 Found 317 hits with Last Name = 'liu' and Initial = 'yc'
Found 317 hits with Last Name = 'liu' and Initial = 'yc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50078163
(CHEMBL3417704)Show SMILES CN(C)S(=O)(=O)N1CCN(CC1)c1ccc(OCc2c(c(C)nn2C)-c2cccc3c(CCCOc4cccc5ccccc45)c(C(O)=O)n(CCN4CCOCC4)c23)cc1 |(-21.22,-16.71,;-19.99,-16.7,;-19.36,-17.76,;-19.23,-15.36,;-19.85,-14.3,;-20.46,-15.37,;-17.68,-15.35,;-16.91,-16.68,;-15.37,-16.67,;-14.6,-15.33,;-15.38,-14,;-16.92,-14.01,;-13.06,-15.32,;-12.28,-16.64,;-10.74,-16.63,;-9.98,-15.29,;-8.44,-15.28,;-7.69,-13.93,;-6.15,-13.92,;-5.26,-12.68,;-3.79,-13.11,;-2.81,-12.36,;-3.77,-14.65,;-5.22,-15.15,;-5.58,-16.33,;-5.74,-11.22,;-7.27,-10.9,;-7.75,-9.44,;-6.72,-8.27,;-5.21,-8.6,;-3.97,-7.71,;-3.98,-6.17,;-2.65,-5.4,;-2.66,-3.85,;-1.33,-3.08,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;1.33,-1.54,;,-.77,;-2.74,-8.57,;-1.29,-8.05,;-.35,-8.85,;-1.06,-6.84,;-3.2,-10.04,;-2.28,-11.27,;-.75,-11.09,;.17,-12.33,;1.7,-12.15,;2.62,-13.39,;2.01,-14.8,;.48,-14.98,;-.44,-13.74,;-4.73,-10.05,;-10.76,-13.96,;-12.3,-13.98,)| Show InChI InChI=1S/C46H55N7O7S/c1-33-43(41(49(4)47-33)32-60-36-19-17-35(18-20-36)51-22-24-52(25-23-51)61(56,57)48(2)3)40-14-8-13-38-39(15-9-29-59-42-16-7-11-34-10-5-6-12-37(34)42)45(46(54)55)53(44(38)40)26-21-50-27-30-58-31-28-50/h5-8,10-14,16-20H,9,15,21-32H2,1-4H3,(H,54,55) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50004708
(FLUMETSULAM)Show InChI InChI=1S/C12H9F2N5O2S/c1-7-5-6-19-11(15-7)16-12(17-19)22(20,21)18-10-8(13)3-2-4-9(10)14/h2-6,18H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487171

(CHEMBL2251948)Show SMILES COC(=O)c1cccc(Cl)c1NS(=O)(=O)c1nc2c(Cl)c(C)nc(C)n2n1 Show InChI InChI=1S/C15H13Cl2N5O4S/c1-7-11(17)13-19-15(20-22(13)8(2)18-7)27(24,25)21-12-9(14(23)26-3)5-4-6-10(12)16/h4-6,21H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487177

(CHEMBL2251959)Show SMILES COC(=O)c1cccc(Cl)c1NS(=O)(=O)c1nc2nc(OC)ccn2n1 Show InChI InChI=1S/C14H12ClN5O5S/c1-24-10-6-7-20-13(16-10)17-14(18-20)26(22,23)19-11-8(12(21)25-2)4-3-5-9(11)15/h3-7,19H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560862

(CHEMBL4796830) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM31712
(HEXACHLOROPHENE | Hexach-lorophene | MLS000028433 ...)Show SMILES Oc1c(Cl)cc(Cl)c(Cl)c1Cc1c(O)c(Cl)cc(Cl)c1Cl Show InChI InChI=1S/C13H6Cl6O2/c14-6-2-8(16)12(20)4(10(6)18)1-5-11(19)7(15)3-9(17)13(5)21/h2-3,20-21H,1H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
| Assay Description
Kinetic parameters were obtained using various concentrations of FRET peptide in the fluorescent assay. The maximal velocity (Vmax) and Michaelis–Men... |
Biochem Biophys Res Commun 333: 194-9 (2005)
Article DOI: 10.1016/j.bbrc.2005.05.095
BindingDB Entry DOI: 10.7270/Q2N58PRD |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560861

