 Found 1802 hits with Last Name = 'liang' and Initial = 'c'
Found 1802 hits with Last Name = 'liang' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50096906
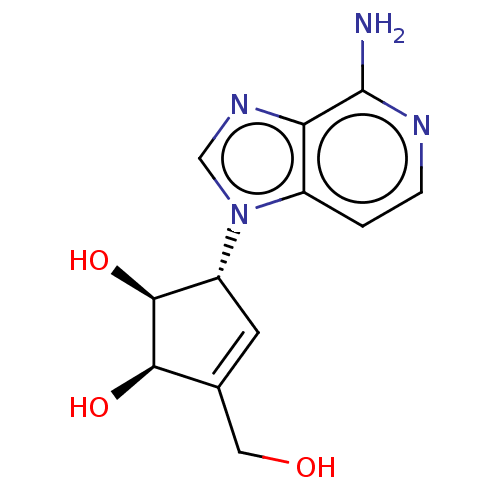
(CHEMBL154745 | US10227373, Compound D-3-Deazaisone...)Show SMILES Nc1nccc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |t:13| Show InChI InChI=1S/C12H14N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-3,5,8,10-11,17-19H,4H2,(H2,13,14)/t8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM155254
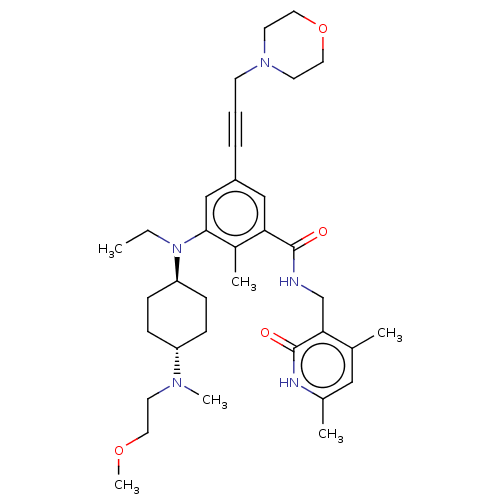
(US10098888, Compound 2 | US9006242, 2)Show SMILES CCN([C@H]1CC[C@@H](CC1)N(C)CCOC)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)C#CCN1CCOCC1 |r,wU:3.2,wD:6.9,(-6,1.54,;-4.67,.77,;-3.33,1.54,;-3.33,3.08,;-4.67,3.85,;-4.67,5.39,;-3.33,6.16,;-2,5.39,;-2,3.85,;-3.33,7.7,;-2,8.47,;-4.67,8.47,;-6,7.7,;-7.34,8.47,;-8.67,7.7,;-2,.77,;-.67,1.54,;.67,.77,;.67,-.77,;-.67,-1.54,;-.67,-3.08,;.67,-3.85,;-2,-3.85,;-2,-5.39,;-3.33,-6.16,;-3.33,-7.7,;-2,-8.47,;-4.67,-8.47,;-6,-7.7,;-7.34,-8.47,;-6,-6.16,;-4.67,-5.39,;-4.67,-3.85,;-2,-.77,;-3.33,-1.54,;2,1.54,;3.33,2.31,;4.67,3.08,;6,2.31,;6,.77,;7.34,,;8.67,.77,;8.67,2.31,;7.34,3.08,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50611954
(CHEMBL5266282) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM50049391
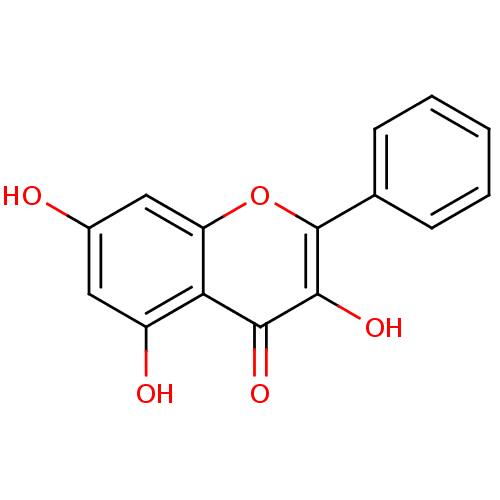
(3,5,7-Trihydroxyflavone | 3,5,7-triOH-flavone | 3,...)Show InChI InChI=1S/C15H10O5/c16-9-6-10(17)12-11(7-9)20-15(14(19)13(12)18)8-4-2-1-3-5-8/h1-7,16-17,19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00080
BindingDB Entry DOI: 10.7270/Q2TQ65MQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50318484

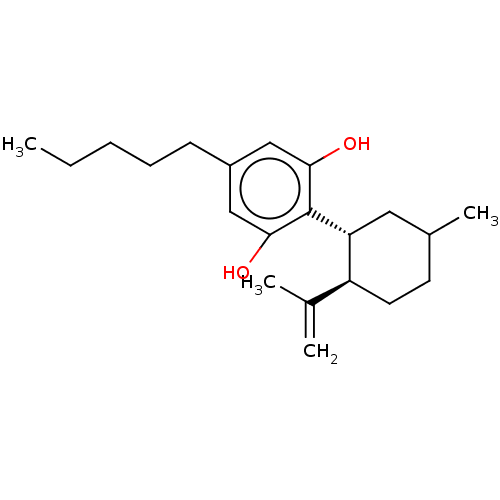
(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM50042944
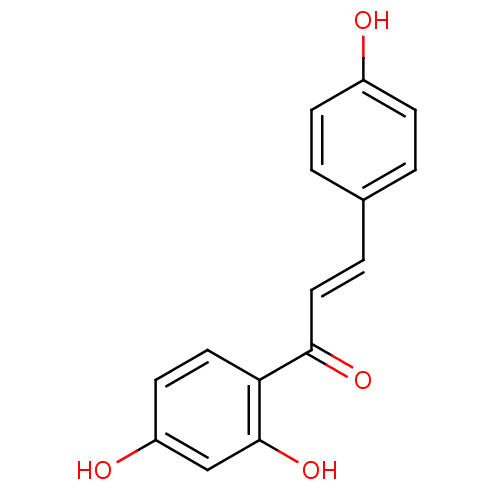
((E)-1-(2,4-Dihydroxy-phenyl)-3-(4-hydroxy-phenyl)-...)Show InChI InChI=1S/C15H12O4/c16-11-4-1-10(2-5-11)3-8-14(18)13-7-6-12(17)9-15(13)19/h1-9,16-17,19H/b8-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00080
BindingDB Entry DOI: 10.7270/Q2TQ65MQ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50116067
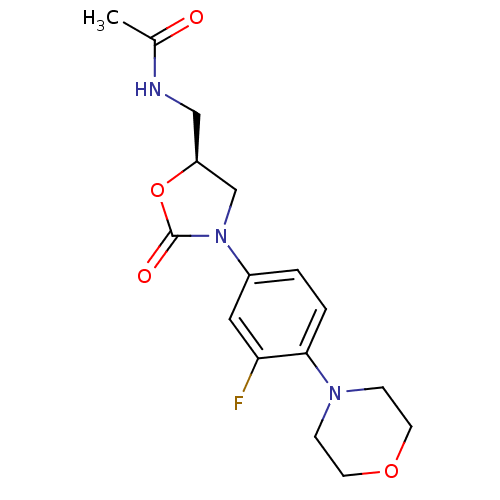
((Linezolid)N-[3-(3-Fluoro-4-morpholin-4-yl-phenyl)...)Show SMILES CC(=O)NC[C@H]1CN(C(=O)O1)c1ccc(N2CCOCC2)c(F)c1 |r| Show InChI InChI=1S/C16H20FN3O4/c1-11(21)18-9-13-10-20(16(22)24-13)12-2-3-15(14(17)8-12)19-4-6-23-7-5-19/h2-3,8,13H,4-7,9-10H2,1H3,(H,18,21)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226485

((R)-3-(3-fluoro-4-(tetrahydro-2H-pyran-4-yl)phenyl...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1ccc(C2CCOCC2)c(F)c1 Show InChI InChI=1S/C15H17FN2O4/c16-12-7-10(18-8-13(14(17)19)22-15(18)20)1-2-11(12)9-3-5-21-6-4-9/h1-2,7,9,13H,3-6,8H2,(H2,17,19)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226479
