 Found 388 hits with Last Name = 'lee' and Initial = 'kn'
Found 388 hits with Last Name = 'lee' and Initial = 'kn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50069447
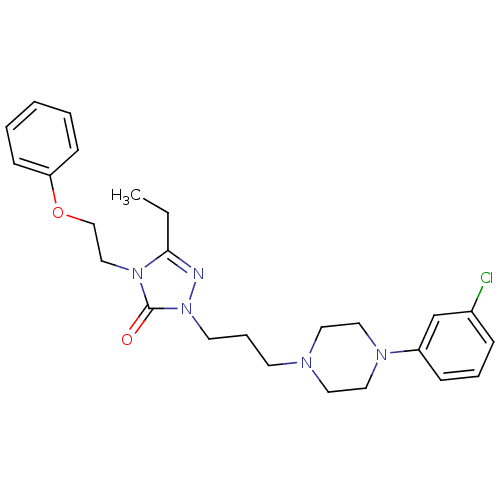
(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50069447

(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352717
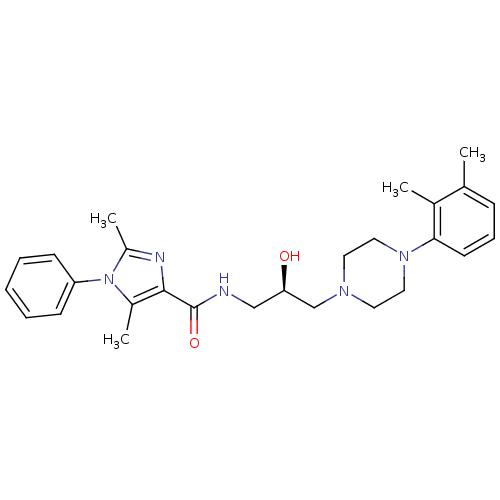
(CHEMBL1823213 | US8835436, Example 151)Show SMILES Cc1nc(C(=O)NC[C@@H](O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1 |r| Show InChI InChI=1S/C27H35N5O2/c1-19-9-8-12-25(20(19)2)31-15-13-30(14-16-31)18-24(33)17-28-27(34)26-21(3)32(22(4)29-26)23-10-6-5-7-11-23/h5-12,24,33H,13-18H2,1-4H3,(H,28,34)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352719
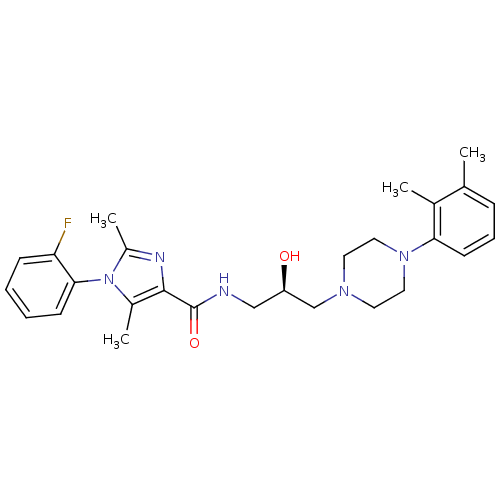
(CHEMBL1823215 | US8835436, Example 152)Show SMILES Cc1nc(C(=O)NC[C@@H](O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1F |r,wD:8.8,(16.59,-9.13,;17.37,-7.8,;18.91,-7.8,;19.39,-6.34,;20.72,-5.57,;20.72,-4.03,;22.05,-6.34,;23.39,-5.57,;24.72,-6.34,;24.72,-7.88,;26.06,-5.57,;27.39,-6.33,;28.71,-5.55,;30.04,-6.32,;30.05,-7.86,;28.71,-8.63,;27.37,-7.87,;31.38,-8.62,;32.71,-7.84,;34.05,-8.61,;34.05,-10.15,;32.71,-10.92,;32.71,-12.46,;31.38,-10.16,;30.05,-10.93,;18.14,-5.43,;18.14,-3.89,;16.89,-6.34,;15.56,-5.56,;15.57,-4.03,;14.24,-3.25,;12.89,-4.02,;12.9,-5.57,;14.23,-6.34,;14.24,-7.88,)| Show InChI InChI=1S/C27H34FN5O2/c1-18-8-7-11-24(19(18)2)32-14-12-31(13-15-32)17-22(34)16-29-27(35)26-20(3)33(21(4)30-26)25-10-6-5-9-23(25)28/h5-11,22,34H,12-17H2,1-4H3,(H,29,35)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351408
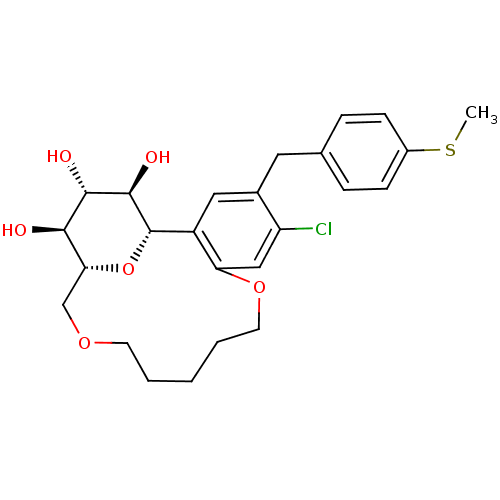
(CHEMBL1819199)Show SMILES CSc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C25H31ClO6S/c1-33-17-7-5-15(6-8-17)11-16-12-18-20(13-19(16)26)31-10-4-2-3-9-30-14-21-22(27)23(28)24(29)25(18)32-21/h5-8,12-13,21-25,27-29H,2-4,9-11,14H2,1H3/t21-,22-,23+,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.778 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351415
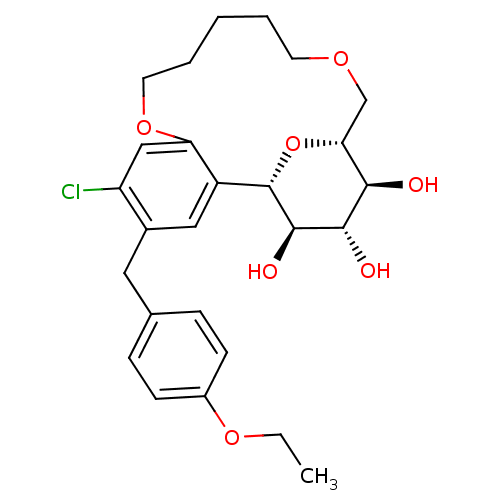
(CHEMBL1819096)Show SMILES CCOc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C26H33ClO7/c1-2-32-18-8-6-16(7-9-18)12-17-13-19-21(14-20(17)27)33-11-5-3-4-10-31-15-22-23(28)24(29)25(30)26(19)34-22/h6-9,13-14,22-26,28-30H,2-5,10-12,15H2,1H3/t22-,23-,24+,25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.899 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351413

