 Found 374 hits with Last Name = 'leung' and Initial = 'c'
Found 374 hits with Last Name = 'leung' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50024544
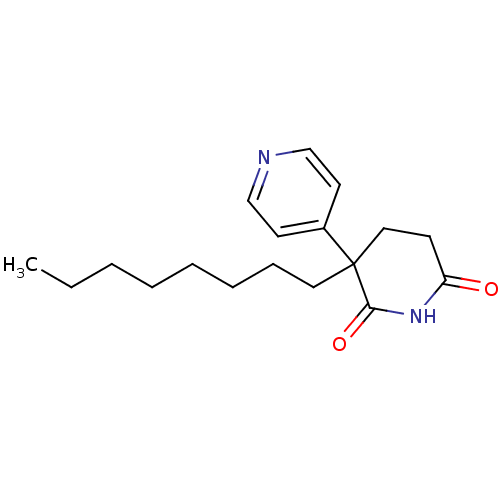
(3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-11-18(15-9-13-19-14-10-15)12-8-16(21)20-17(18)22/h9-10,13-14H,2-8,11-12H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015983
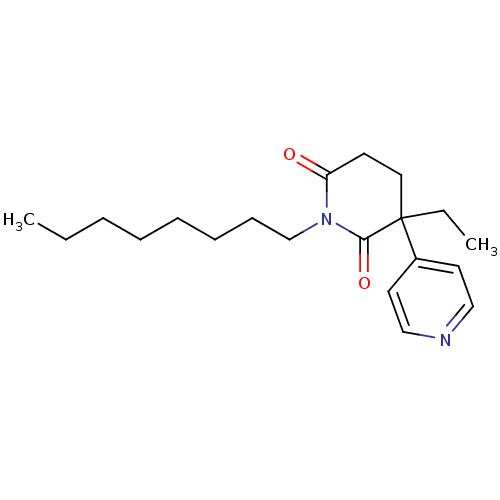
((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with testosterone |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024544

(3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-11-18(15-9-13-19-14-10-15)12-8-16(21)20-17(18)22/h9-10,13-14H,2-8,11-12H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015983