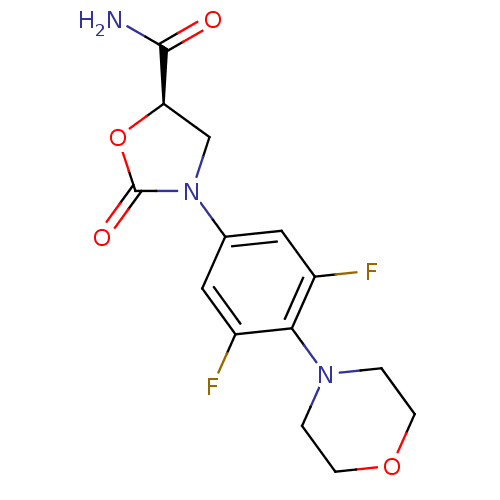
((R)-3-(3,5-difluoro-4-morpholinophenyl)-2-oxooxazo...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1cc(F)c(N2CCOCC2)c(F)c1 Show InChI InChI=1S/C14H15F2N3O4/c15-9-5-8(19-7-11(13(17)20)23-14(19)21)6-10(16)12(9)18-1-3-22-4-2-18/h5-6,11H,1-4,7H2,(H2,17,20)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226484
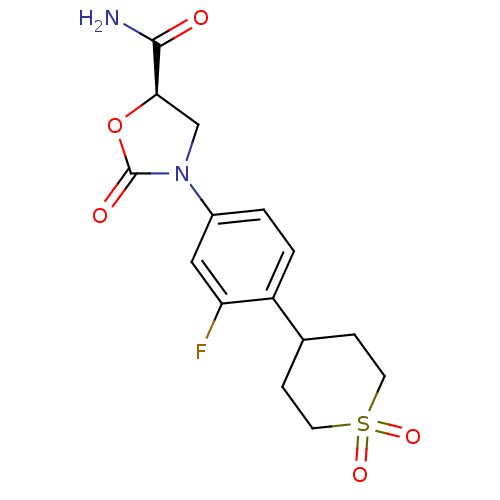
((5R)-3-[4-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1ccc(C2CCS(=O)(=O)CC2)c(F)c1 Show InChI InChI=1S/C15H17FN2O5S/c16-12-7-10(18-8-13(14(17)19)23-15(18)20)1-2-11(12)9-3-5-24(21,22)6-4-9/h1-2,7,9,13H,3-6,8H2,(H2,17,19)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226480
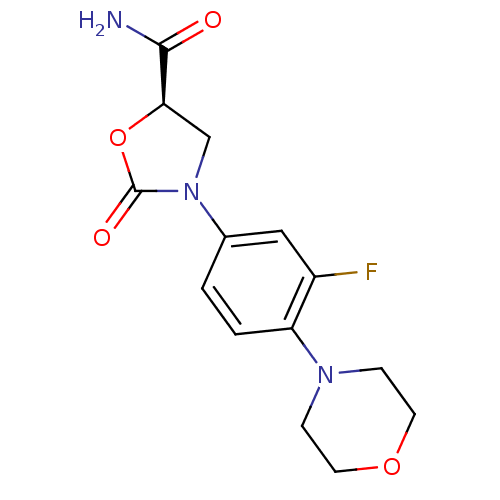
((R)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidi...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1ccc(N2CCOCC2)c(F)c1 Show InChI InChI=1S/C14H16FN3O4/c15-10-7-9(18-8-12(13(16)19)22-14(18)20)1-2-11(10)17-3-5-21-6-4-17/h1-2,7,12H,3-6,8H2,(H2,16,19)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226481
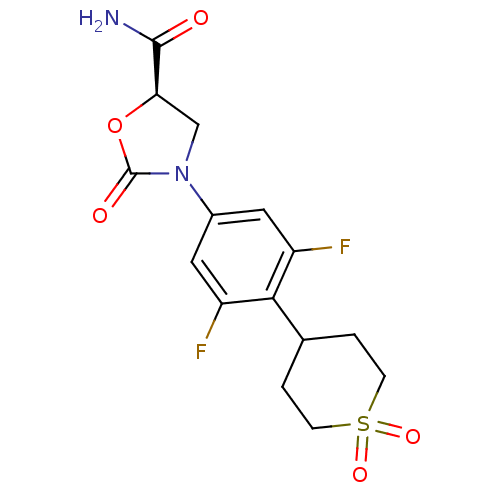
((5R)-3-[4-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1cc(F)c(C2CCS(=O)(=O)CC2)c(F)c1 Show InChI InChI=1S/C15H16F2N2O5S/c16-10-5-9(19-7-12(14(18)20)24-15(19)21)6-11(17)13(10)8-1-3-25(22,23)4-2-8/h5-6,8,12H,1-4,7H2,(H2,18,20)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM50600404
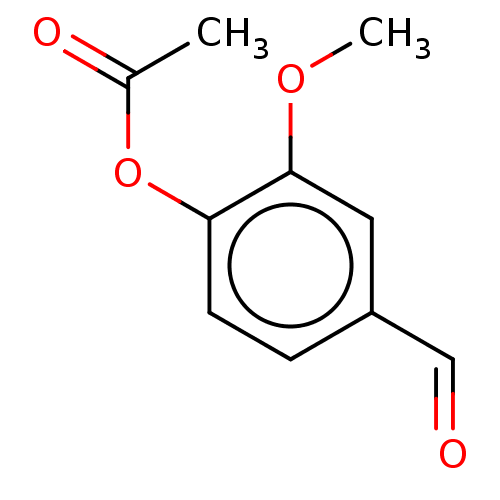
(CHEMBL5201508) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00080
BindingDB Entry DOI: 10.7270/Q2TQ65MQ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226482
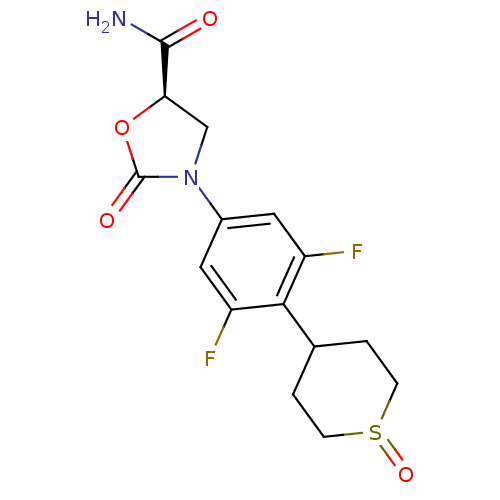
((5R)-3-[3,5-difluoro-4-(1-oxidotetrahydro-2H-thiop...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1cc(F)c(C2CCS(=O)CC2)c(F)c1 Show InChI InChI=1S/C15H16F2N2O4S/c16-10-5-9(19-7-12(14(18)20)23-15(19)21)6-11(17)13(10)8-1-3-24(22)4-2-8/h5-6,8,12H,1-4,7H2,(H2,18,20)/t8?,12-,24?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50594146
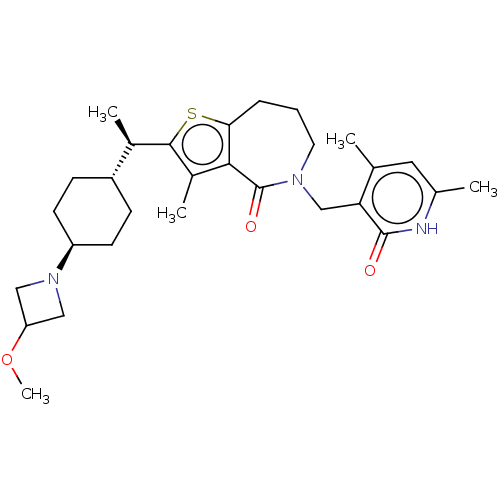
(CHEMBL5206842)Show SMILES COC1CN(C1)[C@H]1CC[C@@H](CC1)[C@@H](C)c1sc2CCCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1C |r,wU:9.9,12.14,wD:6.6,(10.07,6.78,;8.53,6.78,;7.76,5.44,;6.28,5.04,;6.67,3.56,;8.16,3.96,;5.9,2.22,;4.36,2.22,;3.59,.89,;4.36,-.44,;5.9,-.44,;6.67,.89,;3.59,-1.78,;4.36,-3.11,;2.05,-1.78,;1.15,-.53,;-.32,-1.01,;-1.52,-.05,;-3.02,-.39,;-3.69,-1.78,;-3.02,-3.16,;-3.98,-4.37,;-5.5,-4.14,;-6.07,-2.71,;-5.11,-1.5,;-7.59,-2.48,;-8.55,-3.68,;-10.07,-3.45,;-7.99,-5.11,;-6.46,-5.34,;-5.9,-6.78,;-1.52,-3.51,;-1.18,-5.01,;-.32,-2.55,;1.15,-3.02,;1.62,-4.49,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50594109
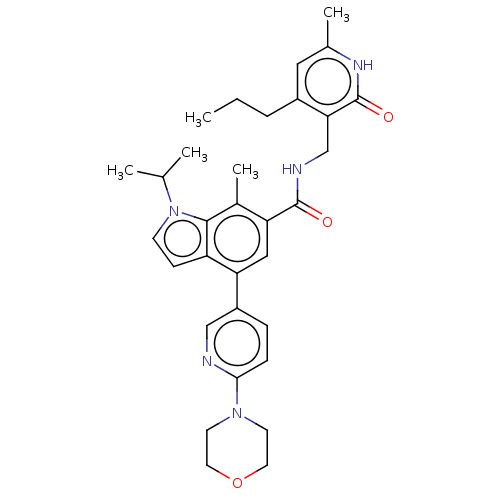