(CHEMBL1819095)Show SMILES CCOc1ccc(Cc2cc3[C@@H]4O[C@H](COC\C=C/COc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r,c:17| Show InChI InChI=1S/C25H29ClO7/c1-2-31-17-7-5-15(6-8-17)11-16-12-18-20(13-19(16)26)32-10-4-3-9-30-14-21-22(27)23(28)24(29)25(18)33-21/h3-8,12-13,21-25,27-29H,2,9-11,14H2,1H3/b4-3-/t21-,22-,23+,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.974 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351411
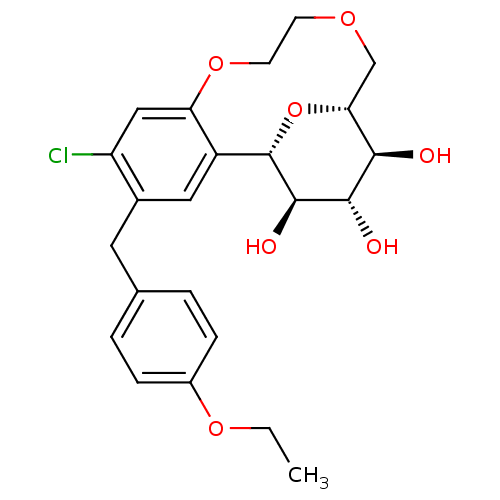
(CHEMBL1819093)Show SMILES CCOc1ccc(Cc2cc3[C@@H]4O[C@H](COCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C23H27ClO7/c1-2-29-15-5-3-13(4-6-15)9-14-10-16-18(11-17(14)24)30-8-7-28-12-19-20(25)21(26)22(27)23(16)31-19/h3-6,10-11,19-23,25-27H,2,7-9,12H2,1H3/t19-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351407

(CHEMBL1819198)Show SMILES CSc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C24H29ClO6S/c1-32-16-6-4-14(5-7-16)10-15-11-17-19(12-18(15)25)30-9-3-2-8-29-13-20-21(26)22(27)23(28)24(17)31-20/h4-7,11-12,20-24,26-28H,2-3,8-10,13H2,1H3/t20-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM20880
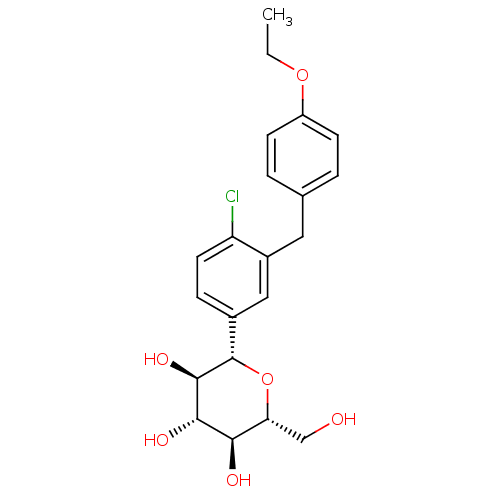
((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351410
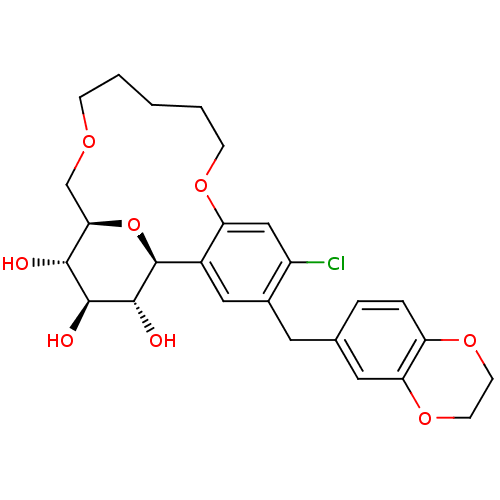
(CHEMBL1819201)Show SMILES O[C@H]1[C@H](O)[C@H]2COCCCCCOc3cc(Cl)c(Cc4ccc5OCCOc5c4)cc3[C@H](O2)[C@@H]1O |r| Show InChI InChI=1S/C26H31ClO8/c27-18-13-20-17(12-16(18)10-15-4-5-19-21(11-15)34-9-8-33-19)26-25(30)24(29)23(28)22(35-26)14-31-6-2-1-3-7-32-20/h4-5,11-13,22-26,28-30H,1-3,6-10,14H2/t22-,23-,24+,25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351405
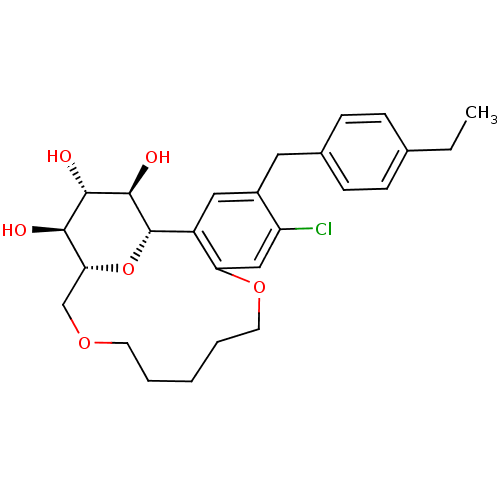
(CHEMBL1819196)Show SMILES CCc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C26H33ClO6/c1-2-16-6-8-17(9-7-16)12-18-13-19-21(14-20(18)27)32-11-5-3-4-10-31-15-22-23(28)24(29)25(30)26(19)33-22/h6-9,13-14,22-26,28-30H,2-5,10-12,15H2,1H3/t22-,23-,24+,25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351406

(CHEMBL1819197)Show SMILES CCc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C27H35ClO6/c1-2-17-7-9-18(10-8-17)13-19-14-20-22(15-21(19)28)33-12-6-4-3-5-11-32-16-23-24(29)25(30)26(31)27(20)34-23/h7-10,14-15,23-27,29-31H,2-6,11-13,16H2,1H3/t23-,24-,25+,26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351404
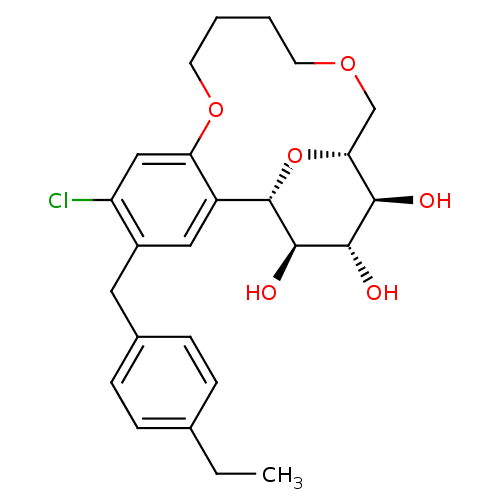