(CHEMBL4757050) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487169

(CHEMBL2251950)Show SMILES Cc1nc(cc2nc(nn12)S(=O)(=O)Nc1c(Cl)cccc1Cl)C(F)(F)F Show InChI InChI=1S/C13H8Cl2F3N5O2S/c1-6-19-9(13(16,17)18)5-10-20-12(21-23(6)10)26(24,25)22-11-7(14)3-2-4-8(11)15/h2-5,22H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560860
(CHEMBL4758555) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560859
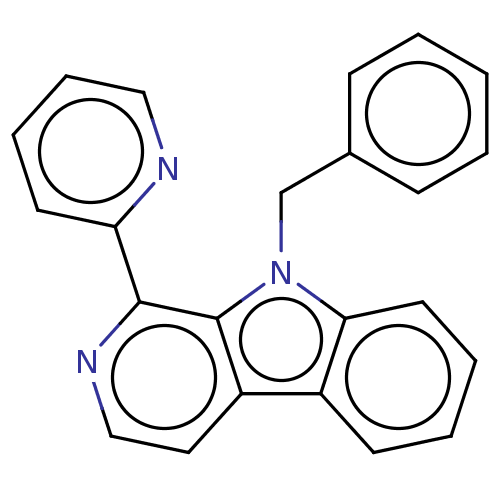
(CHEMBL4744295) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560865

(CHEMBL4778579)Show SMILES C(c1ccc2ccccc2c1)n1c2ccccc2c2ccnc(-c3ccccn3)c12 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487162
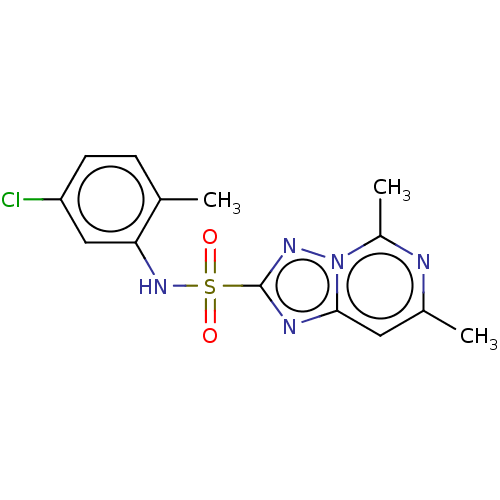
(CHEMBL2251953)Show InChI InChI=1S/C14H14ClN5O2S/c1-8-4-5-11(15)7-12(8)19-23(21,22)14-17-13-6-9(2)16-10(3)20(13)18-14/h4-7,19H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560867
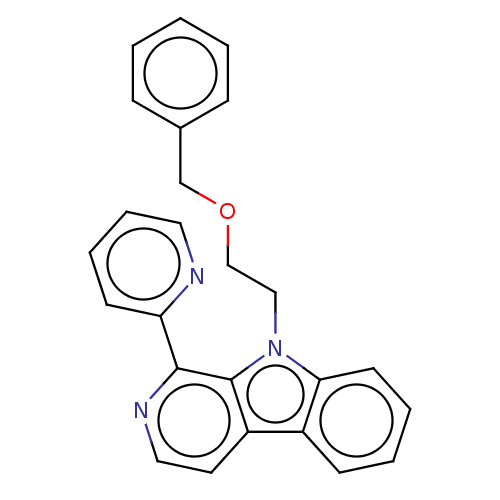
(CHEMBL4794980)Show SMILES C(Cn1c2ccccc2c2ccnc(-c3ccccn3)c12)OCc1ccccc1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560868