(CHEMBL5188827)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(-c2ccc(nc2)N2CCOCC2)c2ccn(C(C)C)c2c1C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50594115

(CHEMBL5172110)Show SMILES [H][C@@]1(CC[C@@H](CC1)N(C)C)[C@]1(C)Oc2c(O1)c(Cl)c1CCN(Cc3c(SC)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:4.7,1.0,wD:10.11,(4.02,.48,;5.35,-.29,;6.84,-.69,;7.93,.4,;7.53,1.89,;6.04,2.29,;4.95,1.2,;8.62,2.98,;8.22,4.46,;10.11,2.58,;4.26,-1.38,;5.6,-2.15,;3.36,-.13,;1.89,-.61,;1.89,-2.15,;3.36,-2.62,;.57,-2.92,;.57,-4.46,;-.77,-2.16,;-2.11,-2.93,;-3.45,-2.16,;-3.44,-.61,;-4.77,.15,;-6.11,-.62,;-6.1,-2.16,;-4.77,-2.93,;-4.77,-4.46,;-7.44,-2.93,;-8.77,-2.16,;-10.11,-2.93,;-8.77,-.62,;-7.44,.15,;-7.44,1.69,;-2.11,.16,;-2.11,1.7,;-.77,-.61,;.56,.16,;.56,1.7,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM542469

(US11274095, Example 4)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2O[C@@](C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N(C)C |r,wU:29.35,wD:20.21,26.29,(-4.77,-4.46,;-4.77,-2.92,;-6.11,-2.15,;-7.44,-2.92,;-8.77,-2.15,;-10.11,-2.92,;-8.77,-.61,;-7.44,.16,;-7.44,1.7,;-6.11,-.61,;-4.77,.16,;-3.44,-.61,;-2.11,.16,;-2.11,1.7,;-.77,-.61,;-.77,-2.15,;.56,-2.92,;.56,-4.46,;1.9,-2.15,;3.36,-2.63,;4.26,-1.38,;5.35,-2.47,;3.36,-.14,;1.9,-.61,;.56,.16,;.56,1.7,;5.35,-.29,;6.84,-.69,;7.93,.4,;7.53,1.89,;6.04,2.28,;4.96,1.19,;8.62,2.97,;10.11,2.58,;8.22,4.46,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM542475
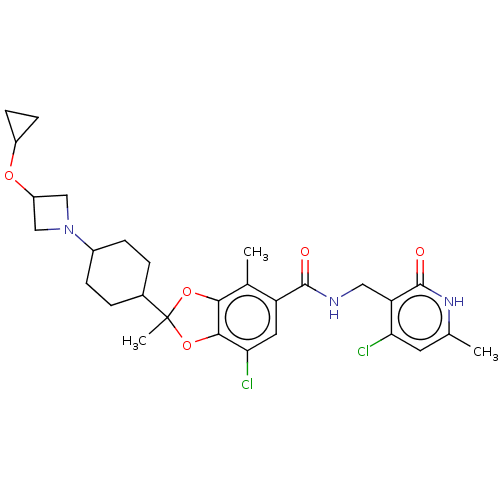
(7-chloro-N-((4-chloro-6-methyl-2-oxo-1,2-dihydropy...)Show SMILES Cc1cc(Cl)c(CNC(=O)c2cc(Cl)c3OC(C)(Oc3c2C)C2CCC(CC2)N2CC(C2)OC2CC2)c(=O)[nH]1 |(-12.17,-3.49,;-10.83,-2.72,;-9.5,-3.49,;-8.17,-2.72,;-6.83,-3.49,;-8.17,-1.18,;-6.83,-.41,;-5.5,-1.18,;-4.16,-.41,;-4.16,1.13,;-2.83,-1.18,;-2.83,-2.72,;-1.5,-3.49,;-1.5,-5.03,;-.16,-2.72,;1.3,-3.2,;2.21,-1.95,;3.3,-3.04,;1.3,-.71,;-.16,-1.18,;-1.5,-.41,;-1.5,1.13,;3.3,-.86,;4.78,-1.26,;5.87,-.17,;5.47,1.31,;3.99,1.71,;2.9,.62,;6.56,2.4,;8.1,2.4,;8.1,3.94,;6.56,3.94,;9.19,5.03,;10.68,4.63,;11.77,3.54,;12.17,5.03,;-9.5,-.41,;-9.5,1.13,;-10.83,-1.18,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50550778
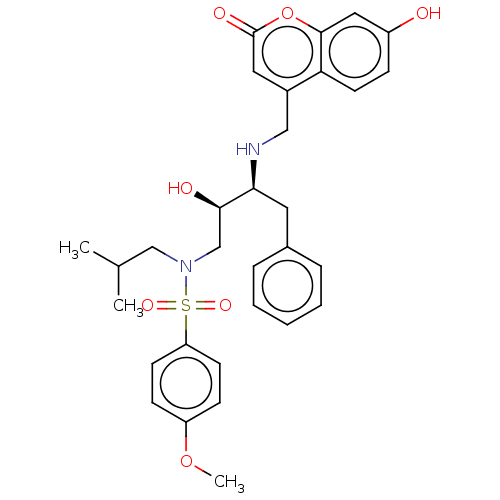
(CHEMBL4741886)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NCc1cc(=O)oc2cc(O)ccc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HIV1 protease measured assessed as hydrolysis of fluorogenic substrate by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111900
BindingDB Entry DOI: 10.7270/Q2D79G2V |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV-1BH10 reverse transcriptase expressed in Escherichia coli assessed as polymerization by real time FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111900