(CHEMBL1819099)Show SMILES CCc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C25H31ClO6/c1-2-15-5-7-16(8-6-15)11-17-12-18-20(13-19(17)26)31-10-4-3-9-30-14-21-22(27)23(28)24(29)25(18)32-21/h5-8,12-13,21-25,27-29H,2-4,9-11,14H2,1H3/t21-,22-,23+,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351403

(CHEMBL1819098)Show SMILES CCc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C24H29ClO6/c1-2-14-4-6-15(7-5-14)10-16-11-17-19(12-18(16)25)30-9-3-8-29-13-20-21(26)22(27)23(28)24(17)31-20/h4-7,11-12,20-24,26-28H,2-3,8-10,13H2,1H3/t20-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351414
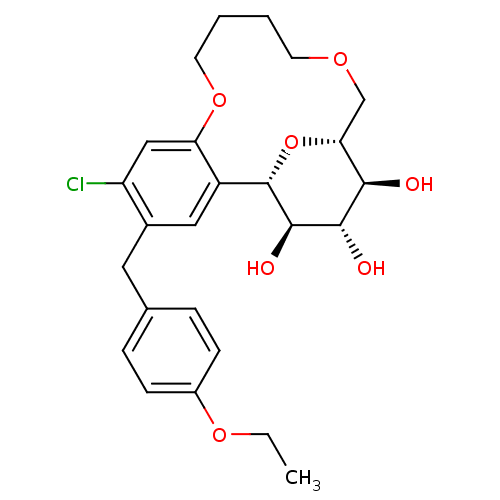
(CHEMBL1817684)Show SMILES CCOc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C25H31ClO7/c1-2-31-17-7-5-15(6-8-17)11-16-12-18-20(13-19(16)26)32-10-4-3-9-30-14-21-22(27)23(28)24(29)25(18)33-21/h5-8,12-13,21-25,27-29H,2-4,9-11,14H2,1H3/t21-,22-,23+,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352682
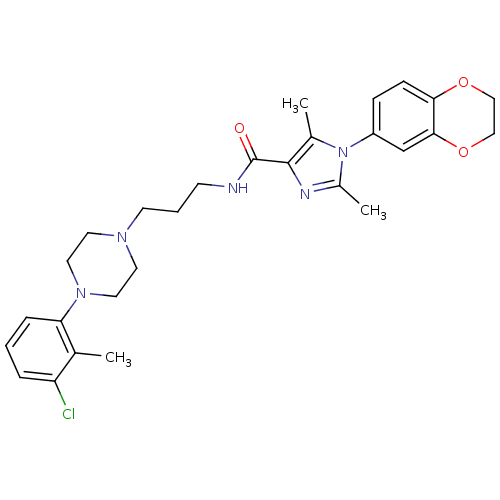
(CHEMBL1823096)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2C)c(C)n1-c1ccc2OCCOc2c1 Show InChI InChI=1S/C28H34ClN5O3/c1-19-23(29)6-4-7-24(19)33-14-12-32(13-15-33)11-5-10-30-28(35)27-20(2)34(21(3)31-27)22-8-9-25-26(18-22)37-17-16-36-25/h4,6-9,18H,5,10-17H2,1-3H3,(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352708
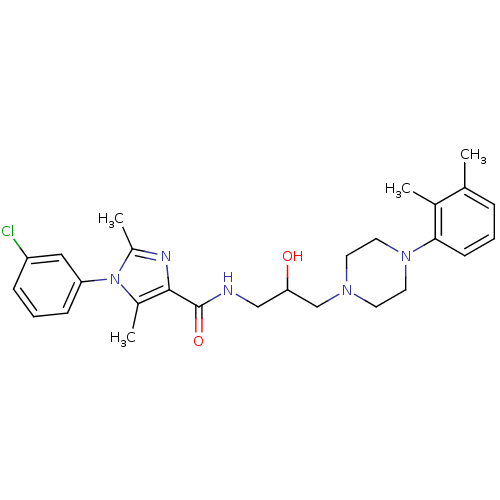
(CHEMBL1823205)Show SMILES Cc1nc(C(=O)NCC(O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C27H34ClN5O2/c1-18-7-5-10-25(19(18)2)32-13-11-31(12-14-32)17-24(34)16-29-27(35)26-20(3)33(21(4)30-26)23-9-6-8-22(28)15-23/h5-10,15,24,34H,11-14,16-17H2,1-4H3,(H,29,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351412

(CHEMBL1819094)Show SMILES CCOc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C24H29ClO7/c1-2-30-16-6-4-14(5-7-16)10-15-11-17-19(12-18(15)25)31-9-3-8-29-13-20-21(26)22(27)23(28)24(17)32-20/h4-7,11-12,20-24,26-28H,2-3,8-10,13H2,1H3/t20-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351416

(CHEMBL1819097)Show SMILES CCOc1ccc(Cc2cc3[C@@H]4O[C@H](COCCCCCCOc3cc2Cl)[C@@H](O)[C@H](O)[C@H]4O)cc1 |r| Show InChI InChI=1S/C27H35ClO7/c1-2-33-19-9-7-17(8-10-19)13-18-14-20-22(15-21(18)28)34-12-6-4-3-5-11-32-16-23-24(29)25(30)26(31)27(20)35-23/h7-10,14-15,23-27,29-31H,2-6,11-13,16H2,1H3/t23-,24-,25+,26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351409

