-dithia-8-aza-benzo[a...)Show InChI InChI=1S/C12H11NO2S2/c1-15-7-2-3-9-8(6-7)10-11(17-9)12(14)13-4-5-16-10/h2-3,6H,4-5H2,1H3,(H,13,14) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD1 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50119286

(3-Methoxy-7,8-dihydro-6H-5,10-dithia-8-aza-benzo[a...)Show InChI InChI=1S/C12H11NO2S2/c1-15-7-2-3-9-8(6-7)10-11(17-9)12(14)13-4-5-16-10/h2-3,6H,4-5H2,1H3,(H,13,14) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM396097

(US10308663, JMS-631-053)Show InChI InChI=1S/C13H8N2O2S/c14-10-11-8(12(16)15-13(10)17)6-9(18-11)7-4-2-1-3-5-7/h1-6,14H,(H,15,16,17) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PTP4A3 |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128167
BindingDB Entry DOI: 10.7270/Q23X8BDD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM81614
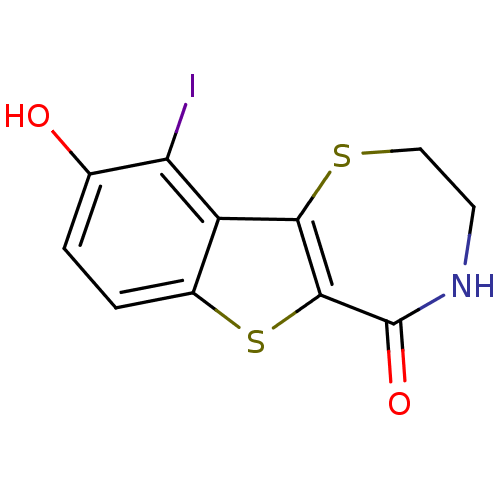
(kb-NB165-31)Show InChI InChI=1S/C11H8INO2S2/c12-8-5(14)1-2-6-7(8)9-10(17-6)11(15)13-3-4-16-9/h1-2,14H,3-4H2,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91.1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM50119286

(3-Methoxy-7,8-dihydro-6H-5,10-dithia-8-aza-benzo[a...)Show InChI InChI=1S/C12H11NO2S2/c1-15-7-2-3-9-8(6-7)10-11(17-9)12(14)13-4-5-16-10/h2-3,6H,4-5H2,1H3,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 98.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D2
(Homo sapiens (Human)) | BDBM50336962

(2,3,4,5-Tetrahydro-10-hydroxybenzo[b]thieno[2,3-f]...)Show InChI InChI=1S/C12H11NO2S2/c14-7-2-3-9-8(6-7)10-11(17-9)12(15)13-4-1-5-16-10/h2-3,6,14H,1,4-5H2,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM50571230
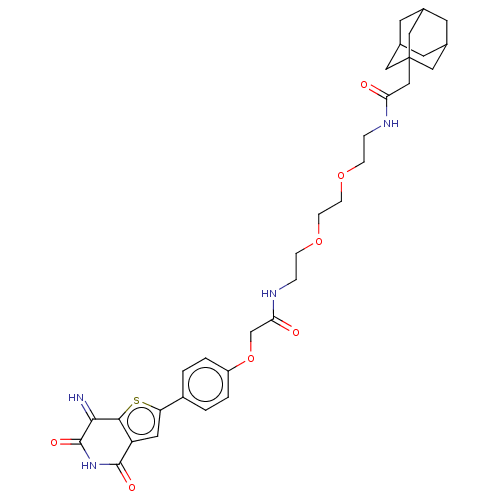
(CHEMBL4862721)Show SMILES N=C1c2sc(cc2C(=O)NC1=O)-c1ccc(OCC(=O)NCCOCCOCCNC(=O)CC23CC4CC(CC(C4)C2)C3)cc1 |TLB:32:33:36.35.40:38,THB:34:35:38:42.33.41,34:33:36.35.40:38,41:33:36:40.39.38,41:39:36:42.34.33,32:33:36:40.39.38| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PTP4A3 |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128167
BindingDB Entry DOI: 10.7270/Q23X8BDD |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50267086

(Adociaquinone B | CHEMBL476648)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C4=NCCS(=O)(=O)C4C(=O)c3cc21 |r,t:19| Show InChI InChI=1S/C22H17NO6S/c1-22-4-2-3-10-9-29-20(15(10)22)18(25)13-7-11-12(8-14(13)22)19(26)21-16(17(11)24)23-5-6-30(21,27)28/h7-9,21H,2-6H2,1H3/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged human recombinant Cdc25B catalytic domain expressed in Escherichia coli |
Bioorg Med Chem 17: 2276-81 (2009)
Article DOI: 10.1016/j.bmc.2008.10.090
BindingDB Entry DOI: 10.7270/Q21J99MN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336962