(CHEMBL4748351) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560866
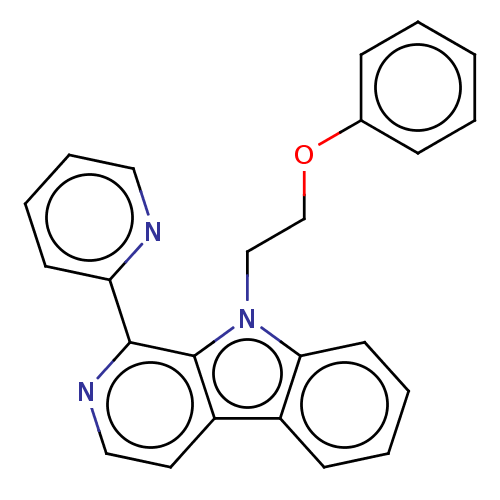
(CHEMBL4760810)Show SMILES C(Cn1c2ccccc2c2ccnc(-c3ccccn3)c12)Oc1ccccc1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560864
(CHEMBL4777267) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560863
(CHEMBL4743955) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50560858
(CHEMBL4578154) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Flu-BID/FAM-BID from His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells incubated... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487176

(CHEMBL2251938)Show InChI InChI=1S/C12H9Cl2N5O3S/c1-22-9-5-6-19-11(15-9)16-12(17-19)23(20,21)18-8-4-2-3-7(13)10(8)14/h2-6,18H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487168
(CHEMBL2251955)Show InChI InChI=1S/C12H9F2N5O3S/c1-22-9-5-6-19-11(15-9)16-12(17-19)23(20,21)18-10-7(13)3-2-4-8(10)14/h2-6,18H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487175
(CHEMBL2251939)Show InChI InChI=1S/C12H10BrN5O3S/c1-21-10-6-7-18-11(14-10)15-12(16-18)22(19,20)17-9-5-3-2-4-8(9)13/h2-7,17H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487165

(CHEMBL2251943)Show InChI InChI=1S/C13H11ClFN5O2S/c1-7-11(14)12-17-13(18-20(12)8(2)16-7)23(21,22)19-10-6-4-3-5-9(10)15/h3-6,19H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487181

(CHEMBL2251958)Show InChI InChI=1S/C12H10FN5O3S/c1-21-10-6-7-18-11(14-10)15-12(16-18)22(19,20)17-9-5-3-2-4-8(9)13/h2-7,17H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487166

(CHEMBL2251947)Show InChI InChI=1S/C13H12FN5O2S/c1-8-7-12-16-13(17-19(12)9(2)15-8)22(20,21)18-11-6-4-3-5-10(11)14/h3-7,18H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487182
(CHEMBL2251954)Show InChI InChI=1S/C13H11F2N5O2S/c1-7-5-12-17-13(18-20(12)8(2)16-7)23(21,22)19-11-6-9(14)3-4-10(11)15/h3-6,19H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487167

(CHEMBL2251957)Show InChI InChI=1S/C12H9F2N5O3S/c1-22-10-4-5-19-11(15-10)16-12(17-19)23(20,21)18-9-6-7(13)2-3-8(9)14/h2-6,18H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487173
(CHEMBL2251942)Show SMILES Cc1nc(cc2nc(nn12)S(=O)(=O)Nc1c(F)cccc1F)C(F)(F)F Show InChI InChI=1S/C13H8F5N5O2S/c1-6-19-9(13(16,17)18)5-10-20-12(21-23(6)10)26(24,25)22-11-7(14)3-2-4-8(11)15/h2-5,22H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487179
(CHEMBL2251952)Show SMILES Cc1cc2nc(nn2c(C)n1)S(=O)(=O)Nc1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C14H11ClF3N5O2S/c1-7-5-12-20-13(21-23(12)8(2)19-7)26(24,25)22-11-4-3-9(6-10(11)15)14(16,17)18/h3-6,22H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487170

(CHEMBL2251949)Show SMILES Cc1nc(cc2nc(nn12)S(=O)(=O)Nc1ccccc1F)C(F)(F)F Show InChI InChI=1S/C13H9F4N5O2S/c1-7-18-10(13(15,16)17)6-11-19-12(20-22(7)11)25(23,24)21-9-5-3-2-4-8(9)14/h2-6,21H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487163

