

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50234418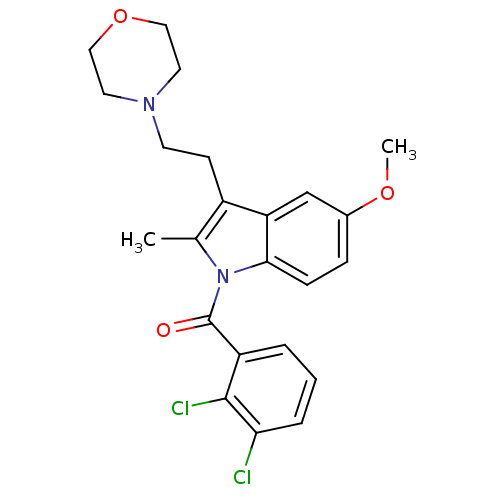 ((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50234418 ((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341928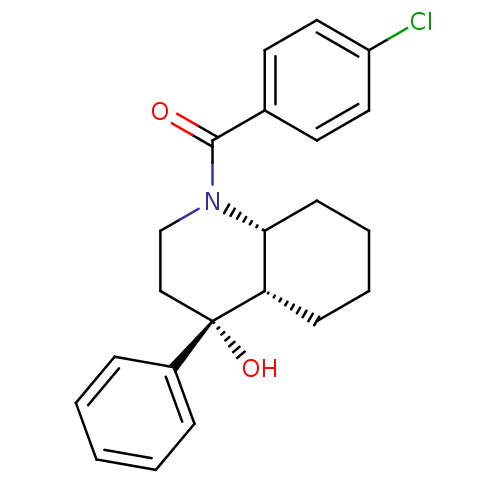 (CHEMBL1765160 | cis-(4-chlorophenyl)((4R,4aS,8aR)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340312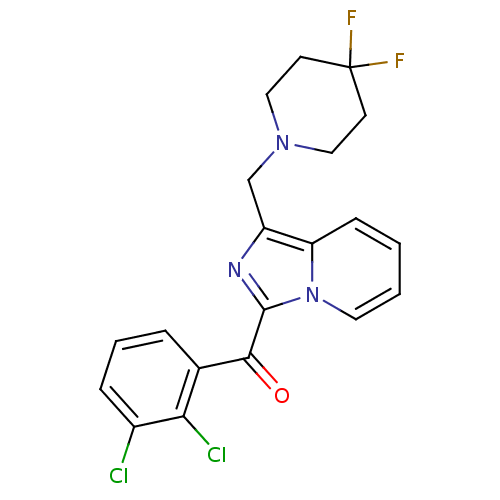 ((2,3-dichlorophenyl)(1-((4,4-difluoropiperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341934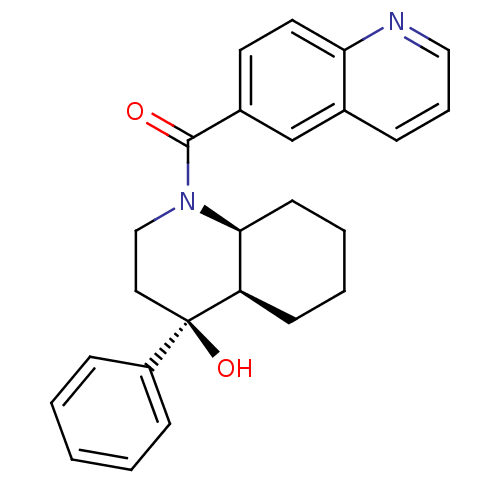 (CHEMBL1765259 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341920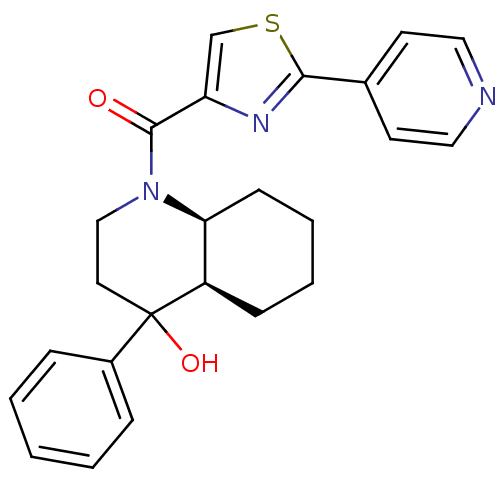 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341920 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341930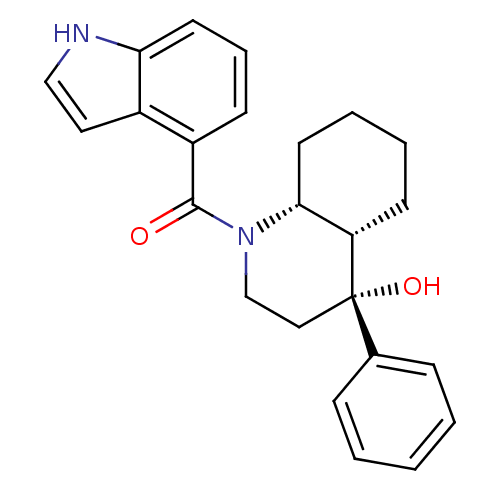 (CHEMBL1765246 | cis-((4R,4aS,8aR)-4-hydroxy-4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340311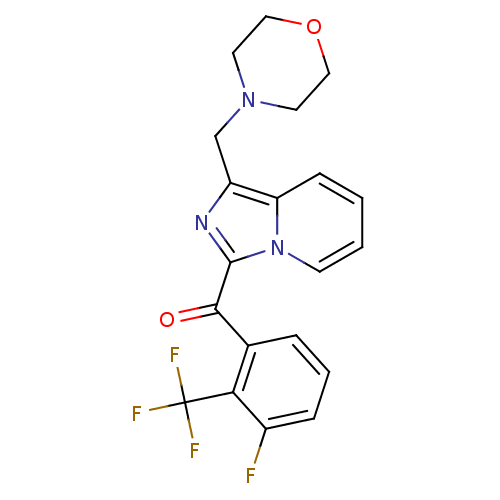 ((3-fluoro-2-(trifluoromethyl)phenyl)(1-(morpholino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341929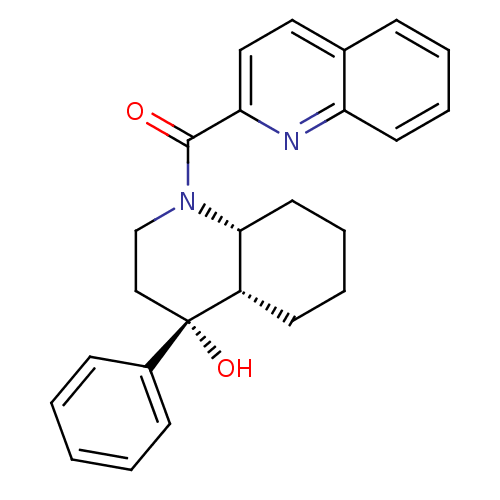 (CHEMBL1765161 | cis-((4R,4aS,8aR)-4-hydroxy-4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341936 (CHEMBL1765261 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022671 (CHEMBL3298265) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340313 (1-((4,4-difluoropiperidin-1-yl)methyl)-N-(6-(trifl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340315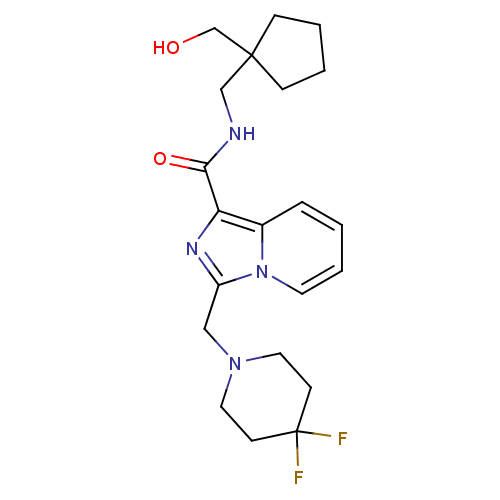 (3-((4,4-difluoropiperidin-1-yl)methyl)-N-((1-(hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446392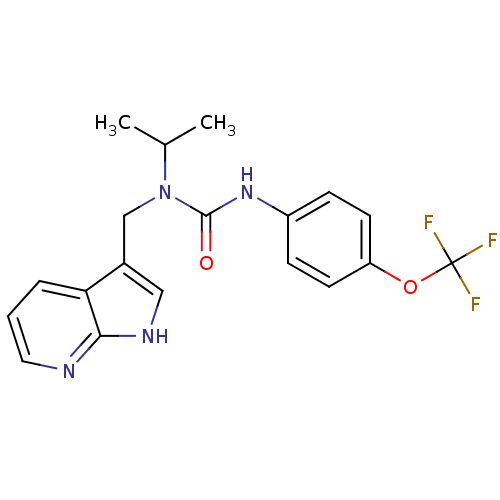 (CHEMBL3109645 | US9181261, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341933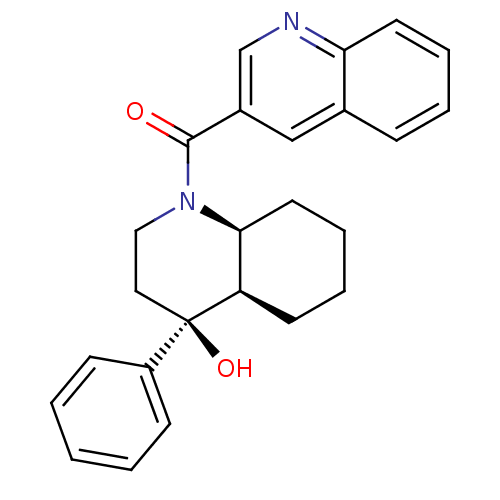 (CHEMBL1765258 