(CHEMBL1819200)Show SMILES O[C@H]1[C@H](O)[C@H]2COCCCCOc3cc(Cl)c(Cc4ccc5OCCOc5c4)cc3[C@H](O2)[C@@H]1O |r| Show InChI InChI=1S/C25H29ClO8/c26-17-12-19-16(11-15(17)9-14-3-4-18-20(10-14)33-8-7-32-18)25-24(29)23(28)22(27)21(34-25)13-30-5-1-2-6-31-19/h3-4,10-12,21-25,27-29H,1-2,5-9,13H2/t21-,22-,23+,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as reduction of [14C]-labeled AMG after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 19: 5468-79 (2011)
Article DOI: 10.1016/j.bmc.2011.07.045
BindingDB Entry DOI: 10.7270/Q2PG1S3W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM9274

(CHEMBL1822893 | US8835436, Example 8)Show SMILES Cc1nc(C(=O)NCCN2CCN(CC2)c2cccc(Cl)c2Cl)c(C)n1-c1ccccc1 Show InChI InChI=1S/C24H27Cl2N5O/c1-17-23(28-18(2)31(17)19-7-4-3-5-8-19)24(32)27-11-12-29-13-15-30(16-14-29)21-10-6-9-20(25)22(21)26/h3-10H,11-16H2,1-2H3,(H,27,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT1A receptor by competition binding assay |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352730
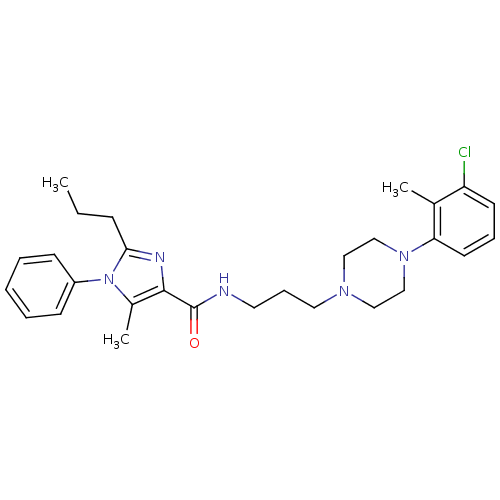
(CHEMBL1823057)Show SMILES CCCc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2C)c(C)n1-c1ccccc1 Show InChI InChI=1S/C28H36ClN5O/c1-4-10-26-31-27(22(3)34(26)23-11-6-5-7-12-23)28(35)30-15-9-16-32-17-19-33(20-18-32)25-14-8-13-24(29)21(25)2/h5-8,11-14H,4,9-10,15-20H2,1-3H3,(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352677
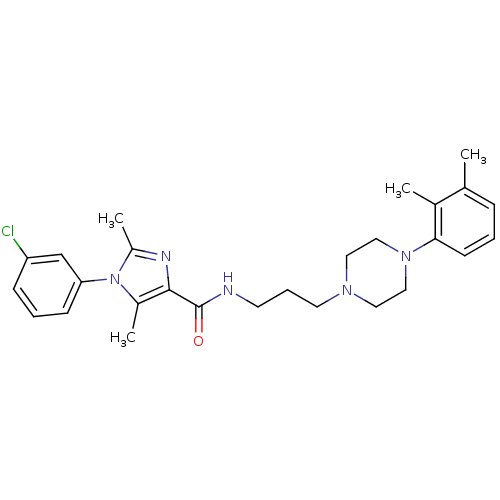
(CHEMBL1823091)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C27H34ClN5O/c1-19-8-5-11-25(20(19)2)32-16-14-31(15-17-32)13-7-12-29-27(34)26-21(3)33(22(4)30-26)24-10-6-9-23(28)18-24/h5-6,8-11,18H,7,12-17H2,1-4H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50352724
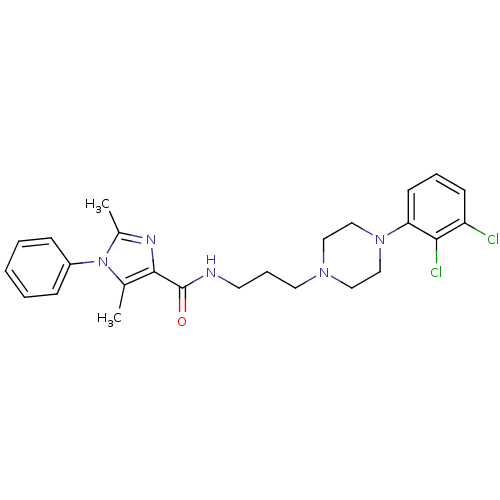
(CHEMBL1822896 | US8835436, Example 6)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2Cl)c(C)n1-c1ccccc1 Show InChI InChI=1S/C25H29Cl2N5O/c1-18-24(29-19(2)32(18)20-8-4-3-5-9-20)25(33)28-12-7-13-30-14-16-31(17-15-30)22-11-6-10-21(26)23(22)27/h3-6,8-11H,7,12-17H2,1-2H3,(H,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT1A receptor by competition binding assay |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352705
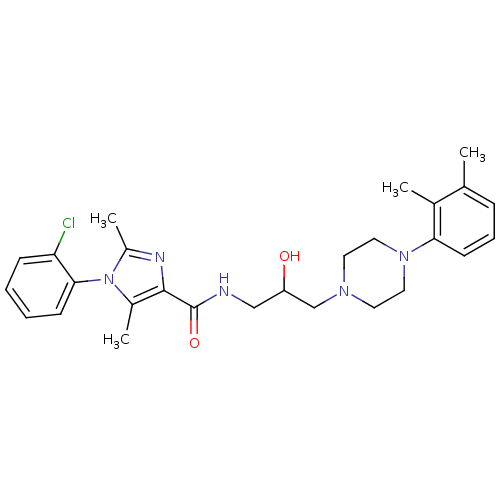