(CHEMBL2251946)Show SMILES Cc1nc(C)n2nc(nc2c1Cl)S(=O)(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C14H11ClF3N5O2S/c1-7-11(15)12-20-13(21-23(12)8(2)19-7)26(24,25)22-10-5-3-9(4-6-10)14(16,17)18/h3-6,22H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487178
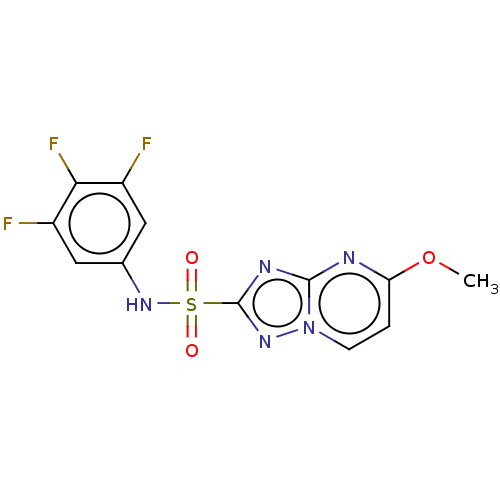
(CHEMBL2251956)Show SMILES COc1ccn2nc(nc2n1)S(=O)(=O)Nc1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C12H8F3N5O3S/c1-23-9-2-3-20-11(16-9)17-12(18-20)24(21,22)19-6-4-7(13)10(15)8(14)5-6/h2-5,19H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487174

(CHEMBL2251941)Show SMILES Cc1nc(C)n2nc(nc2c1Cl)S(=O)(=O)Nc1ccc(F)c(F)c1F Show InChI InChI=1S/C13H9ClF3N5O2S/c1-5-9(14)12-19-13(20-22(12)6(2)18-5)25(23,24)21-8-4-3-7(15)10(16)11(8)17/h3-4,21H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487183
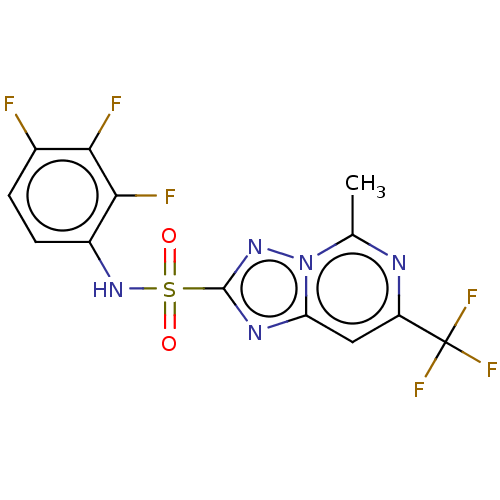
(CHEMBL2251945)Show SMILES Cc1nc(cc2nc(nn12)S(=O)(=O)Nc1ccc(F)c(F)c1F)C(F)(F)F Show InChI InChI=1S/C13H7F6N5O2S/c1-5-20-8(13(17,18)19)4-9-21-12(22-24(5)9)27(25,26)23-7-3-2-6(14)10(15)11(7)16/h2-4,23H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487180

(CHEMBL2251940)Show SMILES COc1ccn2nc(nc2n1)S(=O)(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C13H10F3N5O4S/c1-24-10-6-7-21-11(17-10)18-12(19-21)26(22,23)20-8-2-4-9(5-3-8)25-13(14,15)16/h2-7,20H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487172

(CHEMBL2251944)Show SMILES Cc1cc2nc(nn2c(C)n1)S(=O)(=O)Nc1ccc(F)c(F)c1F Show InChI InChI=1S/C13H10F3N5O2S/c1-6-5-10-18-13(19-21(10)7(2)17-6)24(22,23)20-9-4-3-8(14)11(15)12(9)16/h3-5,20H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50487164