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341938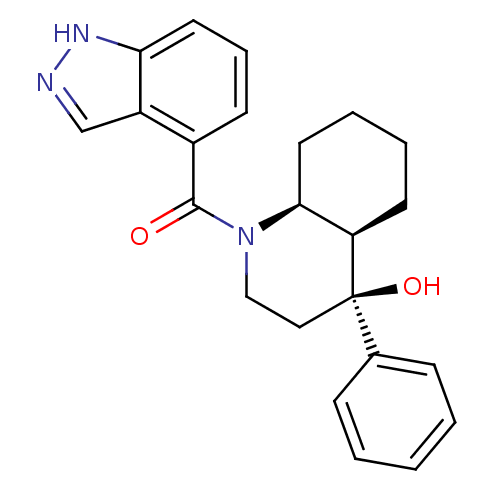 (CHEMBL1765263 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341927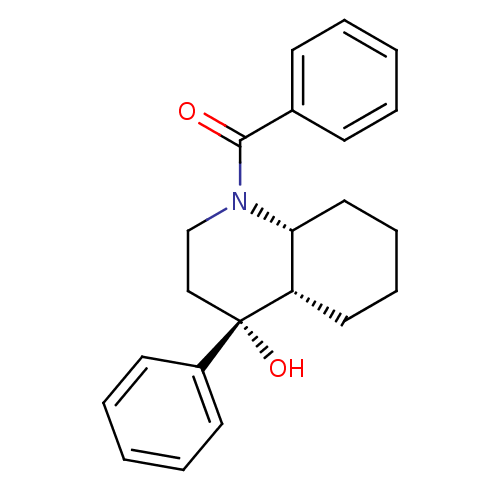 (CHEMBL1765159 | cis-((4R,4aS,8aR)-4-hydroxy-4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50005282 (CHEMBL3114534) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as forskolin-induced cAMP accumulation after 1 hr | Bioorg Med Chem Lett 24: 1218-21 (2014) Article DOI: 10.1016/j.bmcl.2013.12.068 BindingDB Entry DOI: 10.7270/Q2FJ2J9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341919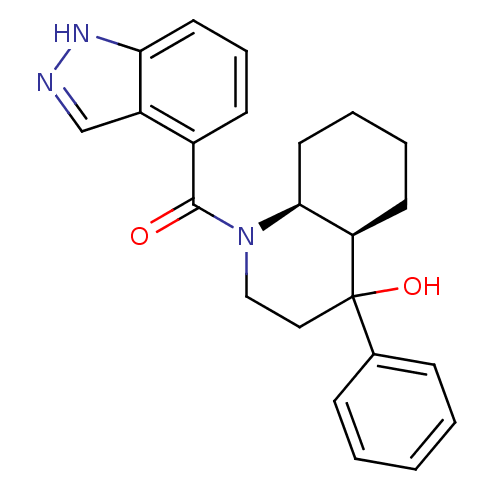 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341919 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340310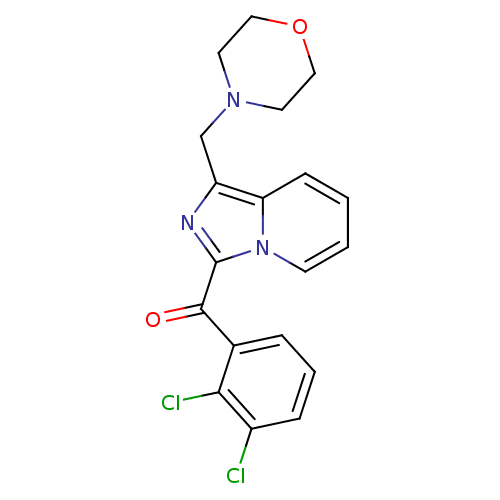 ((2,3-dichlorophenyl)(1-(morpholinomethyl)imidazo[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341935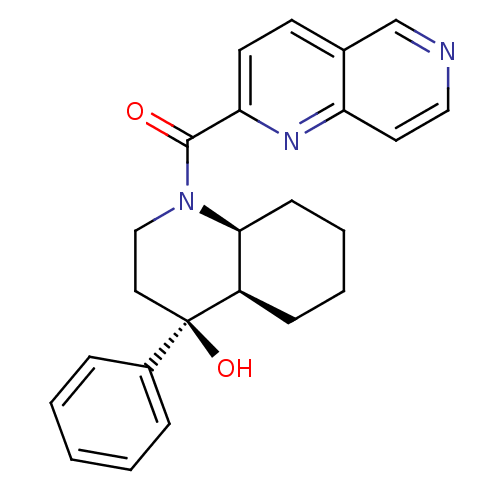 (CHEMBL1765260 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341932 (CHEMBL1765248 | cis-((4R,4aS,8aR)-4-hydroxy-4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341942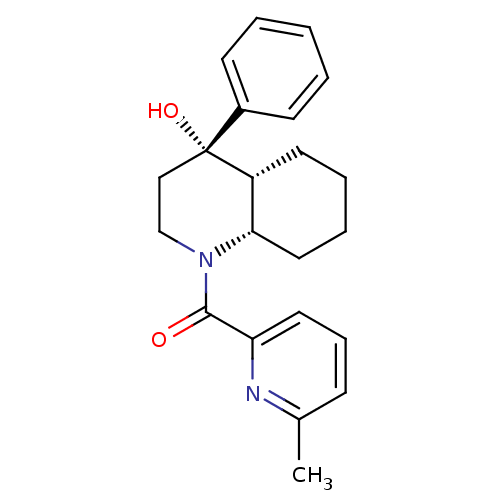 (CHEMBL1765267 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341940 (CHEMBL1765265 | cis-rac-(3-fluoropyridin-4-yl)((4S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50341951 (((4S,4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fors... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022675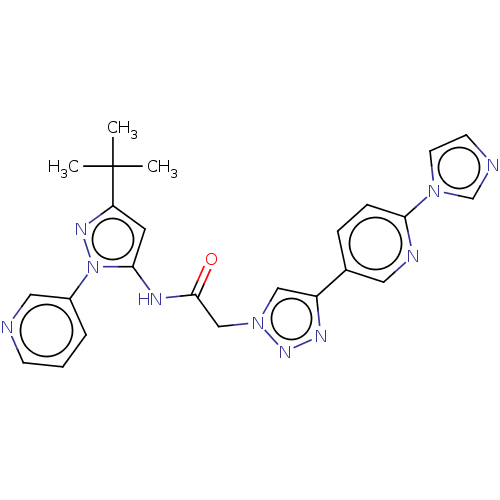 (CHEMBL3298268) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340316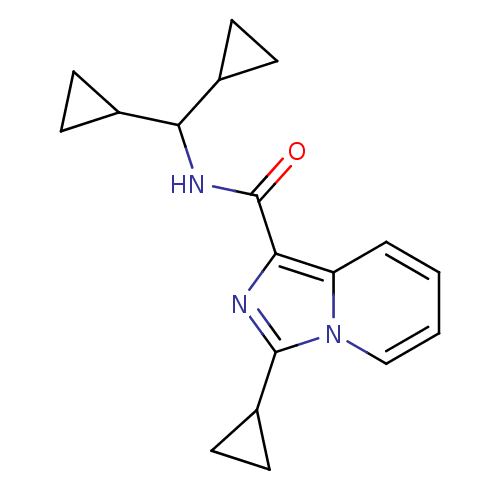 (3-cyclopropyl-N-(dicyclopropylmethyl)imidazo[1,5-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341951 (((4S,4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341951 (((4S,4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341920 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022674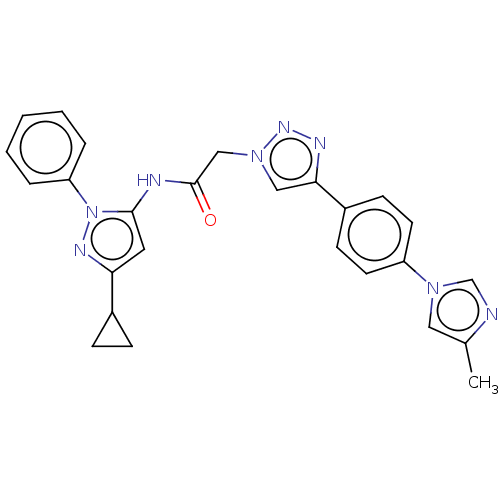 (CHEMBL3298267) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022675 (CHEMBL3298268) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341939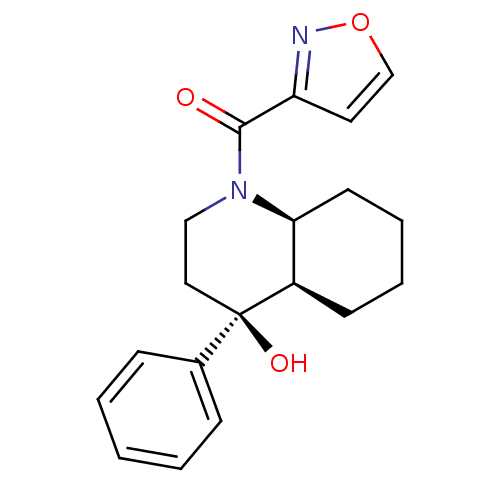 (CHEMBL1765264 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50340310 ((2,3-dichlorophenyl)(1-(morpholinomethyl)imidazo[1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446392 (CHEMBL3109645 | US9181261, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022670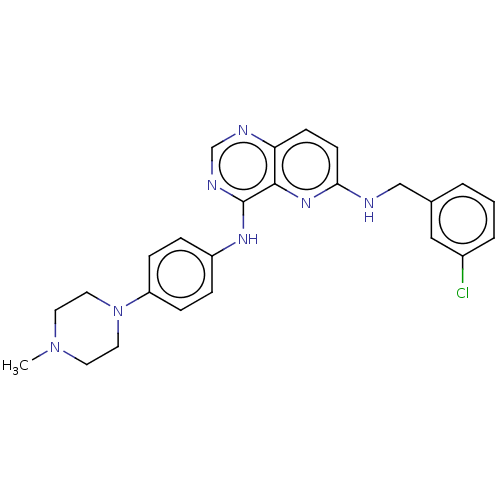 (CHEMBL3297748) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341955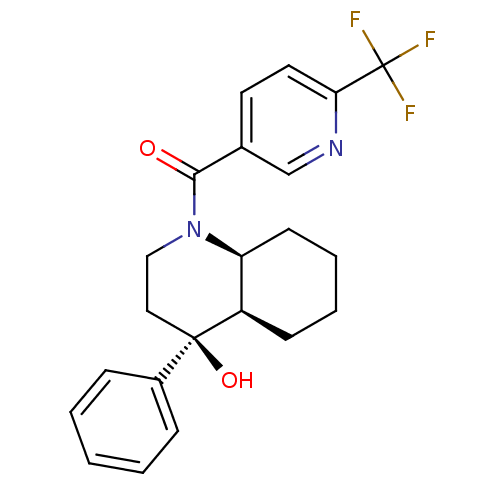 (CHEMBL1765281 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341945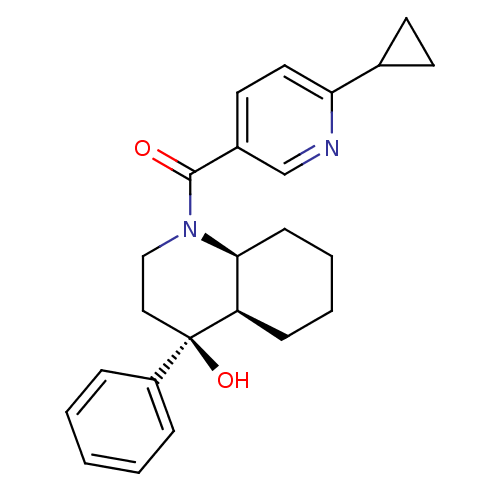 (CHEMBL1765270 | cis-rac-(6-cyclopropylpyridin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022671 (CHEMBL3298265) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341952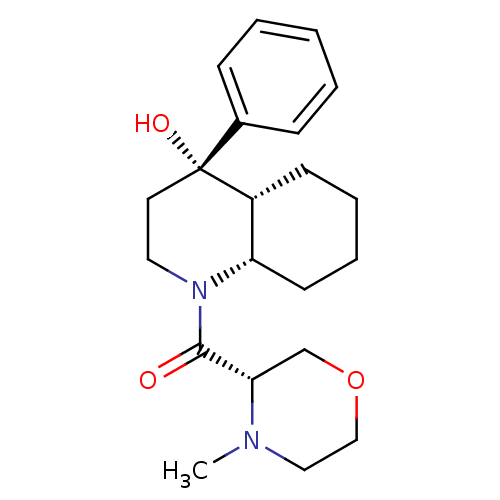 (((4S,4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341943 (CHEMBL1765268 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50460861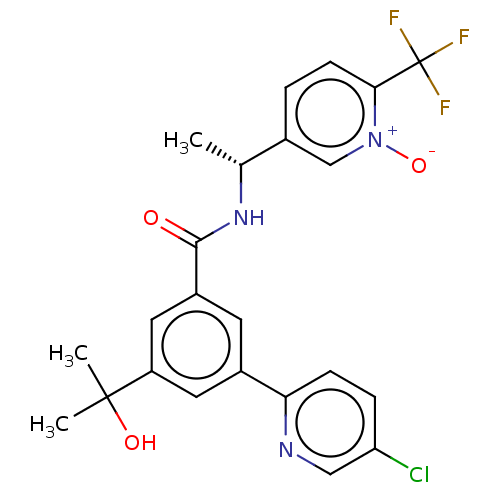 (CHEMBL4229237) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X3 receptor expressed in Flp-In-293 cells assessed as inhibition of CTP-induced chloride current response ... | Bioorg Med Chem Lett 28: 1392-1396 (2018) Article DOI: 10.1016/j.bmcl.2018.02.039 BindingDB Entry DOI: 10.7270/Q25T3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341931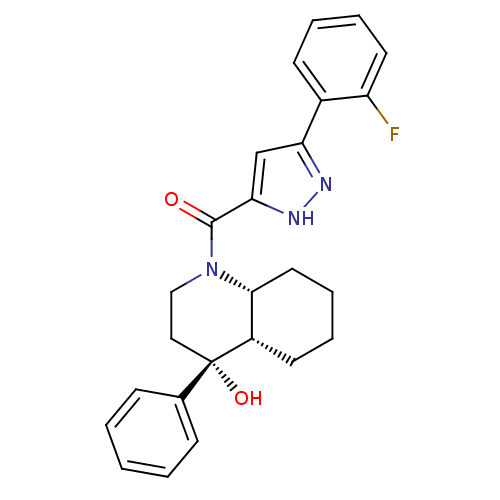 (CHEMBL1765247 | cis-(5-(2-fluorophenyl)-1H-pyrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340318 ((R)-3-morpholino-N-(2,2,2-trifluoro-1-(pyridin-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340317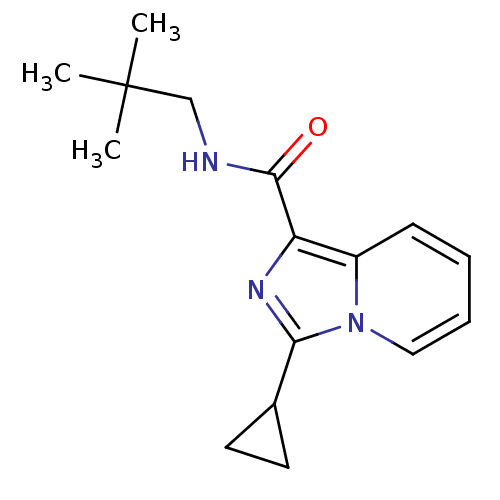 (3-cyclopropyl-N-neopentylimidazo[1,5-a]pyridine-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341924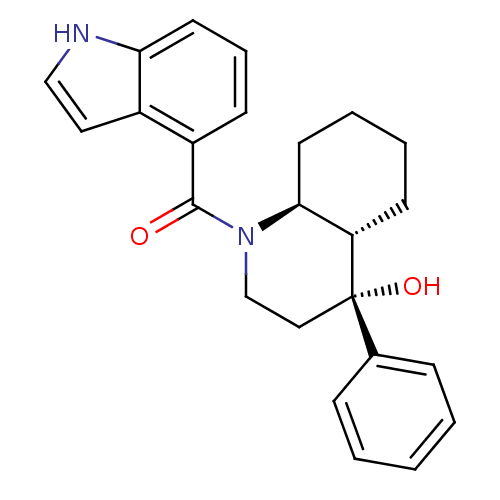 (CHEMBL1765156 | trans-((4R,4aS,8aS)-4-hydroxy-4-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341922 (CHEMBL1765154 | trans-(4-chlorophenyl)((4R,4aS,8aS...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50460863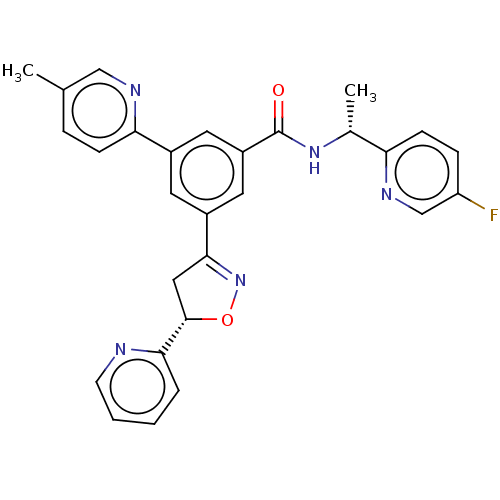 (CHEMBL4227228) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X3 receptor expressed in Flp-In-293 cells assessed as inhibition of CTP-induced chloride current response ... | Bioorg Med Chem Lett 28: 1392-1396 (2018) Article DOI: 10.1016/j.bmcl.2018.02.039 BindingDB Entry DOI: 10.7270/Q25T3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 285 total ) | Next | Last >> |