(CHEMBL1823203)Show SMILES Cc1nc(C(=O)NCC(O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1Cl |(17.54,-.1,;18.32,1.23,;19.86,1.23,;20.34,2.69,;21.67,3.46,;21.67,5,;23,2.69,;24.34,3.46,;25.67,2.69,;25.67,1.15,;27,3.46,;28.33,2.7,;29.66,3.48,;30.99,2.71,;31,1.17,;29.66,.4,;28.32,1.16,;32.33,.41,;33.66,1.19,;35,.42,;35,-1.12,;33.66,-1.89,;33.66,-3.43,;32.33,-1.13,;31,-1.9,;19.09,3.6,;19.08,5.14,;17.84,2.69,;16.51,3.47,;16.52,5,;15.18,5.78,;13.84,5.01,;13.84,3.46,;15.18,2.69,;15.19,1.15,)| Show InChI InChI=1S/C27H34ClN5O2/c1-18-8-7-11-24(19(18)2)32-14-12-31(13-15-32)17-22(34)16-29-27(35)26-20(3)33(21(4)30-26)25-10-6-5-9-23(25)28/h5-11,22,34H,12-17H2,1-4H3,(H,29,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352687
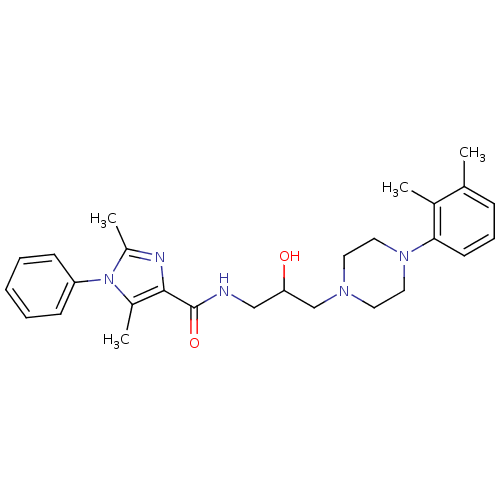
(CHEMBL1823101)Show SMILES Cc1nc(C(=O)NCC(O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1 Show InChI InChI=1S/C27H35N5O2/c1-19-9-8-12-25(20(19)2)31-15-13-30(14-16-31)18-24(33)17-28-27(34)26-21(3)32(22(4)29-26)23-10-6-5-7-11-23/h5-12,24,33H,13-18H2,1-4H3,(H,28,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352685
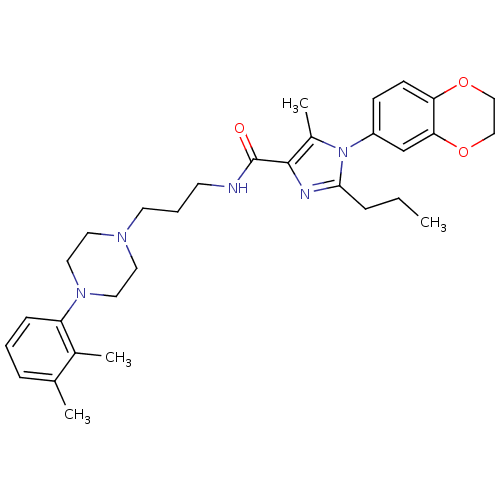
(CHEMBL1823099)Show SMILES CCCc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccc2OCCOc2c1 Show InChI InChI=1S/C31H41N5O3/c1-5-8-29-33-30(24(4)36(29)25-11-12-27-28(21-25)39-20-19-38-27)31(37)32-13-7-14-34-15-17-35(18-16-34)26-10-6-9-22(2)23(26)3/h6,9-12,21H,5,7-8,13-20H2,1-4H3,(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352731
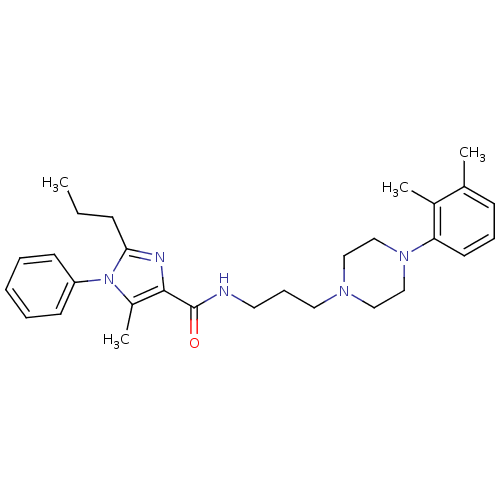
(CHEMBL1823058)Show SMILES CCCc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1 Show InChI InChI=1S/C29H39N5O/c1-5-11-27-31-28(24(4)34(27)25-13-7-6-8-14-25)29(35)30-16-10-17-32-18-20-33(21-19-32)26-15-9-12-22(2)23(26)3/h6-9,12-15H,5,10-11,16-21H2,1-4H3,(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352718

(CHEMBL1823214 | US8835436, Example 139)Show SMILES Cc1nc(C(=O)NC[C@H](O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1 |r| Show InChI InChI=1S/C27H35N5O2/c1-19-9-8-12-25(20(19)2)31-15-13-30(14-16-31)18-24(33)17-28-27(34)26-21(3)32(22(4)29-26)23-10-6-5-7-11-23/h5-12,24,33H,13-18H2,1-4H3,(H,28,34)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50352749
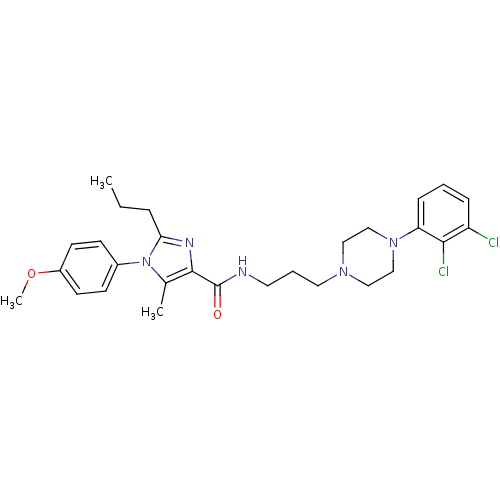
(CHEMBL1822905 | US8835436, Example 143)Show SMILES CCCc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2Cl)c(C)n1-c1ccc(OC)cc1 Show InChI InChI=1S/C28H35Cl2N5O2/c1-4-7-25-32-27(20(2)35(25)21-10-12-22(37-3)13-11-21)28(36)31-14-6-15-33-16-18-34(19-17-33)24-9-5-8-23(29)26(24)30/h5,8-13H,4,6-7,14-19H2,1-3H3,(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant serotonin 5-HT2A receptor expressed in CHO-K1 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352701