((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8885
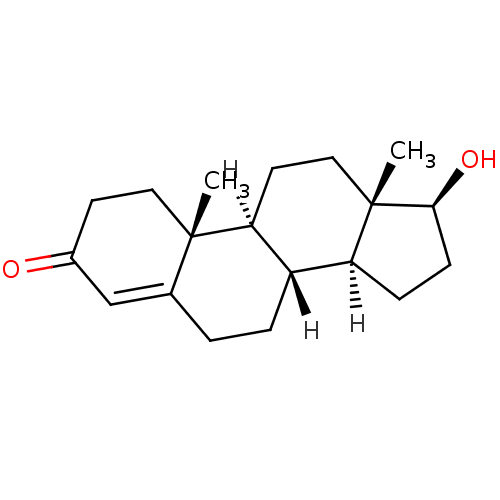
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase Cytochrome P450 19A1 |
J Med Chem 26: 50-4 (1983)
BindingDB Entry DOI: 10.7270/Q2V125CG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant (Ki) for Cytochrome P450 19A1 |
J Med Chem 28: 200-4 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1FK8 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant (Ki) for Cytochrome P450 19A1 |
J Med Chem 28: 200-4 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1FK8 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Klebsiella pneumoniae) | BDBM50548231
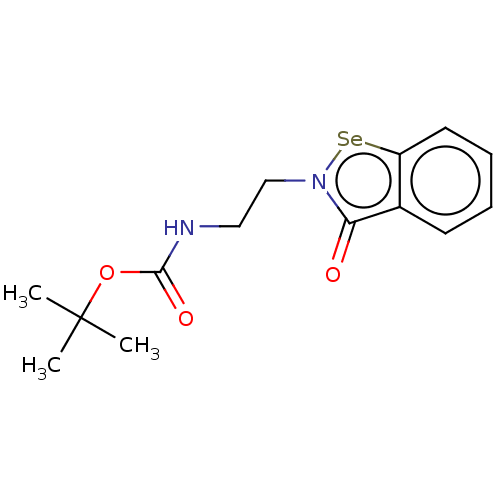
(CHEMBL4753599)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6]-[#6]-n1[se;v2]c2ccccc2c1=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type N-terminal His6-tagged Klebsiella pneumoniae NDM-1 expressed in Escherichia coli BL21 assessed as inhibition constant using n... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.06.007
BindingDB Entry DOI: 10.7270/Q2SB49CC |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM195608
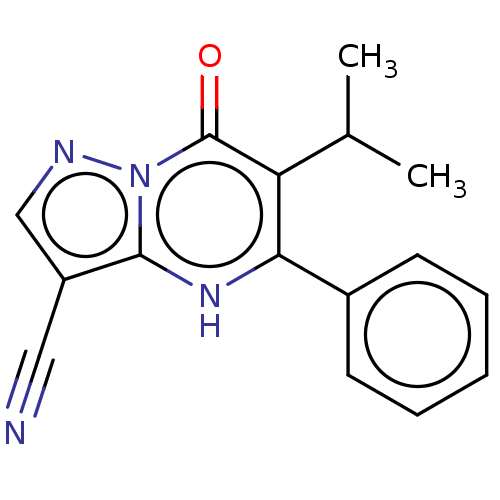
(CPI-455)Show InChI InChI=1S/C16H14N4O/c1-10(2)13-14(11-6-4-3-5-7-11)19-15-12(8-17)9-18-20(15)16(13)21/h3-7,9-10,19H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578
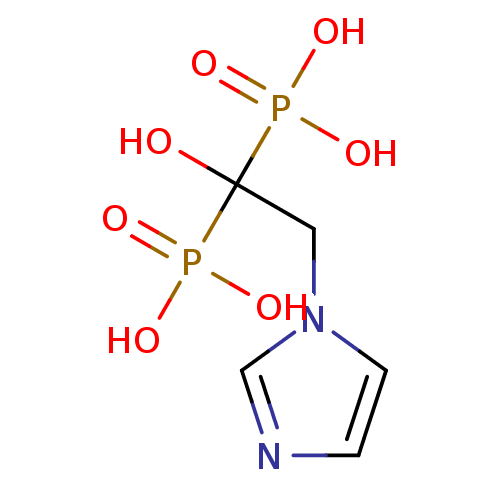
(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566950
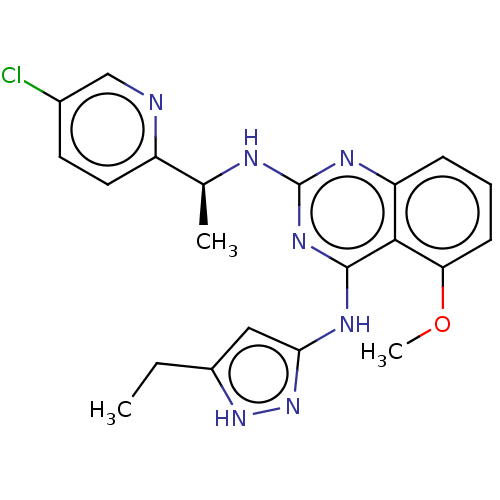
(CHEMBL4852125)Show SMILES CCc1cc(Nc2nc(N[C@@H](C)c3ccc(Cl)cn3)nc3cccc(OC)c23)n[nH]1 |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566949
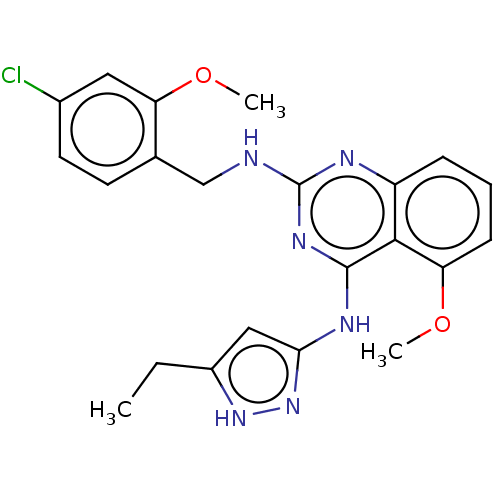
(CHEMBL4877302)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(OC)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK6 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM50609278