BindingDB Entry DOI: 10.7270/Q2D79G2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50593904
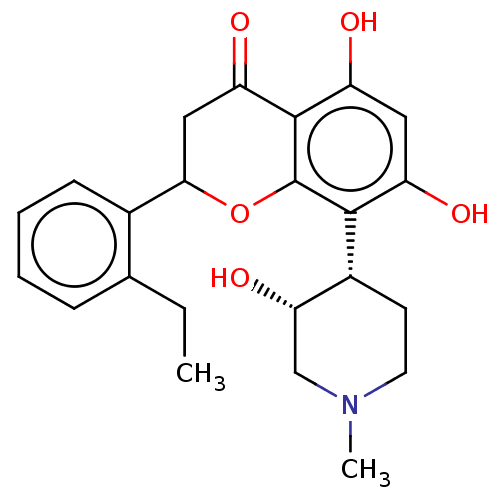
(CHEMBL5198209)Show SMILES CCc1ccccc1C1CC(=O)c2c(O)cc(O)c([C@@H]3CCN(C)C[C@@H]3O)c2O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02064
BindingDB Entry DOI: 10.7270/Q2SB49QV |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141329
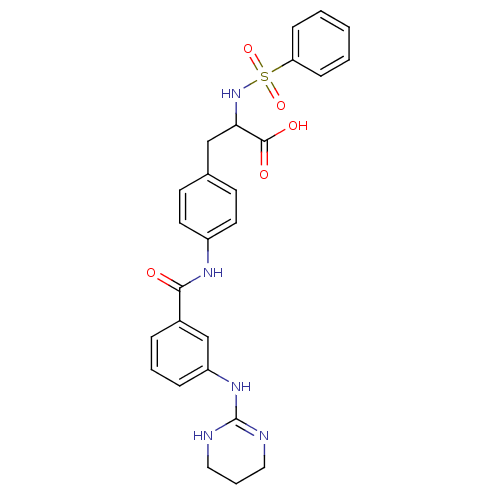
(2-Benzenesulfonylamino-3-{4-[3-(1,4,5,6-tetrahydro...)Show SMILES OC(=O)C(Cc1ccc(NC(=O)c2cccc(NC3=NCCCN3)c2)cc1)NS(=O)(=O)c1ccccc1 |t:18| Show InChI InChI=1S/C26H27N5O5S/c32-24(19-6-4-7-21(17-19)30-26-27-14-5-15-28-26)29-20-12-10-18(11-13-20)16-23(25(33)34)31-37(35,36)22-8-2-1-3-9-22/h1-4,6-13,17,23,31H,5,14-16H2,(H,29,32)(H,33,34)(H2,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V/beta3 receptor by solid-phase receptor binding assays (SPRA) |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141329

(2-Benzenesulfonylamino-3-{4-[3-(1,4,5,6-tetrahydro...)Show SMILES OC(=O)C(Cc1ccc(NC(=O)c2cccc(NC3=NCCCN3)c2)cc1)NS(=O)(=O)c1ccccc1 |t:18| Show InChI InChI=1S/C26H27N5O5S/c32-24(19-6-4-7-21(17-19)30-26-27-14-5-15-28-26)29-20-12-10-18(11-13-20)16-23(25(33)34)31-37(35,36)22-8-2-1-3-9-22/h1-4,6-13,17,23,31H,5,14-16H2,(H,29,32)(H,33,34)(H2,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50550780
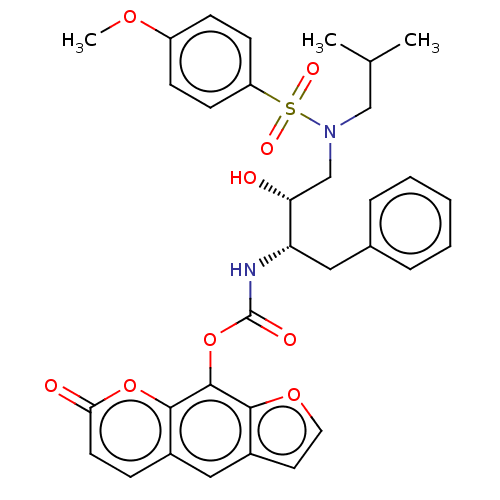
(CHEMBL4744103)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)Oc1c2occc2cc2ccc(=O)oc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HIV1 protease measured assessed as hydrolysis of fluorogenic substrate by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111900
BindingDB Entry DOI: 10.7270/Q2D79G2V |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50550777
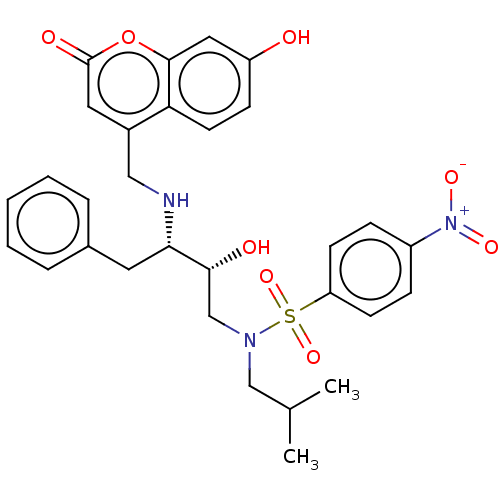
(CHEMBL4760803)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NCc1cc(=O)oc2cc(O)ccc12)S(=O)(=O)c1ccc(cc1)[N+]([O-])=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HIV1 protease measured assessed as hydrolysis of fluorogenic substrate by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111900
BindingDB Entry DOI: 10.7270/Q2D79G2V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110371
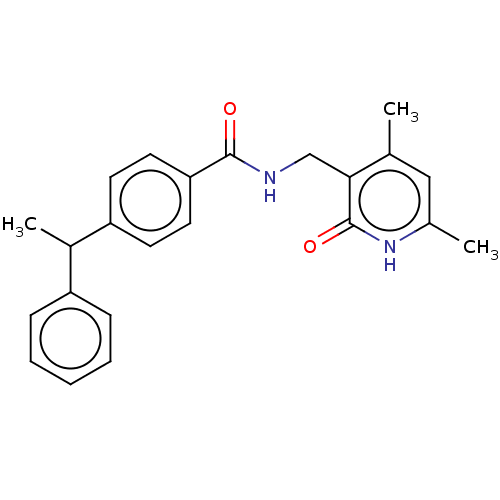
(CHEMBL3605441)Show SMILES CC(c1ccccc1)c1ccc(cc1)C(=O)NCc1c(C)cc(C)[nH]c1=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.153 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141324

(2-Isopropoxycarbonylamino-3-{2-methoxy-4-[3-(1,4,5...)Show SMILES COc1cc(NC(=O)c2cccc(NC3=NCCCN3)c2)ccc1CC(NC(=O)OC(C)C)C(O)=O |t:14| Show InChI InChI=1S/C25H31N5O6/c1-15(2)36-25(34)30-20(23(32)33)13-16-8-9-19(14-21(16)35-3)28-22(31)17-6-4-7-18(12-17)29-24-26-10-5-11-27-24/h4,6-9,12,14-15,20H,5,10-11,13H2,1-3H3,(H,28,31)(H,30,34)(H,32,33)(H2,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V/beta3 receptor by solid-phase receptor binding assays (SPRA) |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50550782
