

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180524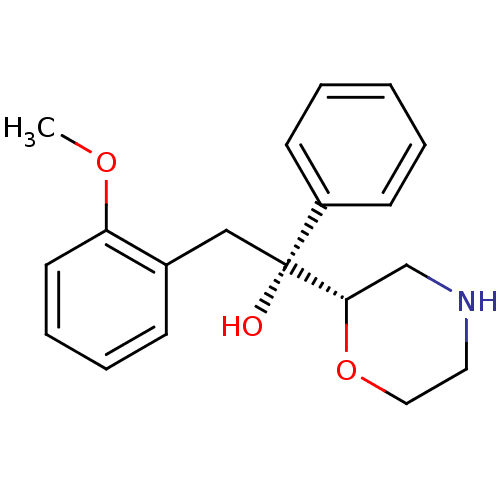 ((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.37 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Eli Lilly and Company US Patent | Assay Description [3H]-Ketanserin binding experiments are carried out in SPA 96-well format. Membranes used in this assay are prepared from AV-12 cells stably expressi... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180521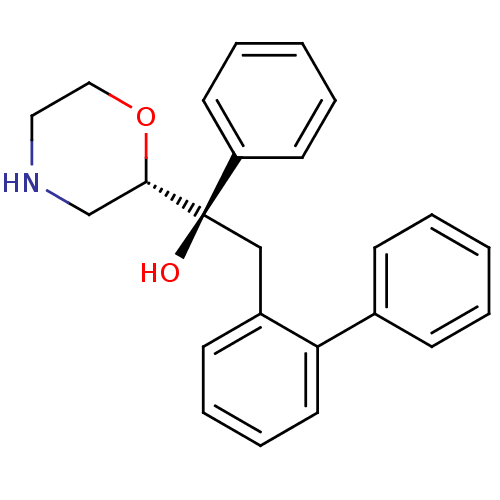 ((R)-2-biphenyl-2-yl-1-(S)-morpholin-2-yl-1-phenyl-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180531 ((R)-2-(2-ethoxyphenyl)-1-((S)-morpholin-2-yl)-1-ph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.01 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Eli Lilly and Company US Patent | Assay Description [3H]-Ketanserin binding experiments are carried out in SPA 96-well format. Membranes used in this assay are prepared from AV-12 cells stably expressi... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180526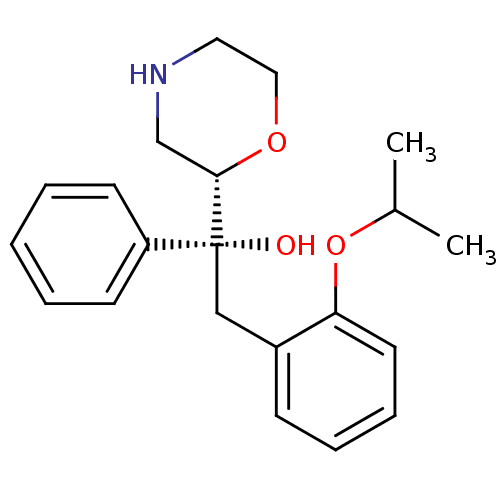 ((R)-2-(2-isopropoxyphenyl)-1-((S)-morpholin-2-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180526 ((R)-2-(2-isopropoxyphenyl)-1-((S)-morpholin-2-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180523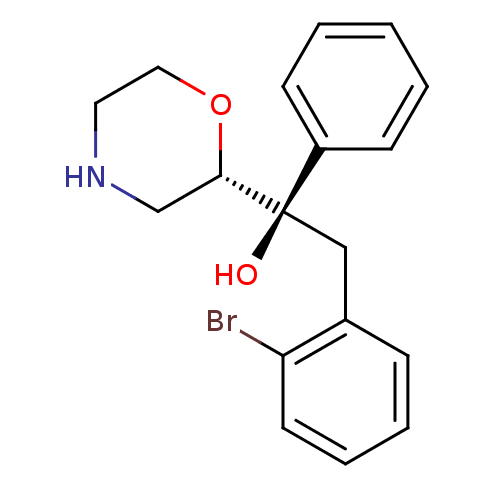 ((R)-2-(2-bromophenyl)-1-((S)-morpholin-2-yl)-1-phe...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.6 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Eli Lilly and Company US Patent | Assay Description [3H]-Pyrilamine binding experiments are carried out in SPA (scintillation proximity assay) 96-well format. Membranes used in this assay are prepared ... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180525 ((R)-2-(2-chlorophenyl)-1-((S)-morpholin-2-yl)-1-ph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180522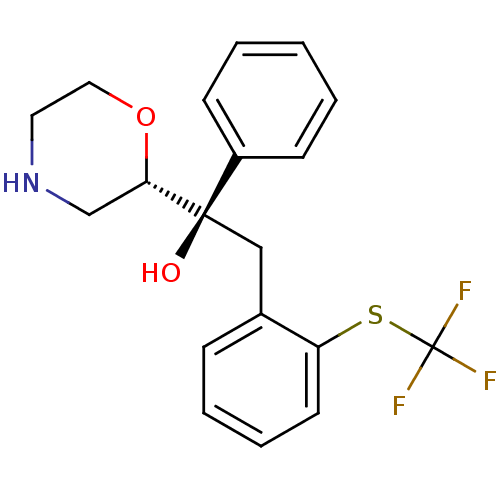 ((R)-1-((S)-morpholin-2-yl)-1-phenyl-2-(2-(trifluor...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 54.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180524 ((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58.8 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Eli Lilly and Company US Patent | Assay Description [3H]-Pyrilamine binding experiments are carried out in SPA (scintillation proximity assay) 96-well format. Membranes used in this assay are prepared ... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 137 | -39.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Eli Lilly and Company US Patent | Assay Description [125I]-(±)DOI binding experiments are carried out in SPA 96-well format. Membranes used in this assay are prepared from AV-12 cells stably expressing... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180527 ((R)-1-((S)-morpholin-2-yl)-1,2-diphenylethanol | C...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180524 ((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50180524 ((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to NET | Bioorg Med Chem Lett 16: 2022-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.061 BindingDB Entry DOI: 10.7270/Q21J9BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 328 | -37.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Eli Lilly and Company US Patent | Assay Description [125I]-(±)DOI binding experiments are carried out in SPA 96-well format. Membranes used in this assay are prepared from AV-12 cells stably expressing... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >5.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >8.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >8.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Activity of compounds on native GABAA receptors is evaluated by monitoring calcium fluxes using a 96 well format FLIPR system (Fluorometric Imaging P... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM255474 (US9481688, 1 | US9481688, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Further, the compounds of the invention may be tested in binding assays and functional activity assays by well known methods for other physiologicall... | US Patent US9481688 (2016) BindingDB Entry DOI: 10.7270/Q25D8QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||