(CHEMBL2251937)Show InChI InChI=1S/C12H10BrN5O3S/c1-21-10-6-7-18-11(14-10)15-12(16-18)22(19,20)17-9-4-2-8(13)3-5-9/h2-7,17H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetolactate synthase by colorimetry |
Bioorg Med Chem 18: 4897-904 (2010)
Article DOI: 10.1016/j.bmc.2010.06.015
BindingDB Entry DOI: 10.7270/Q20G3P19 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 8
(Homo sapiens (Human)) | BDBM50243294
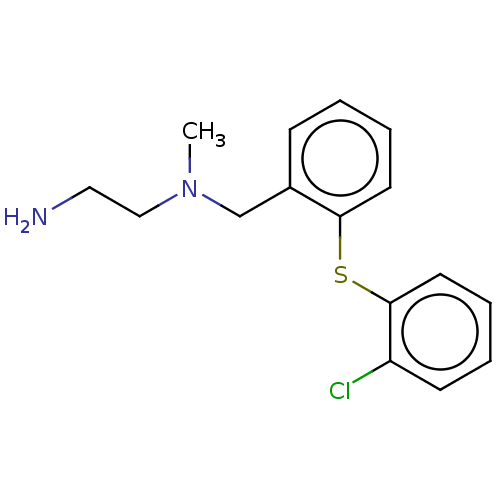
(CHEMBL4094513)Show InChI InChI=1S/C16H19ClN2S/c1-19(11-10-18)12-13-6-2-4-8-15(13)20-16-9-5-3-7-14(16)17/h2-9H,10-12,18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of PRMT8 (unknown origin) incubated for 15 mins followed by substrate addition measured after 60 mins by AlphaLisa method |
J Med Chem 60: 8888-8905 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01134
BindingDB Entry DOI: 10.7270/Q27D2XHK |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Rattus norvegicus) | BDBM50243294

(CHEMBL4094513)Show InChI InChI=1S/C16H19ClN2S/c1-19(11-10-18)12-13-6-2-4-8-15(13)20-16-9-5-3-7-14(16)17/h2-9H,10-12,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of rat His-tagged PRMT1 (11 to 353 residues) expressed in Escherichia coli BL21(DE3) incubated for 15 mins followed by substrate addition ... |
J Med Chem 60: 8888-8905 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01134
BindingDB Entry DOI: 10.7270/Q27D2XHK |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 6
(Homo sapiens (Human)) | BDBM50243294

(CHEMBL4094513)Show InChI InChI=1S/C16H19ClN2S/c1-19(11-10-18)12-13-6-2-4-8-15(13)20-16-9-5-3-7-14(16)17/h2-9H,10-12,18H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of PRMT6 (unknown origin) incubated for 15 mins followed by substrate addition measured after 60 mins by AlphaLisa method |
J Med Chem 60: 8888-8905 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01134
BindingDB Entry DOI: 10.7270/Q27D2XHK |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Rattus norvegicus) | BDBM50243294

(CHEMBL4094513)Show InChI InChI=1S/C16H19ClN2S/c1-19(11-10-18)12-13-6-2-4-8-15(13)20-16-9-5-3-7-14(16)17/h2-9H,10-12,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of rat His-tagged PRMT1 (11 to 353 residues) expressed in Escherichia coli BL21(DE3) using biotinylated H4 peptide (1 to 21 residues) as s... |
J Med Chem 60: 8888-8905 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01134
BindingDB Entry DOI: 10.7270/Q27D2XHK |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1
(Rattus norvegicus) | BDBM50243295
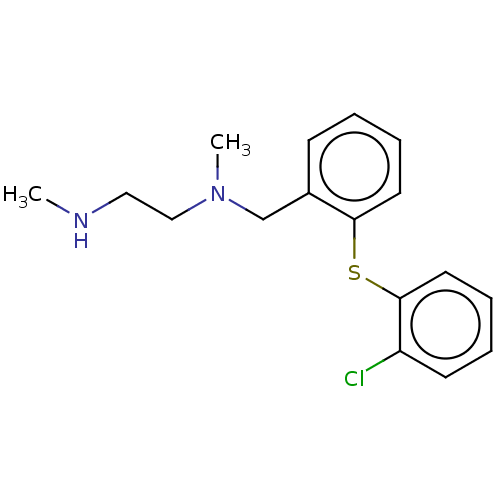
(CHEMBL4084293)Show InChI InChI=1S/C17H21ClN2S/c1-19-11-12-20(2)13-14-7-3-5-9-16(14)21-17-10-6-4-8-15(17)18/h3-10,19H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of rat His-tagged PRMT1 (11 to 353 residues) expressed in Escherichia coli BL21(DE3) using biotinylated H4 peptide (1 to 21 residues) as s... |
J Med Chem 60: 8888-8905 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01134
BindingDB Entry DOI: 10.7270/Q27D2XHK |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50078163