(CHEMBL5272926)Show SMILES CCCCCCCN(C)Cc1ccc(cc1)-c1[nH]c2c(cnn2c(=O)c1C(C)C)C#N | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50158830
(CHEMBL3785583)Show SMILES CC(C)c1c([nH]c2c(cnn2c1=O)C#N)-c1cnn(c1)C(C)(C)C Show InChI InChI=1S/C17H20N6O/c1-10(2)13-14(12-8-19-22(9-12)17(3,4)5)21-15-11(6-18)7-20-23(15)16(13)24/h7-10,21H,1-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576
(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566953
(CHEMBL4860332)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(C)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rhodopsin kinase GRK7
(Homo sapiens (Human)) | BDBM50566949

(CHEMBL4877302)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(OC)c23)n[nH]1 | NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human GRK7 using casein as substrate incubated for 40 mins in presence of [gamma-33ATP] by scintillation counti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50481825

(CHEMBL5277258)Show SMILES NS(=O)(=O)c1ccc(C\N=C\c2ccccc2[N+]([O-])=O)cc1 Show InChI InChI=1S/C14H13N3O4S/c15-22(20,21)13-7-5-11(6-8-13)9-16-10-12-3-1-2-4-14(12)17(18)19/h1-8,10H,9H2,(H2,15,20,21)/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566946
(CHEMBL4854871)Show SMILES CCc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)nc3cccc(OC)c23)n[nH]1 |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566949

(CHEMBL4877302)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(OC)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM195608

(CPI-455)Show InChI InChI=1S/C16H14N4O/c1-10(2)13-14(11-6-4-3-5-7-11)19-15-12(8-17)9-18-20(15)16(13)21/h3-7,9-10,19H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566923

(CHEMBL4855000)Show SMILES CCc1cc(Nc2nc(NCCc3ccccc3)nc3cccc(OC)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM50481825

(CHEMBL5277258)Show SMILES NS(=O)(=O)c1ccc(C\N=C\c2ccccc2[N+]([O-])=O)cc1 Show InChI InChI=1S/C14H13N3O4S/c15-22(20,21)13-7-5-11(6-8-13)9-16-10-12-3-1-2-4-14(12)17(18)19/h1-8,10H,9H2,(H2,15,20,21)/b16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443050
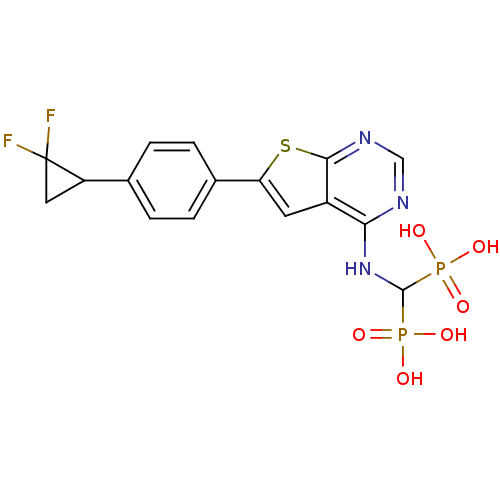
(CHEMBL3087938)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(cc1)C1CC1(F)F)P(O)(O)=O Show InChI InChI=1S/C16H15F2N3O6P2S/c17-16(18)6-11(16)8-1-3-9(4-2-8)12-5-10-13(19-7-20-14(10)30-12)21-15(28(22,23)24)29(25,26)27/h1-5,7,11,15H,6H2,(H,19,20,21)(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition by scintillation counting analy... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566951
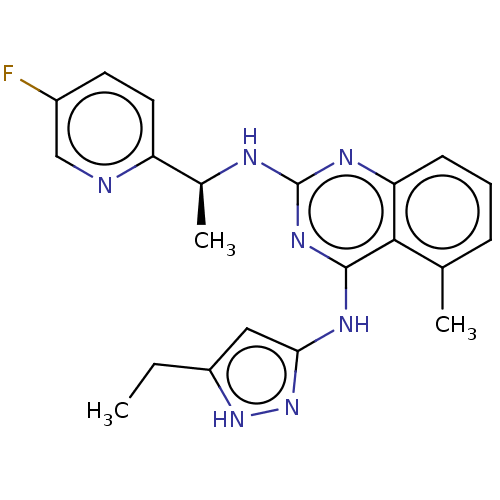
(CHEMBL4875929)Show SMILES CCc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)nc3cccc(C)c23)n[nH]1 |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50566915
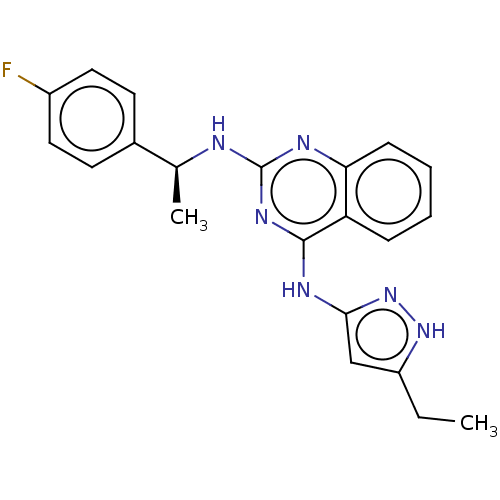
(CHEMBL4848826)Show SMILES CCc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cc3)nc3ccccc23)n[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human Aurora A using LRRASLG as substrate incubated for 40 mins in presence of [gamma-33ATP] by radiometric sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 5
(Homo sapiens (Human)) | BDBM50566949