(2,3,4,5-Tetrahydro-10-hydroxybenzo[b]thieno[2,3-f]...)Show InChI InChI=1S/C12H11NO2S2/c14-7-2-3-9-8(6-7)10-11(17-9)12(15)13-4-1-5-16-10/h2-3,6,14H,1,4-5H2,(H,13,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD1 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336962

(2,3,4,5-Tetrahydro-10-hydroxybenzo[b]thieno[2,3-f]...)Show InChI InChI=1S/C12H11NO2S2/c14-7-2-3-9-8(6-7)10-11(17-9)12(15)13-4-1-5-16-10/h2-3,6,14H,1,4-5H2,(H,13,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM81614

(kb-NB165-31)Show InChI InChI=1S/C11H8INO2S2/c12-8-5(14)1-2-6-7(8)9-10(17-6)11(15)13-3-4-16-9/h1-2,14H,3-4H2,(H,13,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336958

(2-Methoxy-7H,8H,9H-1,4-thiazepino[7',6'-5,4]thioph...)Show InChI InChI=1S/C10H9N3O2S2/c1-15-10-12-4-5-6(13-10)7-8(17-5)9(14)11-2-3-16-7/h4H,2-3H2,1H3,(H,11,14) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD1 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336959
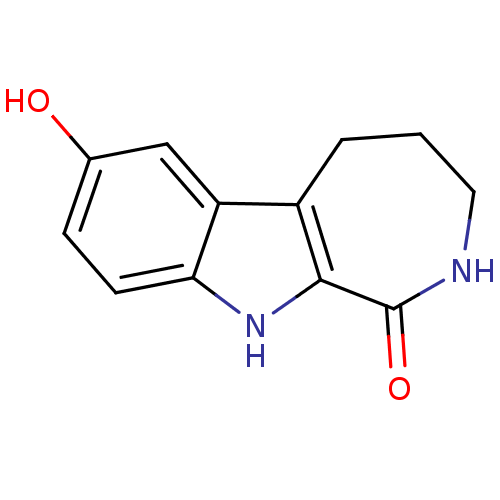
(3,4,5,10-Tetrahydro-7-hydroxy-azepino[3,4-b]indol-...)Show InChI InChI=1S/C12H12N2O2/c15-7-3-4-10-9(6-7)8-2-1-5-13-12(16)11(8)14-10/h3-4,6,14-15H,1-2,5H2,(H,13,16) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD1 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM50059084
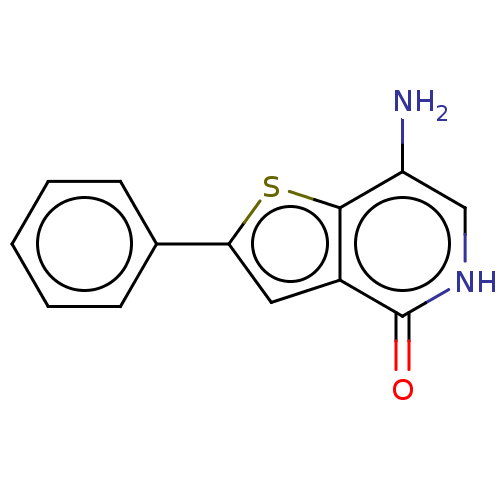
(CHEMBL3393171 | US10308663, Thienopyridone (5))Show InChI InChI=1S/C13H10N2OS/c14-10-7-15-13(16)9-6-11(17-12(9)10)8-4-2-1-3-5-8/h1-7H,14H2,(H,15,16) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-6-tagged PTP4A3 expressed in Escherichia coli assessed as increase in polarization using TAMRAThr-Ala-Asp-Ile-Tyr... |
Bioorg Med Chem Lett 29: 2008-2015 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.048
BindingDB Entry DOI: 10.7270/Q2VH5SBF |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM50059084

(CHEMBL3393171 | US10308663, Thienopyridone (5))Show InChI InChI=1S/C13H10N2OS/c14-10-7-15-13(16)9-6-11(17-12(9)10)8-4-2-1-3-5-8/h1-7H,14H2,(H,15,16) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The in vitro biochemical evaluation of all compounds was carried out using recombinant human PTP4A3, overexpressed as a His6-tag fusion protein in E.... |
Bioorg Med Chem Lett 17: 4664-9 (2007)
BindingDB Entry DOI: 10.7270/Q20867N2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D2
(Homo sapiens (Human)) | BDBM50119286