(CHEMBL3417704)Show SMILES CN(C)S(=O)(=O)N1CCN(CC1)c1ccc(OCc2c(c(C)nn2C)-c2cccc3c(CCCOc4cccc5ccccc45)c(C(O)=O)n(CCN4CCOCC4)c23)cc1 |(-21.22,-16.71,;-19.99,-16.7,;-19.36,-17.76,;-19.23,-15.36,;-19.85,-14.3,;-20.46,-15.37,;-17.68,-15.35,;-16.91,-16.68,;-15.37,-16.67,;-14.6,-15.33,;-15.38,-14,;-16.92,-14.01,;-13.06,-15.32,;-12.28,-16.64,;-10.74,-16.63,;-9.98,-15.29,;-8.44,-15.28,;-7.69,-13.93,;-6.15,-13.92,;-5.26,-12.68,;-3.79,-13.11,;-2.81,-12.36,;-3.77,-14.65,;-5.22,-15.15,;-5.58,-16.33,;-5.74,-11.22,;-7.27,-10.9,;-7.75,-9.44,;-6.72,-8.27,;-5.21,-8.6,;-3.97,-7.71,;-3.98,-6.17,;-2.65,-5.4,;-2.66,-3.85,;-1.33,-3.08,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;1.33,-1.54,;,-.77,;-2.74,-8.57,;-1.29,-8.05,;-.35,-8.85,;-1.06,-6.84,;-3.2,-10.04,;-2.28,-11.27,;-.75,-11.09,;.17,-12.33,;1.7,-12.15,;2.62,-13.39,;2.01,-14.8,;.48,-14.98,;-.44,-13.74,;-4.73,-10.05,;-10.76,-13.96,;-12.3,-13.98,)| Show InChI InChI=1S/C46H55N7O7S/c1-33-43(41(49(4)47-33)32-60-36-19-17-35(18-20-36)51-22-24-52(25-23-51)61(56,57)48(2)3)40-14-8-13-38-39(15-9-29-59-42-16-7-11-34-10-5-6-12-37(34)42)45(46(54)55)53(44(38)40)26-21-50-27-30-58-31-28-50/h5-8,10-14,16-20H,9,15,21-32H2,1-4H3,(H,54,55) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal biotin-labeled BIM BH3 peptide (141 to 166 residues) binding to His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM151611
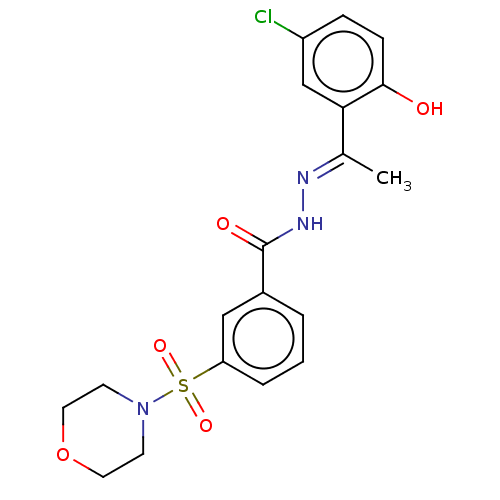
(SP-2509 | US11433053, Example HCI-2509 | US8987335...)Show SMILES C\C(=N/NC(=O)c1cccc(c1)S(=O)(=O)N1CCOCC1)c1cc(Cl)ccc1O Show InChI InChI=1S/C19H20ClN3O5S/c1-13(17-12-15(20)5-6-18(17)24)21-22-19(25)14-3-2-4-16(11-14)29(26,27)23-7-9-28-10-8-23/h2-6,11-12,24H,7-10H2,1H3,(H,22,25)/b21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 25 |
China Pharmaceutical University
| Assay Description
The LSD1 screening biochemical assay was performed by Shanghai ChemPartner Co. Ltd and the detailed protocol was shown as followed. The AlphaLISA ass... |
Bioorg Chem 72: 182-189 (2017)
Article DOI: 10.1016/j.bioorg.2017.04.006
BindingDB Entry DOI: 10.7270/Q2PC3179 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50078163