(CHEMBL4877302)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(OC)c23)n[nH]1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human GRK5 using casein as substrate incubated for 40 mins in presence of [gamma-33ATP] by scintillation counti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5C
(Homo sapiens (Human)) | BDBM195608

(CPI-455)Show InChI InChI=1S/C16H14N4O/c1-10(2)13-14(11-6-4-3-5-7-11)19-15-12(8-17)9-18-20(15)16(13)21/h3-7,9-10,19H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443052
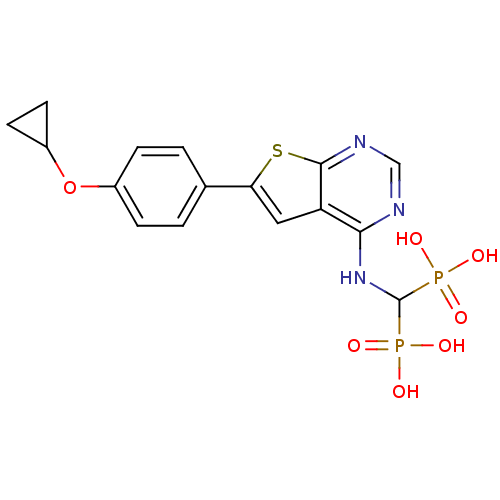
(CHEMBL3087936 | US11279719, Example C-13)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C16H17N3O7P2S/c20-27(21,22)16(28(23,24)25)19-14-12-7-13(29-15(12)18-8-17-14)9-1-3-10(4-2-9)26-11-5-6-11/h1-4,7-8,11,16H,5-6H2,(H,17,18,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443052

(CHEMBL3087936 | US11279719, Example C-13)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C16H17N3O7P2S/c20-27(21,22)16(28(23,24)25)19-14-12-7-13(29-15(12)18-8-17-14)9-1-3-10(4-2-9)26-11-5-6-11/h1-4,7-8,11,16H,5-6H2,(H,17,18,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM50158830

(CHEMBL3785583)Show SMILES CC(C)c1c([nH]c2c(cnn2c1=O)C#N)-c1cnn(c1)C(C)(C)C Show InChI InChI=1S/C17H20N6O/c1-10(2)13-14(12-8-19-22(9-12)17(3,4)5)21-15-11(6-18)7-20-23(15)16(13)24/h7-10,21H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rhodopsin kinase GRK7
(Homo sapiens (Human)) | BDBM50566946