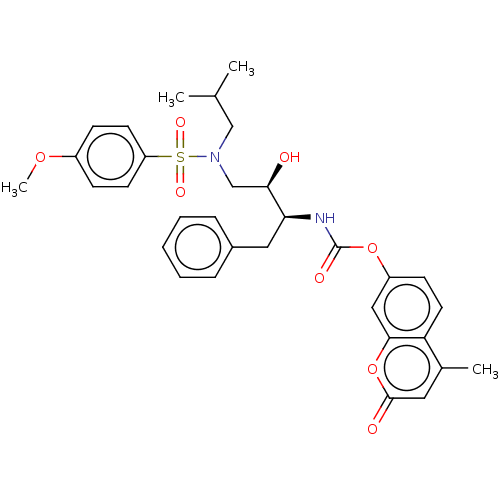
(CHEMBL4784196)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)Oc1ccc2c(C)cc(=O)oc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HIV1 protease measured assessed as hydrolysis of fluorogenic substrate by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111900
BindingDB Entry DOI: 10.7270/Q2D79G2V |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141309
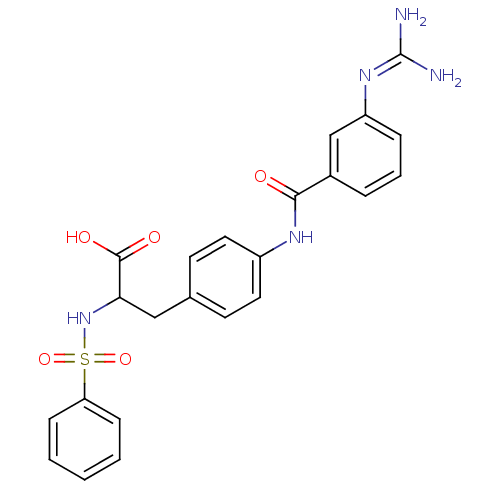
(2-Benzenesulfonylamino-3-[4-(3-guanidino-benzoylam...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1cccc(c1)-[#6](=O)-[#7]-c1ccc(-[#6]-[#6](-[#7]S(=O)(=O)c2ccccc2)-[#6](-[#8])=O)cc1 Show InChI InChI=1S/C23H23N5O5S/c24-23(25)27-18-6-4-5-16(14-18)21(29)26-17-11-9-15(10-12-17)13-20(22(30)31)28-34(32,33)19-7-2-1-3-8-19/h1-12,14,20,28H,13H2,(H,26,29)(H,30,31)(H4,24,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V/beta3 receptor by solid-phase receptor binding assays (SPRA) |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50594135
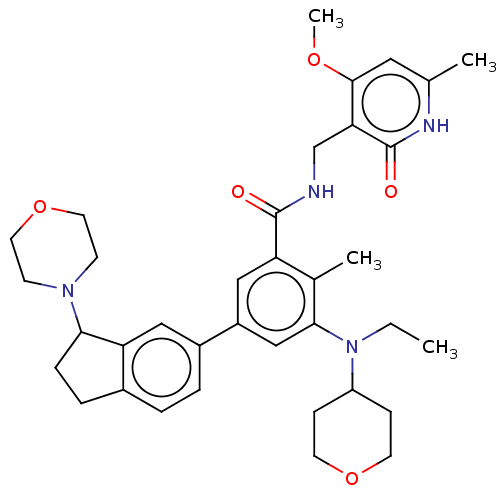
(CHEMBL5188577)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C)-c1ccc2CCC(N3CCOCC3)c2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141325
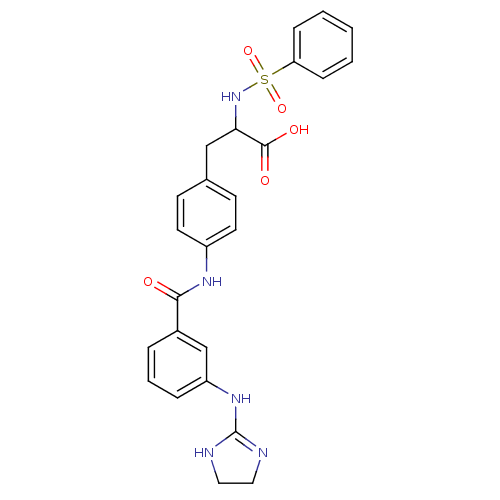
(2-Benzenesulfonylamino-3-{4-[3-(4,5-dihydro-1H-imi...)Show SMILES OC(=O)C(Cc1ccc(NC(=O)c2cccc(NC3=NCCN3)c2)cc1)NS(=O)(=O)c1ccccc1 |t:18| Show InChI InChI=1S/C25H25N5O5S/c31-23(18-5-4-6-20(16-18)29-25-26-13-14-27-25)28-19-11-9-17(10-12-19)15-22(24(32)33)30-36(34,35)21-7-2-1-3-8-21/h1-12,16,22,30H,13-15H2,(H,28,31)(H,32,33)(H2,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V/beta3 receptor by solid-phase receptor binding assays (SPRA) |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141356
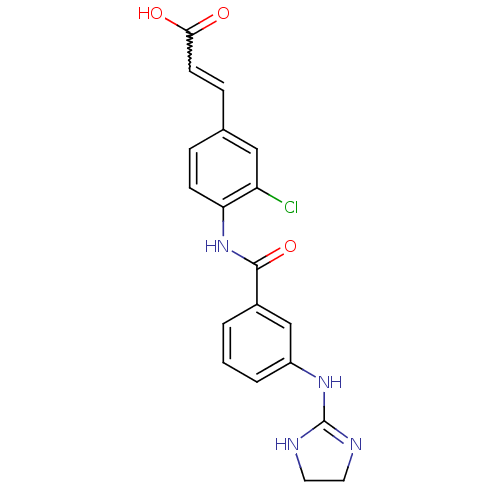
(3-{3-Chloro-4-[3-(4,5-dihydro-1H-imidazol-2-ylamin...)Show SMILES OC(=O)C=Cc1ccc(NC(=O)c2cccc(NC3=NCCN3)c2)c(Cl)c1 |w:3.2,t:18| Show InChI InChI=1S/C19H17ClN4O3/c20-15-10-12(5-7-17(25)26)4-6-16(15)24-18(27)13-2-1-3-14(11-13)23-19-21-8-9-22-19/h1-7,10-11H,8-9H2,(H,24,27)(H,25,26)(H2,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141355
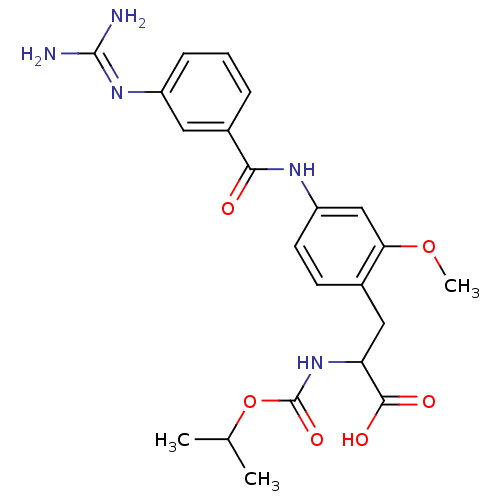
(3-[4-(3-Guanidino-benzoylamino)-2-methoxy-phenyl]-...)Show SMILES [#6]-[#8]-c1cc(-[#7]-[#6](=O)-c2cccc(c2)\[#7]=[#6](/[#7])-[#7])ccc1-[#6]-[#6](-[#7]-[#6](=O)-[#8]-[#6](-[#6])-[#6])-[#6](-[#8])=O Show InChI InChI=1S/C22H27N5O6/c1-12(2)33-22(31)27-17(20(29)30)10-13-7-8-16(11-18(13)32-3)25-19(28)14-5-4-6-15(9-14)26-21(23)24/h4-9,11-12,17H,10H2,1-3H3,(H,25,28)(H,27,31)(H,29,30)(H4,23,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V/beta3 receptor by solid-phase receptor binding assays (SPRA) |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141324