(CHEMBL3417704)Show SMILES CN(C)S(=O)(=O)N1CCN(CC1)c1ccc(OCc2c(c(C)nn2C)-c2cccc3c(CCCOc4cccc5ccccc45)c(C(O)=O)n(CCN4CCOCC4)c23)cc1 |(-21.22,-16.71,;-19.99,-16.7,;-19.36,-17.76,;-19.23,-15.36,;-19.85,-14.3,;-20.46,-15.37,;-17.68,-15.35,;-16.91,-16.68,;-15.37,-16.67,;-14.6,-15.33,;-15.38,-14,;-16.92,-14.01,;-13.06,-15.32,;-12.28,-16.64,;-10.74,-16.63,;-9.98,-15.29,;-8.44,-15.28,;-7.69,-13.93,;-6.15,-13.92,;-5.26,-12.68,;-3.79,-13.11,;-2.81,-12.36,;-3.77,-14.65,;-5.22,-15.15,;-5.58,-16.33,;-5.74,-11.22,;-7.27,-10.9,;-7.75,-9.44,;-6.72,-8.27,;-5.21,-8.6,;-3.97,-7.71,;-3.98,-6.17,;-2.65,-5.4,;-2.66,-3.85,;-1.33,-3.08,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;1.33,-1.54,;,-.77,;-2.74,-8.57,;-1.29,-8.05,;-.35,-8.85,;-1.06,-6.84,;-3.2,-10.04,;-2.28,-11.27,;-.75,-11.09,;.17,-12.33,;1.7,-12.15,;2.62,-13.39,;2.01,-14.8,;.48,-14.98,;-.44,-13.74,;-4.73,-10.05,;-10.76,-13.96,;-12.3,-13.98,)| Show InChI InChI=1S/C46H55N7O7S/c1-33-43(41(49(4)47-33)32-60-36-19-17-35(18-20-36)51-22-24-52(25-23-51)61(56,57)48(2)3)40-14-8-13-38-39(15-9-29-59-42-16-7-11-34-10-5-6-12-37(34)42)45(46(54)55)53(44(38)40)26-21-50-27-30-58-31-28-50/h5-8,10-14,16-20H,9,15,21-32H2,1-4H3,(H,54,55) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of biotinylated Bim peptide (81 to 106 residues) binding to His-tagged Mcl-1 (171 to 327 residues) (unknown origin) expressed in Escherich... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02047
BindingDB Entry DOI: 10.7270/Q22J6GKF |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396980
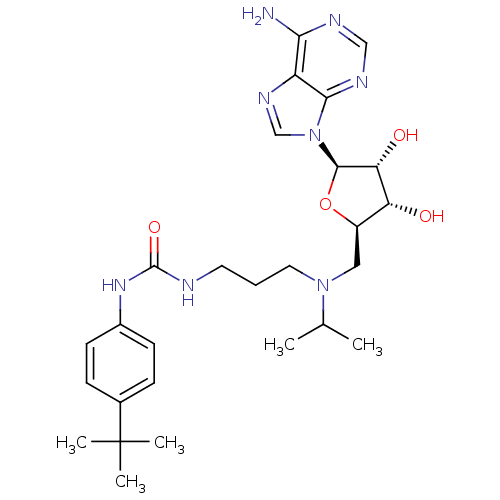
(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6-SUMO tagged DOT1L (1 to 416 residues) expressed in Escherichia coli BL21(DE3) using oligonucleosome as substrate... |
Bioorg Med Chem 26: 1751-1758 (2018)
Article DOI: 10.1016/j.bmc.2018.02.020
BindingDB Entry DOI: 10.7270/Q2319ZHM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 3
(Homo sapiens) | BDBM50243294

(CHEMBL4094513)Show InChI InChI=1S/C16H19ClN2S/c1-19(11-10-18)12-13-6-2-4-8-15(13)20-16-9-5-3-7-14(16)17/h2-9H,10-12,18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of PRMT3 (unknown origin) incubated for 15 mins followed by substrate addition measured after 60 mins by AlphaLisa method |
J Med Chem 60: 8888-8905 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01134
BindingDB Entry DOI: 10.7270/Q27D2XHK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50242394