(3-Methoxy-7,8-dihydro-6H-5,10-dithia-8-aza-benzo[a...)Show InChI InChI=1S/C12H11NO2S2/c1-15-7-2-3-9-8(6-7)10-11(17-9)12(14)13-4-5-16-10/h2-3,6H,4-5H2,1H3,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D2
(Homo sapiens (Human)) | BDBM81614

(kb-NB165-31)Show InChI InChI=1S/C11H8INO2S2/c12-8-5(14)1-2-6-7(8)9-10(17-6)11(15)13-3-4-16-9/h1-2,14H,3-4H2,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 1
(Homo sapiens (Human)) | BDBM50059084

(CHEMBL3393171 | US10308663, Thienopyridone (5))Show InChI InChI=1S/C13H10N2OS/c14-10-7-15-13(16)9-6-11(17-12(9)10)8-4-2-1-3-5-8/h1-7H,14H2,(H,15,16) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-6-tagged PTP4A1 expressed in Escherichia coli assessed as increase in polarization using TAMRAThr-Ala-Asp-Ile-Tyr... |
Bioorg Med Chem Lett 29: 2008-2015 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.048
BindingDB Entry DOI: 10.7270/Q2VH5SBF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM32334

(7-hydroxy-2,3,4,5-tetrahydro-1H-[1]benzofuro[2,3-c...)Show InChI InChI=1S/C12H11NO3/c14-7-3-4-10-9(6-7)8-2-1-5-13-12(15)11(8)16-10/h3-4,6,14H,1-2,5H2,(H,13,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
The radiometric kinase assay using PKD kinase. |
J Biol Chem 283: 33516-26 (2008)
Article DOI: 10.1074/jbc.M805358200
BindingDB Entry DOI: 10.7270/Q20863WJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM32334

(7-hydroxy-2,3,4,5-tetrahydro-1H-[1]benzofuro[2,3-c...)Show InChI InChI=1S/C12H11NO3/c14-7-3-4-10-9(6-7)8-2-1-5-13-12(15)11(8)16-10/h3-4,6,14H,1-2,5H2,(H,13,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD1 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM32334

(7-hydroxy-2,3,4,5-tetrahydro-1H-[1]benzofuro[2,3-c...)Show InChI InChI=1S/C12H11NO3/c14-7-3-4-10-9(6-7)8-2-1-5-13-12(15)11(8)16-10/h3-4,6,14H,1-2,5H2,(H,13,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336963

(2,3,4,5-Tetrahydro-10-methoxybenzo[b]thieno[2,3-f]...)Show InChI InChI=1S/C13H13NO2S2/c1-16-8-3-4-10-9(7-8)11-12(18-10)13(15)14-5-2-6-17-11/h3-4,7H,2,5-6H2,1H3,(H,14,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
In vitro radiometric kinase assay were conducted using PKD1 from Biomol International, PKD2 from SignalChem and PKD3 Enzo Life Sciences. |
BMC Chem Biol 10: 5 (2010)
Article DOI: 10.1186/1472-6769-10-5
BindingDB Entry DOI: 10.7270/Q2VQ315D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50336963

(2,3,4,5-Tetrahydro-10-methoxybenzo[b]thieno[2,3-f]...)Show InChI InChI=1S/C13H13NO2S2/c1-16-8-3-4-10-9(7-8)11-12(18-10)13(15)14-5-2-6-17-11/h3-4,7H,2,5-6H2,1H3,(H,14,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKD1 after 10 mins by radiometric assay |
ACS Med Chem Lett 2: 154-159 (2012)
Article DOI: 10.1021/ml100230n
BindingDB Entry DOI: 10.7270/Q29G5NTR |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase type IVA 3
(Homo sapiens (Human)) | BDBM50571225

(CHEMBL4862502)Show SMILES N=C1c2sc(cc2C(=O)NC1=O)-c1ccc(OCC(=O)NCCCNC(=O)CC23CC4CC(CC(C4)C2)C3)cc1 |TLB:27:28:31.30.35:33,THB:29:30:33:37.28.36,29:28:31.30.35:33,36:28:31:35.34.33,36:34:31:37.29.28,27:28:31:35.34.33| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PTP4A3 |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128167
BindingDB Entry DOI: 10.7270/Q23X8BDD |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D3
(Homo sapiens (Human)) | BDBM32334

(7-hydroxy-2,3,4,5-tetrahydro-1H-[1]benzofuro[2,3-c...)Show InChI InChI=1S/C12H11NO3/c14-7-3-4-10-9(6-7)8-2-1-5-13-12(15)11(8)16-10/h3-4,6,14H,1-2,5H2,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Pittsburgh
| Assay Description
The radiometric kinase assay using PKD kinase. |
J Biol Chem 283: 33516-26 (2008)
Article DOI: 10.1074/jbc.M805358200
BindingDB Entry DOI: 10.7270/Q20863WJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data