(2-Isopropoxycarbonylamino-3-{2-methoxy-4-[3-(1,4,5...)Show SMILES COc1cc(NC(=O)c2cccc(NC3=NCCCN3)c2)ccc1CC(NC(=O)OC(C)C)C(O)=O |t:14| Show InChI InChI=1S/C25H31N5O6/c1-15(2)36-25(34)30-20(23(32)33)13-16-8-9-19(14-21(16)35-3)28-22(31)17-6-4-7-18(12-17)29-24-26-10-5-11-27-24/h4,6-9,12,14-15,20H,5,10-11,13H2,1-3H3,(H,28,31)(H,30,34)(H,32,33)(H2,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50594116
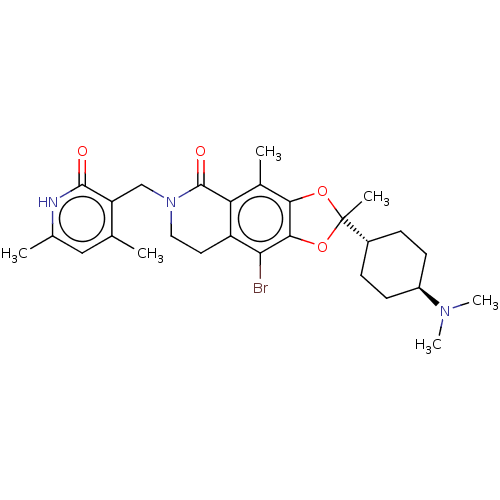
(CHEMBL5185991)Show SMILES [H][C@@]1(CC[C@@H](CC1)N(C)C)C1(C)Oc2c(O1)c(Br)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:4.7,1.0,(4.02,.48,;5.35,-.29,;6.84,-.69,;7.93,.4,;7.53,1.89,;6.04,2.28,;4.95,1.2,;8.62,2.97,;8.22,4.46,;10.11,2.58,;4.26,-1.38,;5.6,-2.15,;3.36,-.14,;1.89,-.61,;1.89,-2.15,;3.36,-2.63,;.57,-2.92,;.57,-4.46,;-.77,-2.16,;-2.11,-2.94,;-3.45,-2.16,;-3.44,-.62,;-4.77,.15,;-6.11,-.62,;-6.1,-2.16,;-4.77,-2.93,;-7.44,-2.93,;-8.77,-2.16,;-10.11,-2.93,;-8.77,-.62,;-7.44,.15,;-7.44,1.69,;-2.11,.15,;-2.11,1.69,;-.77,-.61,;.56,.16,;.56,1.7,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50141355

(3-[4-(3-Guanidino-benzoylamino)-2-methoxy-phenyl]-...)Show SMILES [#6]-[#8]-c1cc(-[#7]-[#6](=O)-c2cccc(c2)\[#7]=[#6](/[#7])-[#7])ccc1-[#6]-[#6](-[#7]-[#6](=O)-[#8]-[#6](-[#6])-[#6])-[#6](-[#8])=O Show InChI InChI=1S/C22H27N5O6/c1-12(2)33-22(31)27-17(20(29)30)10-13-7-8-16(11-18(13)32-3)25-19(28)14-5-4-6-15(9-14)26-21(23)24/h4-9,11-12,17H,10H2,1-3H3,(H,25,28)(H,27,31)(H,29,30)(H4,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50594114
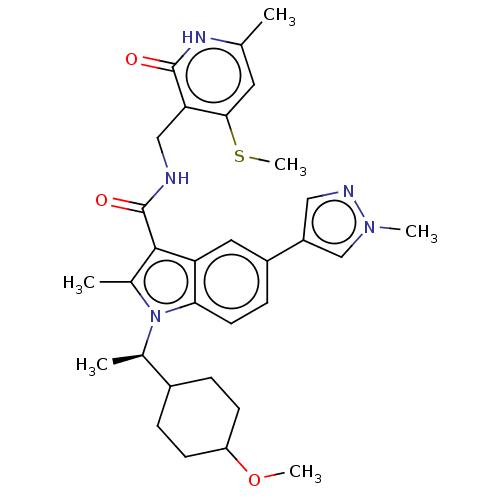
(CHEMBL5191375)Show SMILES [2H]C([2H])([2H])Sc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCC(CC2)OC)c2ccc(cc12)-c1cnn(C)c1 |r,wD:21.22,(-2.68,5.46,;-4.01,4.69,;-5.35,5.46,;-4.01,6.23,;-4.01,3.15,;-5.35,2.38,;-6.68,3.15,;-8.02,2.38,;-9.35,3.15,;-8.02,.84,;-6.68,.07,;-6.68,-1.47,;-5.35,.84,;-4.01,.07,;-2.68,.84,;-1.35,.07,;-1.35,-1.47,;-.01,.84,;.15,2.37,;-.94,3.46,;1.65,2.69,;2.42,4.03,;1.65,5.36,;3.96,4.03,;4.73,5.36,;6.27,5.36,;7.04,4.02,;6.28,2.7,;4.74,2.69,;8.58,4.02,;9.35,5.36,;2.42,1.36,;3.92,1.04,;4.4,-.43,;3.38,-1.57,;1.87,-1.26,;1.39,.21,;3.78,-3.05,;2.81,-4.25,;3.65,-5.54,;5.13,-5.14,;6.22,-6.23,;5.21,-3.61,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50550807
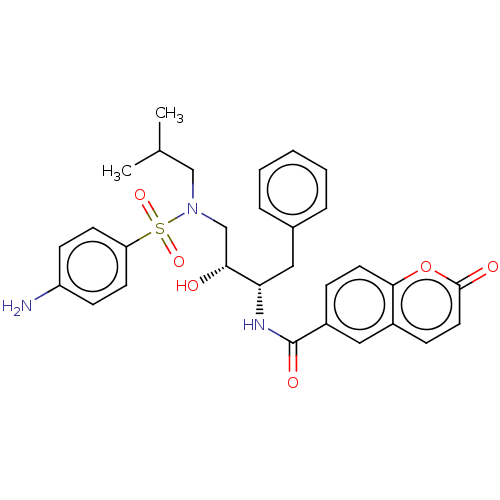
(CHEMBL4785296)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccc2oc(=O)ccc2c1)S(=O)(=O)c1ccc(N)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HIV1 protease measured assessed as hydrolysis of fluorogenic substrate by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111900
BindingDB Entry DOI: 10.7270/Q2D79G2V |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50141324