(CHEMBL1823189 | US8835436, Example 137)Show SMILES CCCc1nc(C(=O)NCC(O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccc(OC)cc1 Show InChI InChI=1S/C30H41N5O3/c1-6-8-28-32-29(23(4)35(28)24-11-13-26(38-5)14-12-24)30(37)31-19-25(36)20-33-15-17-34(18-16-33)27-10-7-9-21(2)22(27)3/h7,9-14,25,36H,6,8,15-20H2,1-5H3,(H,31,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352729
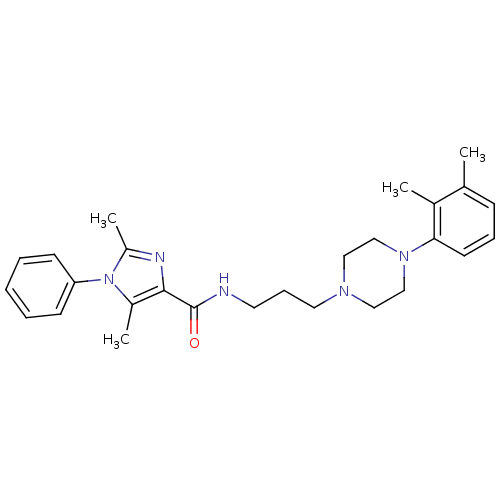
(CHEMBL1823056)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1 Show InChI InChI=1S/C27H35N5O/c1-20-10-8-13-25(21(20)2)31-18-16-30(17-19-31)15-9-14-28-27(33)26-22(3)32(23(4)29-26)24-11-6-5-7-12-24/h5-8,10-13H,9,14-19H2,1-4H3,(H,28,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352683
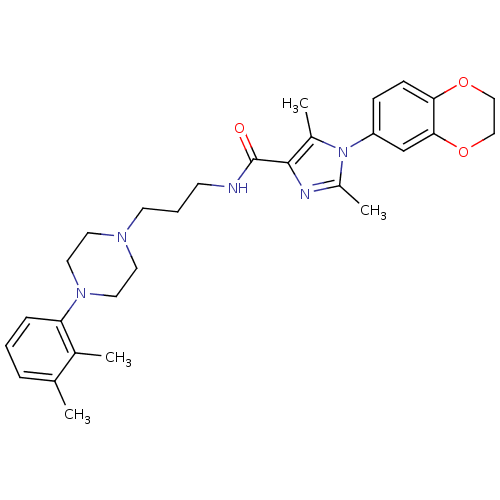
(CHEMBL1823097)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccc2OCCOc2c1 Show InChI InChI=1S/C29H37N5O3/c1-20-7-5-8-25(21(20)2)33-15-13-32(14-16-33)12-6-11-30-29(35)28-22(3)34(23(4)31-28)24-9-10-26-27(19-24)37-18-17-36-26/h5,7-10,19H,6,11-18H2,1-4H3,(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352665
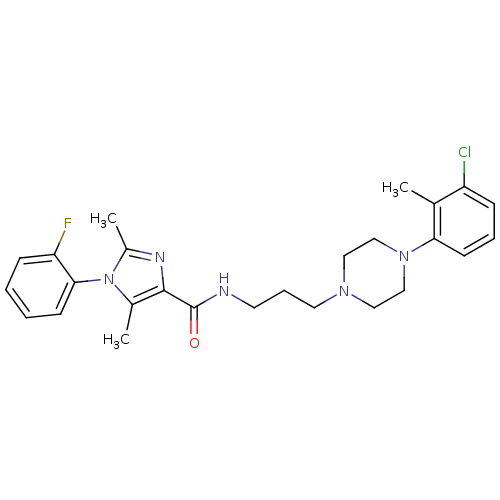
(CHEMBL1823079 | US8835436, Example 153)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2C)c(C)n1-c1ccccc1F |(13.53,.69,;14.3,2.02,;15.84,2.02,;16.32,3.49,;17.66,4.26,;17.66,5.8,;18.99,3.49,;20.32,4.26,;21.66,3.49,;22.99,4.26,;24.32,3.49,;25.64,4.27,;26.97,3.51,;26.98,1.96,;25.65,1.19,;24.31,1.96,;28.32,1.2,;29.65,1.98,;30.98,1.22,;30.99,-.33,;29.65,-1.1,;29.65,-2.64,;28.32,-.33,;26.99,-1.1,;15.07,4.39,;15.07,5.93,;13.83,3.49,;12.5,4.26,;12.5,5.8,;11.17,6.57,;9.83,5.8,;9.84,4.25,;11.17,3.49,;11.17,1.95,)| Show InChI InChI=1S/C26H31ClFN5O/c1-18-21(27)8-6-11-23(18)32-16-14-31(15-17-32)13-7-12-29-26(34)25-19(2)33(20(3)30-25)24-10-5-4-9-22(24)28/h4-6,8-11H,7,12-17H2,1-3H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352688
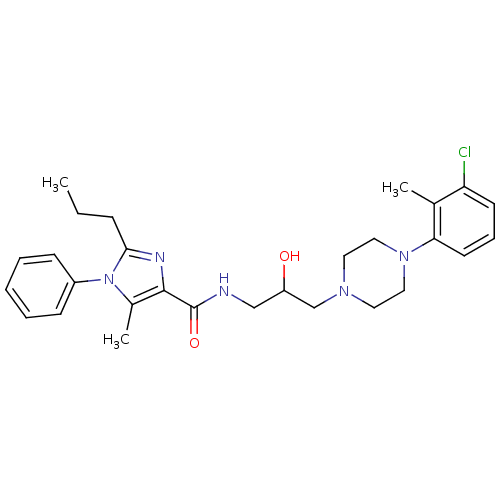
(CHEMBL1823102)Show SMILES CCCc1nc(C(=O)NCC(O)CN2CCN(CC2)c2cccc(Cl)c2C)c(C)n1-c1ccccc1 Show InChI InChI=1S/C28H36ClN5O2/c1-4-9-26-31-27(21(3)34(26)22-10-6-5-7-11-22)28(36)30-18-23(35)19-32-14-16-33(17-15-32)25-13-8-12-24(29)20(25)2/h5-8,10-13,23,35H,4,9,14-19H2,1-3H3,(H,30,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352656
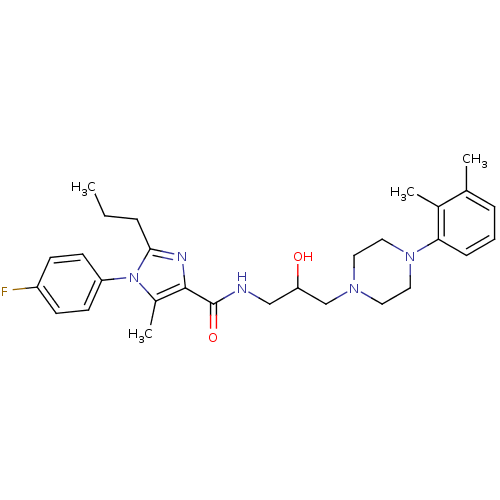
