

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496900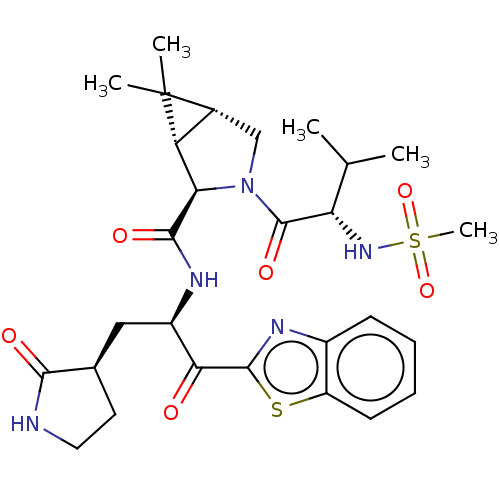 (science.abl4784, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 7.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496901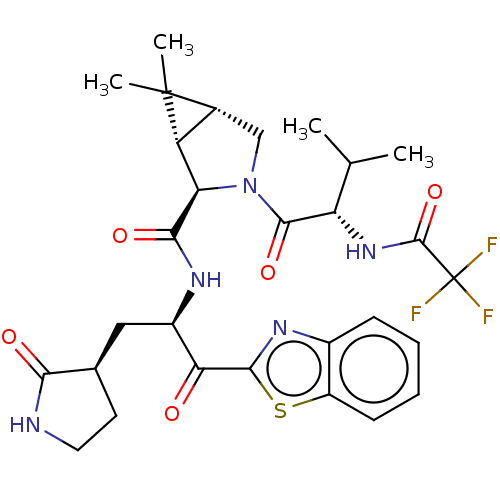 (science.abl4784, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496896 (US11312704, Compound 101 | US11351149, Example 49 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496897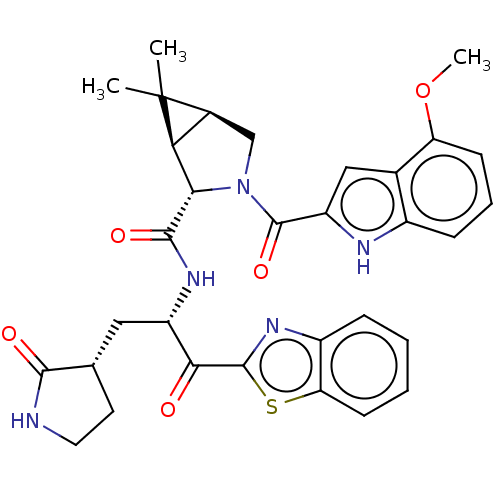 (science.abl4784, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM537926 (US11248003, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,C797S] (Homo sapiens (Human)) | BDBM537926 (US11248003, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM537926 (US11248003, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50117930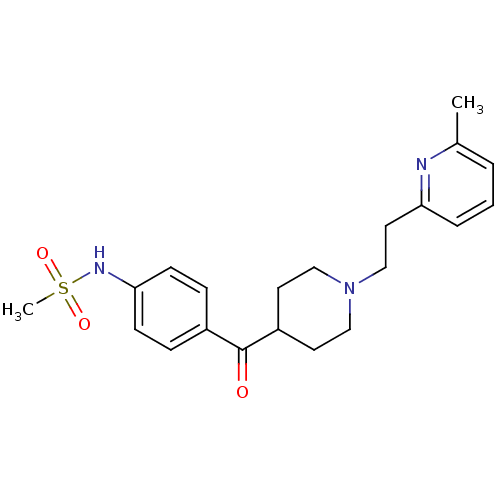 ((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of human ERG by fluorescently labeled tracer binding method | J Med Chem 60: 9205-9221 (2017) Article DOI: 10.1021/acs.jmedchem.7b01039 BindingDB Entry DOI: 10.7270/Q26D5WF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin A1 (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105748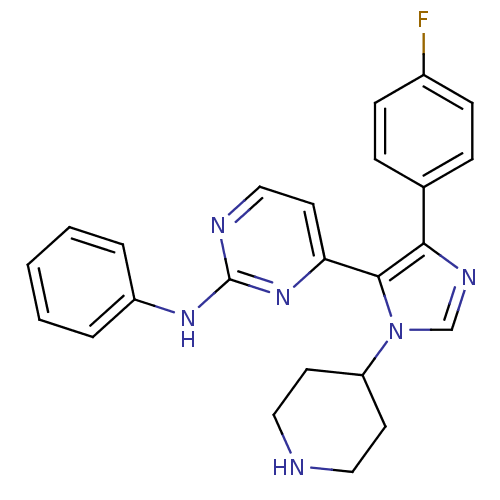 (4-(4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105762 (4-[4-(4-Fluoro-phenyl)-5-(2-phenylamino-pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin-O (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of human wild type ALK using YRRAAVPPSPSLSRHSSPHQ(pS)EDEEE as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition afte... | J Med Chem 60: 9205-9221 (2017) Article DOI: 10.1021/acs.jmedchem.7b01039 BindingDB Entry DOI: 10.7270/Q26D5WF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM50185140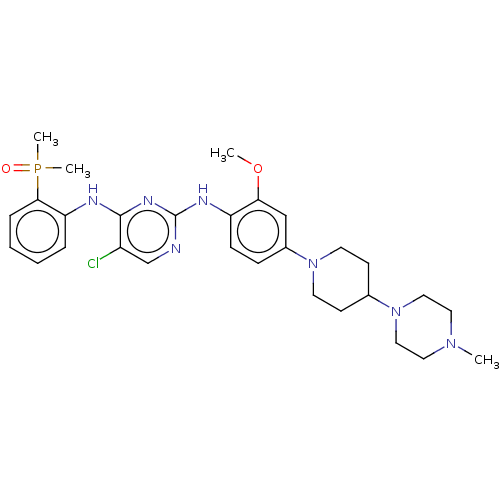 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50563174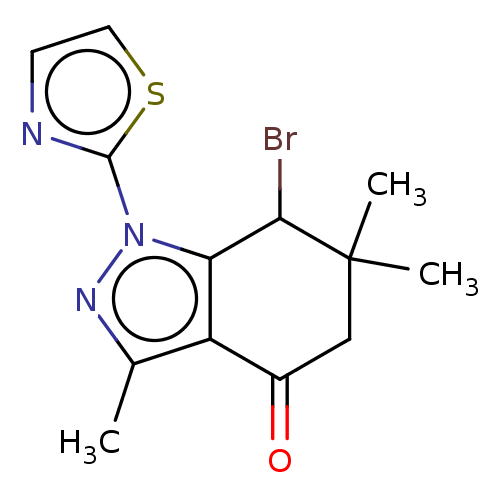 (CHEMBL1708376) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105746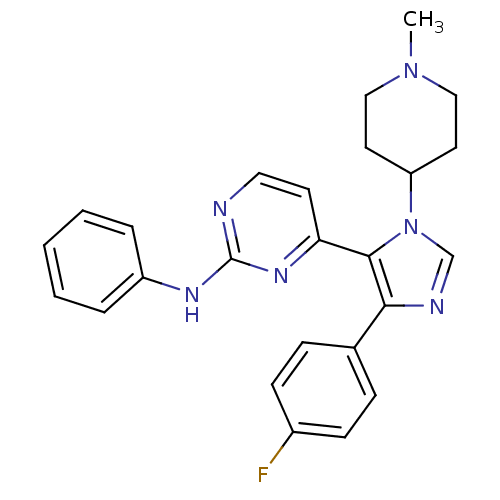 (CHEMBL318892 | {4-[5-(4-Fluoro-phenyl)-3-(1-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM276763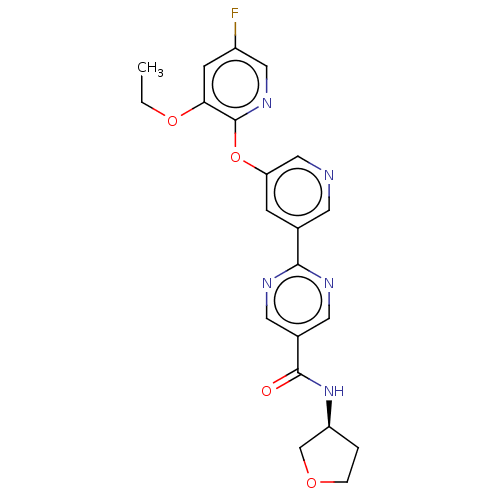 (US10071992, Example 8 | US11034678, Example 8 | US...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q27D2ZDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM276763 (US10071992, Example 8 | US11034678, Example 8 | US...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B9255 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504497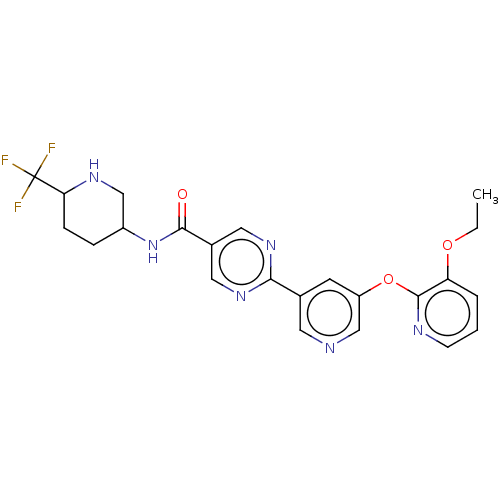 (First-eluting enantiomer from separation of Exampl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q27D2ZDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504497 (First-eluting enantiomer from separation of Exampl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B9255 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50105742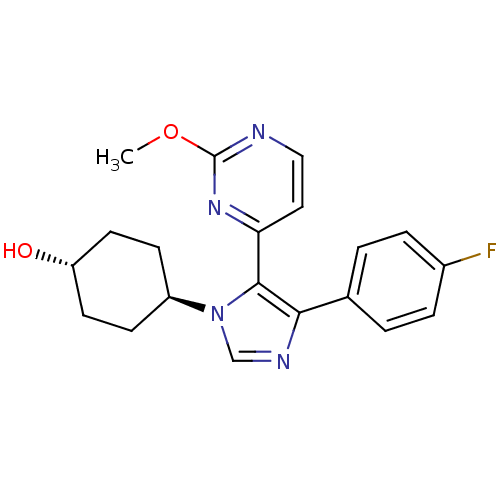 (4-[4-(4-Fluoro-phenyl)-5-(2-methoxy-pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of JNK2beta2 kinase | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50246545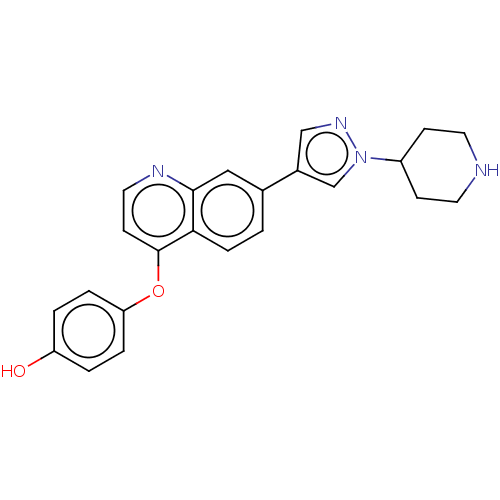 (CHEMBL4065208) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of human wild type ALK using YRRAAVPPSPSLSRHSSPHQ(pS)EDEEE as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition afte... | J Med Chem 60: 9205-9221 (2017) Article DOI: 10.1021/acs.jmedchem.7b01039 BindingDB Entry DOI: 10.7270/Q26D5WF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504419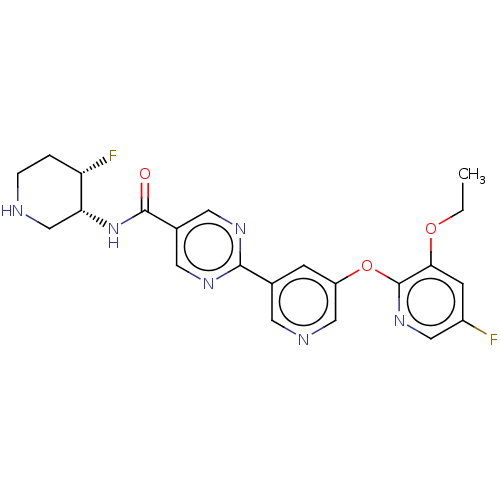 (2-{5-[(3-Ethoxy-5-fluoropyridin-2-yl)oxy]pyridin-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q27D2ZDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504419 (2-{5-[(3-Ethoxy-5-fluoropyridin-2-yl)oxy]pyridin-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B9255 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM537979 (US11248003, Example TRE-069) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity on a C797S-containing epidermal growth factor receptor (EGFR) kinase and an MET kinase was measured for compound 1 obtained i... | Citation and Details BindingDB Entry DOI: 10.7270/Q28G8PWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105743 (4-[3-(1-Benzyl-piperidin-4-yl)-5-(4-fluoro-phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504424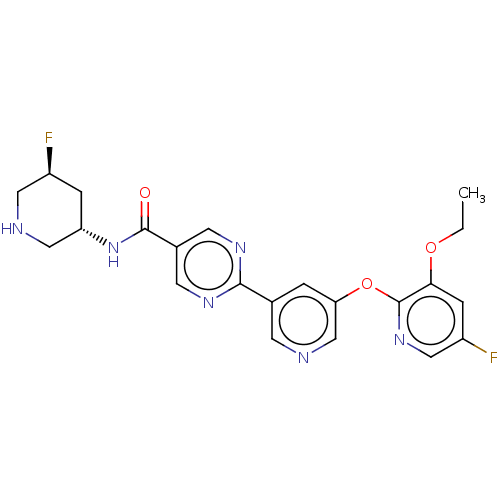 (2-{5-[(3-Ethoxy-5-fluoropyridin-2-yl)oxy]pyridin-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q27D2ZDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504424 (2-{5-[(3-Ethoxy-5-fluoropyridin-2-yl)oxy]pyridin-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B9255 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50563177 (CHEMBL4763182) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105763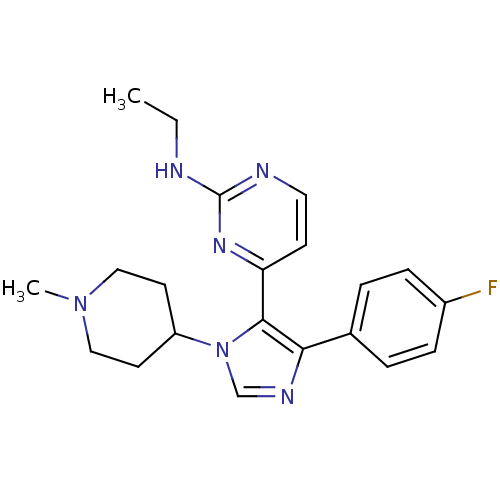 (CHEMBL420081 | D3RKN_10 | Ethyl-{4-[5-(4-fluoro-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM15458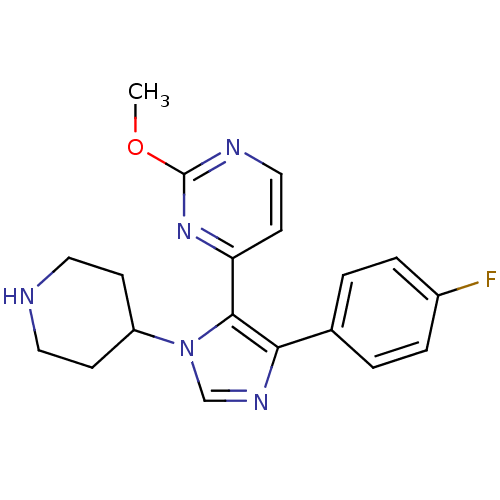 (4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of EGFR kinase | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM15458 (4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck kinase | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504484 (2-{5-[(3-Ethoxypyridin-2-yl)oxy]pyridin-3-yl}-N-[(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B9255 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504484 (2-{5-[(3-Ethoxypyridin-2-yl)oxy]pyridin-3-yl}-N-[(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q27D2ZDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105751 (4-[4-(4-Fluoro-phenyl)-5-(2-phenoxy-pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM276752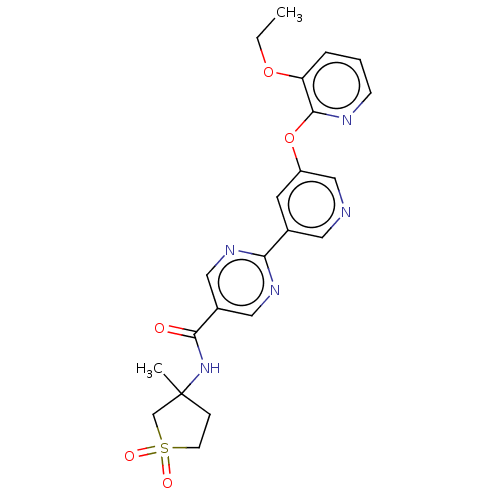 (2-(5-((3-ethoxypyridin- 2-yl)oxy)pyridin-3-yl)-N-(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B9255 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM276752 (2-(5-((3-ethoxypyridin- 2-yl)oxy)pyridin-3-yl)-N-(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q27D2ZDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50125604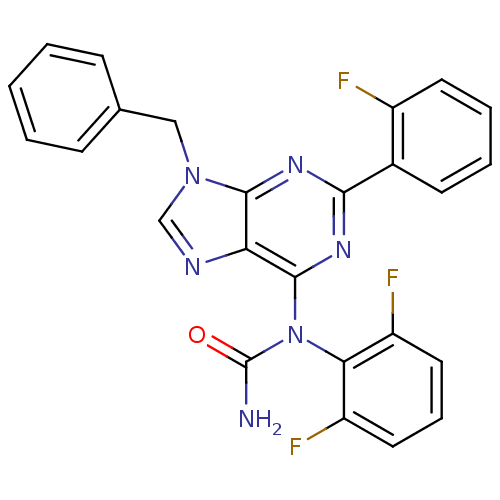 (1-[9-Benzyl-2-(2-fluoro-phenyl)-9H-purin-6-yl]-1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human MAP p38-alpha kinase in vitro. | Bioorg Med Chem Lett 13: 1191-4 (2003) BindingDB Entry