(2-Isopropoxycarbonylamino-3-{2-methoxy-4-[3-(1,4,5...)Show SMILES COc1cc(NC(=O)c2cccc(NC3=NCCCN3)c2)ccc1CC(NC(=O)OC(C)C)C(O)=O |t:14| Show InChI InChI=1S/C25H31N5O6/c1-15(2)36-25(34)30-20(23(32)33)13-16-8-9-19(14-21(16)35-3)28-22(31)17-6-4-7-18(12-17)29-24-26-10-5-11-27-24/h4,6-9,12,14-15,20H,5,10-11,13H2,1-3H3,(H,28,31)(H,30,34)(H,32,33)(H2,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta5 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141354
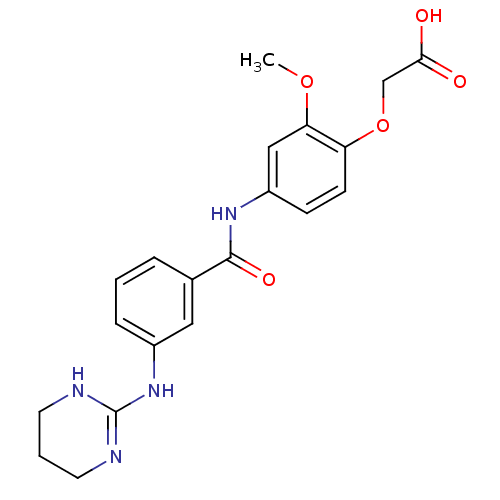
(CHEMBL285192 | {2-Methoxy-4-[3-(1,4,5,6-tetrahydro...)Show SMILES COc1cc(NC(=O)c2cccc(NC3=NCCCN3)c2)ccc1OCC(O)=O |t:14| Show InChI InChI=1S/C20H22N4O5/c1-28-17-11-15(6-7-16(17)29-12-18(25)26)23-19(27)13-4-2-5-14(10-13)24-20-21-8-3-9-22-20/h2,4-7,10-11H,3,8-9,12H2,1H3,(H,23,27)(H,25,26)(H2,21,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V/beta3 receptor by solid-phase receptor binding assays (SPRA) |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588052
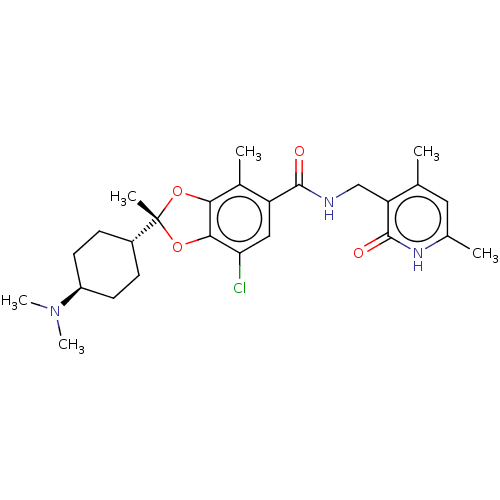
((2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@@]1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(C)cc(C)[nH]c1=O |r,wU:6.6,wD:9.10,3.2,(5.78,-4.8,;4.69,-5.89,;5.09,-7.38,;3.2,-5.49,;2.8,-4.01,;1.32,-3.61,;.23,-4.7,;.63,-6.18,;2.11,-6.58,;-1.26,-4.3,;-2.03,-5.63,;-2.79,-4.14,;-3.11,-2.63,;-1.78,-1.86,;-.63,-2.89,;-1.78,-.32,;-.44,.45,;-3.11,.45,;-4.45,-.32,;-4.45,-1.86,;-5.78,-2.63,;-3.11,1.99,;-4.45,2.76,;-1.78,2.76,;-1.78,4.3,;-.44,5.07,;-.44,6.61,;-1.78,7.38,;.89,7.38,;2.22,6.61,;3.56,7.38,;2.22,5.07,;.89,4.3,;.89,2.76,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141319
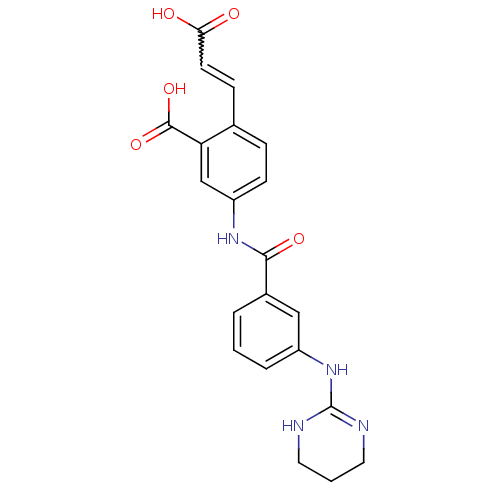
(2-(2-Carboxy-vinyl)-5-[3-(1,4,5,6-tetrahydro-pyrim...)Show SMILES OC(=O)C=Cc1ccc(NC(=O)c2cccc(NC3=NCCCN3)c2)cc1C(O)=O |w:3.2,t:18| Show InChI InChI=1S/C21H20N4O5/c26-18(27)8-6-13-5-7-16(12-17(13)20(29)30)24-19(28)14-3-1-4-15(11-14)25-21-22-9-2-10-23-21/h1,3-8,11-12H,2,9-10H2,(H,24,28)(H,26,27)(H,29,30)(H2,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta5 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5140
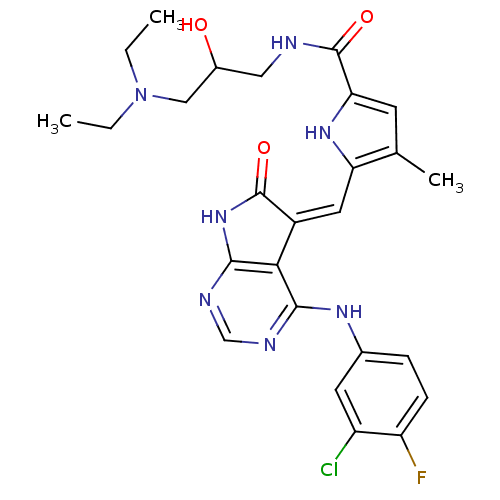
(5-{[(5Z)-4-[(3-chloro-4-fluorophenyl)amino]-6-oxo-...)Show SMILES CCN(CC)CC(O)CNC(=O)c1cc(C)c(\C=C2/C(=O)Nc3ncnc(Nc4ccc(F)c(Cl)c4)c23)[nH]1 Show InChI InChI=1S/C26H29ClFN7O3/c1-4-35(5-2)12-16(36)11-29-26(38)21-8-14(3)20(33-21)10-17-22-23(30-13-31-24(22)34-25(17)37)32-15-6-7-19(28)18(27)9-15/h6-10,13,16,33,36H,4-5,11-12H2,1-3H3,(H,29,38)(H2,30,31,32,34,37)/b17-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
Bioorg Med Chem Lett 12: 2153-7 (2002)
Article DOI: 10.1016/s0960-894x(02)00364-5
BindingDB Entry DOI: 10.7270/Q200009N |
More data for this
Ligand-Target Pair | |
Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM7533