(CHEMBL4854871)Show SMILES CCc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)nc3cccc(OC)c23)n[nH]1 |r| | NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human GRK7 using casein as substrate incubated for 40 mins in presence of [gamma-33ATP] by scintillation counti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443055
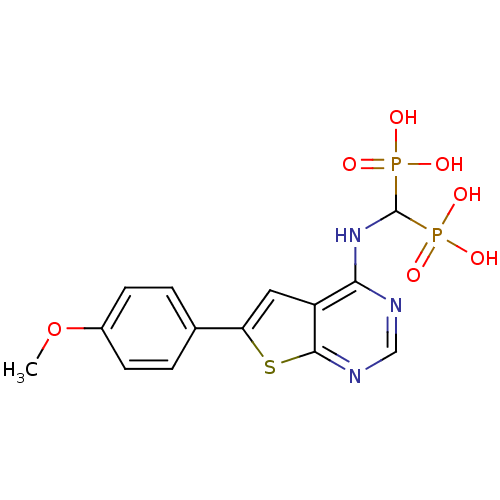
(CHEMBL3087933)Show SMILES COc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O7P2S/c1-24-9-4-2-8(3-5-9)11-6-10-12(15-7-16-13(10)27-11)17-14(25(18,19)20)26(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM50540061
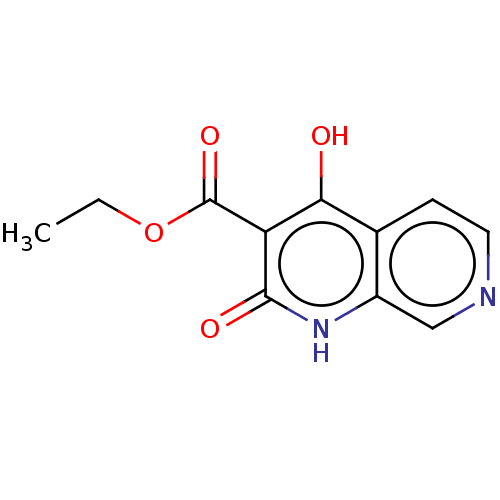
(CHEMBL4637168)Show InChI InChI=1S/C11H10N2O4/c1-2-17-11(16)8-9(14)6-3-4-12-5-7(6)13-10(8)15/h3-5H,2H2,1H3,(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566948

(CHEMBL4874424)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3)nc3cccc(OC)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50421094
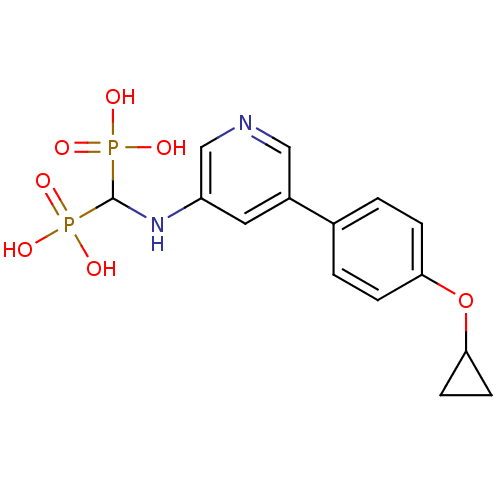
(CHEMBL2088339)Show SMILES OP(O)(=O)C(Nc1cncc(c1)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C15H18N2O7P2/c18-25(19,20)15(26(21,22)23)17-12-7-11(8-16-9-12)10-1-3-13(4-2-10)24-14-5-6-14/h1-4,7-9,14-15,17H,5-6H2,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition by scintillation counting analy... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566946

(CHEMBL4854871)Show SMILES CCc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)nc3cccc(OC)c23)n[nH]1 |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK6 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566947
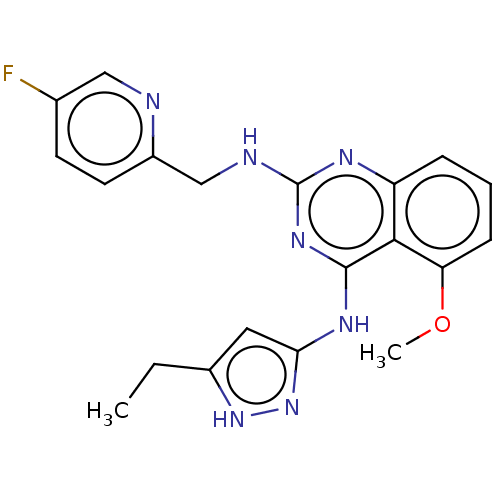
(CHEMBL4847703)Show SMILES CCc1cc(Nc2nc(NCc3ccc(F)cn3)nc3cccc(OC)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50421094