(CHEMBL1823199)Show SMILES CCCc1nc(C(=O)NCC(O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccc(F)cc1 Show InChI InChI=1S/C29H38FN5O2/c1-5-7-27-32-28(22(4)35(27)24-12-10-23(30)11-13-24)29(37)31-18-25(36)19-33-14-16-34(17-15-33)26-9-6-8-20(2)21(26)3/h6,8-13,25,36H,5,7,14-19H2,1-4H3,(H,31,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352670
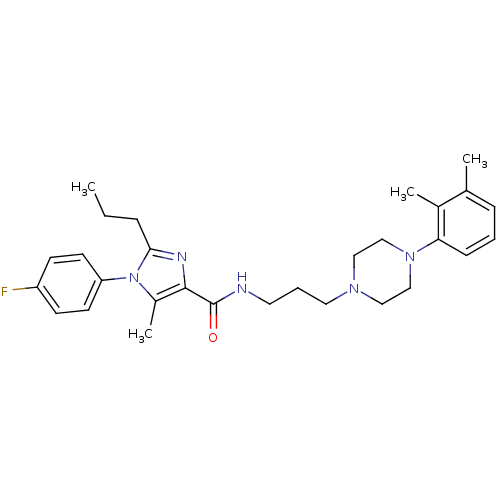
(CHEMBL1823084)Show SMILES CCCc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccc(F)cc1 Show InChI InChI=1S/C29H38FN5O/c1-5-8-27-32-28(23(4)35(27)25-13-11-24(30)12-14-25)29(36)31-15-7-16-33-17-19-34(20-18-33)26-10-6-9-21(2)22(26)3/h6,9-14H,5,7-8,15-20H2,1-4H3,(H,31,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM9274

(CHEMBL1822893 | US8835436, Example 8)Show SMILES Cc1nc(C(=O)NCCN2CCN(CC2)c2cccc(Cl)c2Cl)c(C)n1-c1ccccc1 Show InChI InChI=1S/C24H27Cl2N5O/c1-17-23(28-18(2)31(17)19-7-4-3-5-8-19)24(32)27-11-12-29-13-15-30(16-14-29)21-10-6-9-20(25)22(21)26/h3-10H,11-16H2,1-2H3,(H,27,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor by competition binding assay |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352666
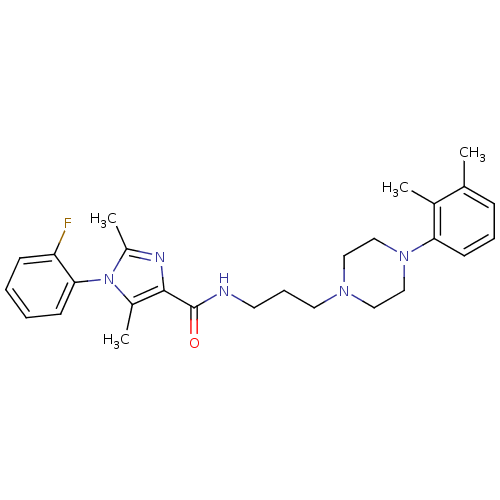
(CHEMBL1823080)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1F |(-6.05,-10.44,;-5.27,-9.11,;-3.73,-9.11,;-3.25,-7.64,;-1.92,-6.87,;-1.92,-5.33,;-.59,-7.64,;.75,-6.87,;2.08,-7.64,;3.41,-6.87,;4.74,-7.64,;6.07,-6.86,;7.4,-7.62,;7.41,-9.17,;6.07,-9.94,;4.73,-9.17,;8.74,-9.93,;10.07,-9.15,;11.41,-9.91,;11.41,-11.46,;10.07,-12.23,;10.07,-13.77,;8.74,-11.46,;7.41,-12.23,;-4.5,-6.74,;-4.51,-5.2,;-5.75,-7.64,;-7.08,-6.87,;-7.07,-5.33,;-8.41,-4.56,;-9.75,-5.33,;-9.74,-6.88,;-8.41,-7.64,;-8.41,-9.18,)| Show InChI InChI=1S/C27H34FN5O/c1-19-9-7-12-24(20(19)2)32-17-15-31(16-18-32)14-8-13-29-27(34)26-21(3)33(22(4)30-26)25-11-6-5-10-23(25)28/h5-7,9-12H,8,13-18H2,1-4H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352676
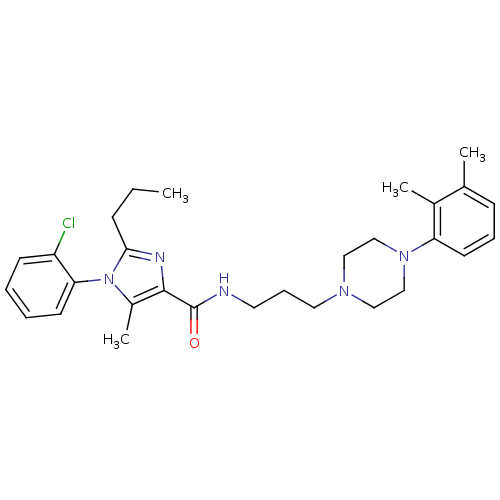
(CHEMBL1823090)Show SMILES CCCc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1Cl |(-8.42,-1.08,;-7.64,.25,;-6.1,.25,;-5.33,1.58,;-3.79,1.58,;-3.31,3.04,;-1.97,3.81,;-1.97,5.35,;-.64,3.04,;.69,3.81,;2.03,3.04,;3.36,3.81,;4.69,3.05,;6.01,3.83,;7.34,3.06,;7.35,1.52,;6.02,.75,;4.68,1.51,;8.68,.76,;10.02,1.54,;11.35,.77,;11.36,-.77,;10.02,-1.54,;10.02,-3.08,;8.69,-.78,;7.35,-1.55,;-4.56,3.95,;-4.56,5.49,;-5.8,3.04,;-7.13,3.82,;-7.13,5.35,;-8.46,6.13,;-9.8,5.36,;-9.8,3.81,;-8.46,3.04,;-8.46,1.5,)| Show InChI InChI=1S/C29H38ClN5O/c1-5-10-27-32-28(23(4)35(27)26-13-7-6-12-24(26)30)29(36)31-15-9-16-33-17-19-34(20-18-33)25-14-8-11-21(2)22(25)3/h6-8,11-14H,5,9-10,15-20H2,1-4H3,(H,31,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352652