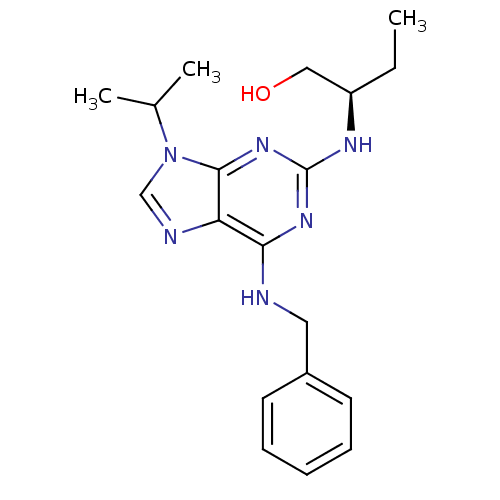
((2R)-2-[[6-(benzylamino)-9-isopropyl-purin-2-yl]am...)Show SMILES CC[C@H](CO)Nc1nc(NCc2ccccc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C19H26N6O/c1-4-15(11-26)22-19-23-17(20-10-14-8-6-5-7-9-14)16-18(24-19)25(12-21-16)13(2)3/h5-9,12-13,15,26H,4,10-11H2,1-3H3,(H2,20,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02064
BindingDB Entry DOI: 10.7270/Q2SB49QV |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141327

(3-{3-Methyl-4-[3-(1,4,5,6-tetrahydro-pyrimidin-2-y...)Show SMILES Cc1cc(C=CC(O)=O)ccc1NC(=O)c1cccc(NC2=NCCCN2)c1 |w:5.5,t:22| Show InChI InChI=1S/C21H22N4O3/c1-14-12-15(7-9-19(26)27)6-8-18(14)25-20(28)16-4-2-5-17(13-16)24-21-22-10-3-11-23-21/h2,4-9,12-13H,3,10-11H2,1H3,(H,25,28)(H,26,27)(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50594132
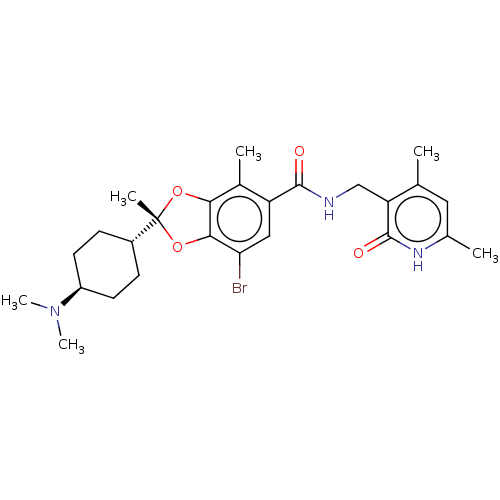
(CHEMBL5197386)Show SMILES [H][C@@]1(CC[C@@H](CC1)N(C)C)[C@@]1(C)Oc2c(O1)c(C)c(cc2Br)C(=O)NCc1c(C)cc(C)[nH]c1=O |r,wU:4.7,1.0,wD:10.11,(3.73,.93,;5.06,.16,;6.39,-.61,;7.73,.16,;7.73,1.7,;6.39,2.47,;5.06,1.7,;9.06,2.47,;9.06,4.01,;10.4,1.7,;3.97,-.93,;5.31,-1.7,;3.07,-2.18,;1.6,-1.7,;1.6,-.16,;3.07,.32,;.27,.61,;.27,2.15,;-1.06,-.16,;-1.06,-1.71,;.28,-2.47,;.28,-4.01,;-2.4,.61,;-2.4,2.15,;-3.73,-.16,;-5.06,.6,;-6.4,-.17,;-6.4,-1.71,;-5.06,-2.48,;-7.73,-2.48,;-9.06,-1.71,;-10.4,-2.48,;-9.06,-.17,;-7.73,.6,;-7.73,2.14,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50594135

(CHEMBL5188577)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C)-c1ccc2CCC(N3CCOCC3)c2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-1
(Homo sapiens (Human)) | BDBM50141334

(3-[4-(3-Guanidino-benzoylamino)-phenyl]-2-isopropo...)Show SMILES [#6]-[#6](-[#6])-[#8]-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(-[#7]-[#6](=O)-c2cccc(c2)\[#7]=[#6](/[#7])-[#7])cc1)-[#6](-[#8])=O Show InChI InChI=1S/C21H25N5O5/c1-12(2)31-21(30)26-17(19(28)29)10-13-6-8-15(9-7-13)24-18(27)14-4-3-5-16(11-14)25-20(22)23/h3-9,11-12,17H,10H2,1-2H3,(H,24,27)(H,26,30)(H,28,29)(H4,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta5 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50141343
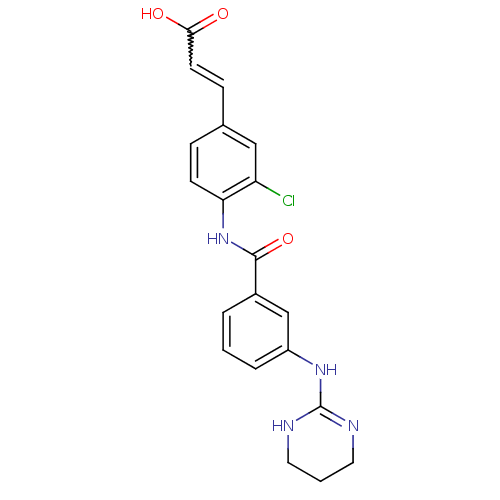
(3-{3-Chloro-4-[3-(1,4,5,6-tetrahydro-pyrimidin-2-y...)Show SMILES OC(=O)C=Cc1ccc(NC(=O)c2cccc(NC3=NCCCN3)c2)c(Cl)c1 |w:3.2,t:18| Show InChI InChI=1S/C20H19ClN4O3/c21-16-11-13(6-8-18(26)27)5-7-17(16)25-19(28)14-3-1-4-15(12-14)24-20-22-9-2-10-23-20/h1,3-8,11-12H,2,9-10H2,(H,25,28)(H,26,27)(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha V-beta1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 14: 1471-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.015
BindingDB Entry DOI: 10.7270/Q2RJ4HXS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data