(CHEMBL2088339)Show SMILES OP(O)(=O)C(Nc1cncc(c1)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C15H18N2O7P2/c18-25(19,20)15(26(21,22)23)17-12-7-11(8-16-9-12)10-1-3-13(4-2-10)24-14-5-6-14/h1-4,7-9,14-15,17H,5-6H2,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50421094

(CHEMBL2088339)Show SMILES OP(O)(=O)C(Nc1cncc(c1)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C15H18N2O7P2/c18-25(19,20)15(26(21,22)23)17-12-7-11(8-16-9-12)10-1-3-13(4-2-10)24-14-5-6-14/h1-4,7-9,14-15,17H,5-6H2,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM223320
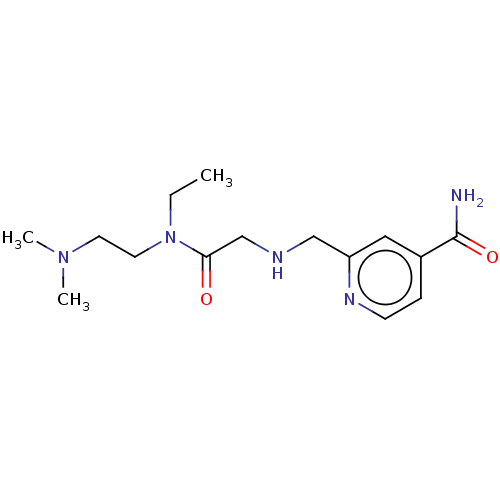
(KDOAM-25)Show InChI InChI=1S/C15H25N5O2/c1-4-20(8-7-19(2)3)14(21)11-17-10-13-9-12(15(16)22)5-6-18-13/h5-6,9,17H,4,7-8,10-11H2,1-3H3,(H2,16,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566922

(CHEMBL4876072)Show SMILES CCc1cc(Nc2nc(NCCc3ccccc3)nc3cccc(F)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50481821
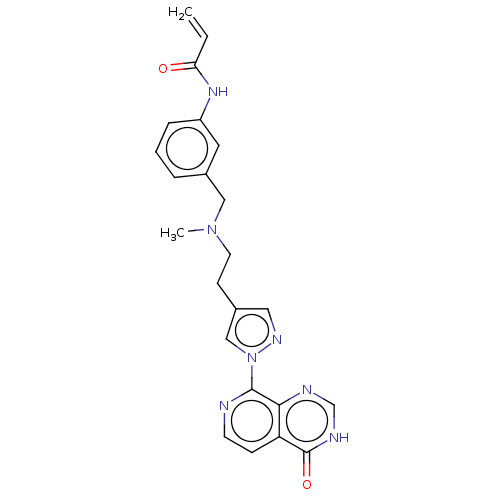
(CHEMBL5277129)Show InChI InChI=1S/C14H14N2O3S/c1-19-14-5-3-2-4-11(14)10-16-12-6-8-13(9-7-12)20(15,17)18/h2-10H,1H3,(H2,15,17,18)/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443054
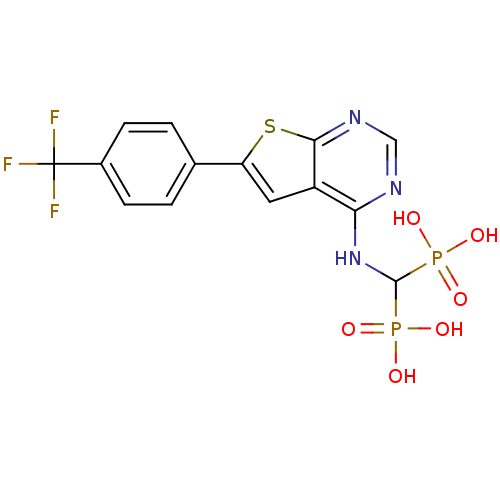
(CHEMBL3087934 | US11279719, Example C-12)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(cc1)C(F)(F)F)P(O)(O)=O Show InChI InChI=1S/C14H12F3N3O6P2S/c15-14(16,17)8-3-1-7(2-4-8)10-5-9-11(18-6-19-12(9)29-10)20-13(27(21,22)23)28(24,25)26/h1-6,13H,(H,18,19,20)(H2,21,22,23)(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data