(CHEMBL4074788)Show SMILES Cc1nc2c(ncnc2n1Cc1ccc(cc1)-c1ccccc1Cl)N1CCOCC1 Show InChI InChI=1S/C23H22ClN5O/c1-16-27-21-22(28-10-12-30-13-11-28)25-15-26-23(21)29(16)14-17-6-8-18(9-7-17)19-4-2-3-5-20(19)24/h2-9,15H,10-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged PDE10A1 (2 to 789 residues) expressed in baculovirus infected Sf9 cells using fluorescein-label... |
Bioorg Med Chem 25: 3315-3329 (2017)
Article DOI: 10.1016/j.bmc.2017.04.019
BindingDB Entry DOI: 10.7270/Q25X2CB8 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50242395
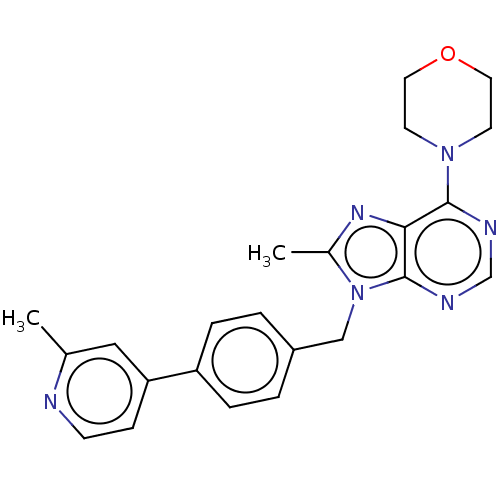
(CHEMBL4079798)Show SMILES Cc1nc2c(ncnc2n1Cc1ccc(cc1)-c1ccnc(C)c1)N1CCOCC1 Show InChI InChI=1S/C23H24N6O/c1-16-13-20(7-8-24-16)19-5-3-18(4-6-19)14-29-17(2)27-21-22(25-15-26-23(21)29)28-9-11-30-12-10-28/h3-8,13,15H,9-12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged PDE10A1 (2 to 789 residues) expressed in baculovirus infected Sf9 cells using fluorescein-label... |
Bioorg Med Chem 25: 3315-3329 (2017)
Article DOI: 10.1016/j.bmc.2017.04.019
BindingDB Entry DOI: 10.7270/Q25X2CB8 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50365262

((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...)Show SMILES Cc1nnc2[C@H](CC(=O)OC(C)(C)C)N=C(c3c(C)c(C)sc3-n12)c1ccc(Cl)cc1 |r,c:14| Show InChI InChI=1S/C23H25ClN4O2S/c1-12-13(2)31-22-19(12)20(15-7-9-16(24)10-8-15)25-17(11-18(29)30-23(4,5)6)21-27-26-14(3)28(21)22/h7-10,17H,11H2,1-6H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of BRD4(1) (unknown origin) incubated for 4 hrs by (+)-JQ1 fluorescent ligand based fluorescence anisotrophy |
J Med Chem 58: 1281-97 (2015)
Article DOI: 10.1021/jm501504k
BindingDB Entry DOI: 10.7270/Q2P270TX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50071105

(CHEMBL3410003)Show SMILES O=C(Nc1cc(cc(c1)S(=O)(=O)NC1CCCC1)-c1csc(=O)[nH]1)C1CC1 Show InChI InChI=1S/C18H21N3O4S2/c22-17(11-5-6-11)19-14-7-12(16-10-26-18(23)20-16)8-15(9-14)27(24,25)21-13-3-1-2-4-13/h7-11,13,21H,1-6H2,(H,19,22)(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of BRD4(1) (unknown origin) incubated for 4 hrs by (+)-JQ1 fluorescent ligand based fluorescence anisotrophy |
J Med Chem 58: 1281-97 (2015)
Article DOI: 10.1021/jm501504k
BindingDB Entry DOI: 10.7270/Q2P270TX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data