DOI: 10.7270/Q2MG7NW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM276750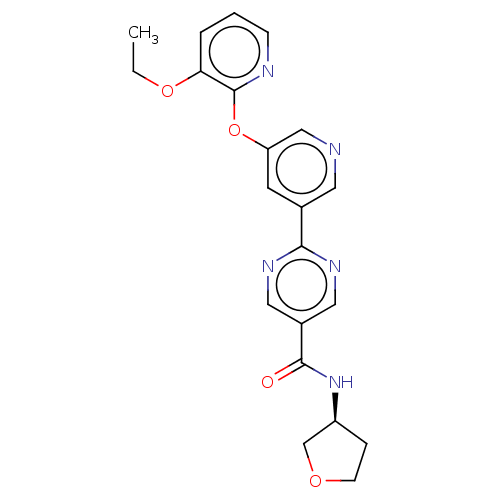 ((S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B9255 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM276750 ((S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q27D2ZDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM15458 (4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of PKC-beta2 kinase | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105761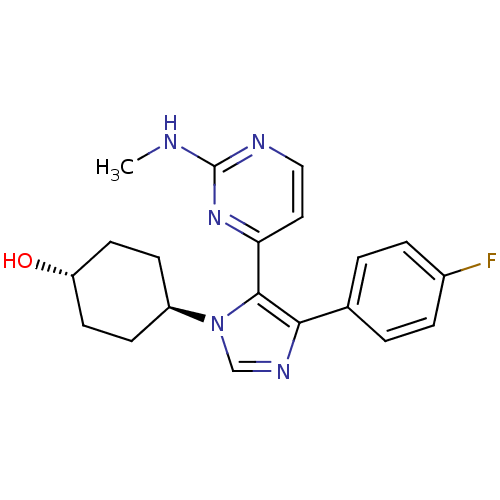 (4-[4-(4-Fluoro-phenyl)-5-(2-methylamino-pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50105742 (4-[4-(4-Fluoro-phenyl)-5-(2-methoxy-pyrimidin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck kinase | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504495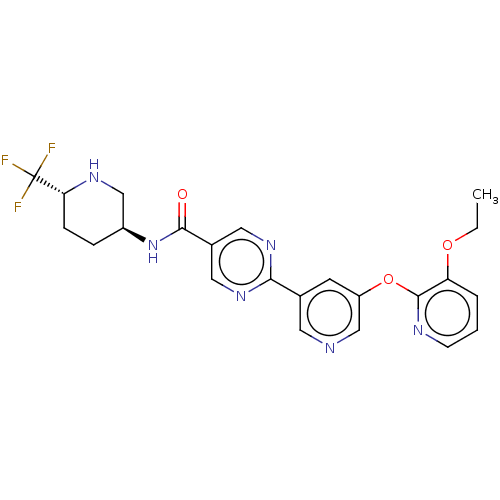 (First-eluting isomer (see footnote 4 in Table 1); ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q27D2ZDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM504495 (First-eluting isomer (see footnote 4 in Table 1); ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white polypropylene plates (Nunc) in a total volume of 20 μL. To 1 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22B9255 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15239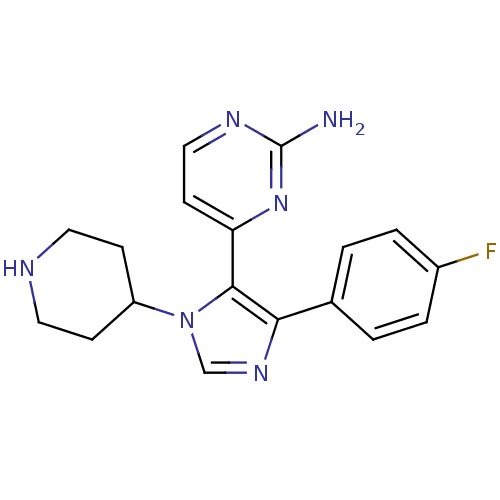 (4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p38 alpha kinase | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15458 (4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 561 total ) | Next | Last >> |