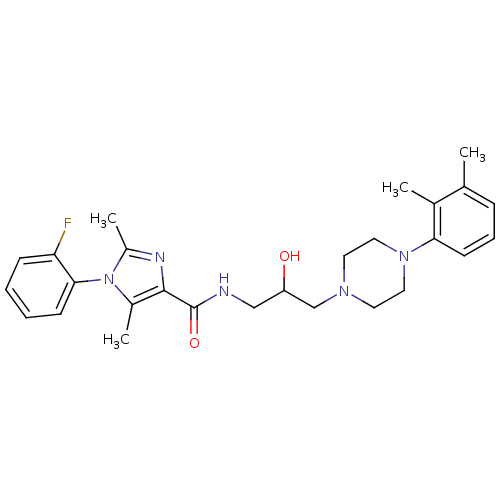
(CHEMBL1823195)Show SMILES Cc1nc(C(=O)NCC(O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1F |(17.63,-21.49,;18.4,-20.16,;19.94,-20.16,;20.42,-18.7,;21.76,-17.93,;21.76,-16.39,;23.09,-18.7,;24.42,-17.93,;25.76,-18.7,;25.76,-20.24,;27.09,-17.93,;28.42,-18.69,;29.74,-17.91,;31.07,-18.68,;31.08,-20.22,;29.75,-20.99,;28.41,-20.23,;32.41,-20.98,;33.75,-20.2,;35.08,-20.97,;35.09,-22.51,;33.75,-23.28,;33.75,-24.82,;32.42,-22.52,;31.08,-23.29,;19.17,-17.79,;19.17,-16.25,;17.93,-18.7,;16.6,-17.92,;16.6,-16.38,;15.27,-15.61,;13.93,-16.38,;13.93,-17.93,;15.27,-18.69,;15.27,-20.23,)| Show InChI InChI=1S/C27H34FN5O2/c1-18-8-7-11-24(19(18)2)32-14-12-31(13-15-32)17-22(34)16-29-27(35)26-20(3)33(21(4)30-26)25-10-6-5-9-23(25)28/h5-11,22,34H,12-17H2,1-4H3,(H,29,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50352727

(CHEMBL1822913 | US8835436, Example 150)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2Cl)c(C)n1-c1ccc2OCCOc2c1 Show InChI InChI=1S/C27H31Cl2N5O3/c1-18-26(31-19(2)34(18)20-7-8-23-24(17-20)37-16-15-36-23)27(35)30-9-4-10-32-11-13-33(14-12-32)22-6-3-5-21(28)25(22)29/h3,5-8,17H,4,9-16H2,1-2H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant serotonin 5-HT2A receptor expressed in CHO-K1 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50352756

(CHEMBL1823069 | US8835436, Example 99)Show SMILES COc1ccccc1-n1c(C)nc(C(=O)NCCCN2CCN(CC2)c2cccc(C)c2C)c1C |(15.99,-4.6,;15.99,-6.14,;14.65,-6.9,;13.32,-6.13,;11.97,-6.9,;11.98,-8.45,;13.32,-9.21,;14.64,-8.44,;15.98,-9.22,;16.45,-10.68,;15.68,-12.01,;17.99,-10.68,;18.47,-9.22,;19.8,-8.45,;19.8,-6.91,;21.14,-9.22,;22.47,-8.45,;23.8,-9.22,;25.14,-8.45,;26.47,-9.21,;27.79,-8.43,;29.12,-9.2,;29.13,-10.74,;27.8,-11.51,;26.45,-10.74,;30.46,-11.5,;31.79,-10.72,;33.13,-11.49,;33.14,-13.03,;31.8,-13.8,;31.8,-15.34,;30.47,-13.03,;29.13,-13.81,;17.22,-8.31,;17.22,-6.77,)| Show InChI InChI=1S/C28H37N5O2/c1-20-10-8-12-24(21(20)2)32-18-16-31(17-19-32)15-9-14-29-28(34)27-22(3)33(23(4)30-27)25-11-6-7-13-26(25)35-5/h6-8,10-13H,9,14-19H2,1-5H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data