 Found 1294 hits with Last Name = 'loo' and Initial = 'd'
Found 1294 hits with Last Name = 'loo' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617046

(US11752155, Compound E1-38.6B')Show SMILES FC(F)[H]c1ccc(COC(=O)N2CC[C@H](CNc3ccncc3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617044

(US11752155, Compound E1-38.2B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ccncc3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205787

((E)-5-fluoro-2-(2-(5-(2-fluorophenylsulfonyl)pyrid...)Show SMILES Oc1cc(F)ccc1\C=C\c1ccc(cn1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H13F2NO3S/c20-14-7-5-13(18(23)11-14)6-8-15-9-10-16(12-22-15)26(24,25)19-4-2-1-3-17(19)21/h1-12,23H/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617051

(US11752155, Compound III-E1-38.1B')Show SMILES FC1(F)CN(CC[C@@H]1CNc1ccncc1)C(=O)OC1Cc2ccccc2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617045

(US11752155, Compound E1-38.4B')Show SMILES Fc1ccc(COC(=O)N2CC[C@H](CNc3ccncc3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50169842
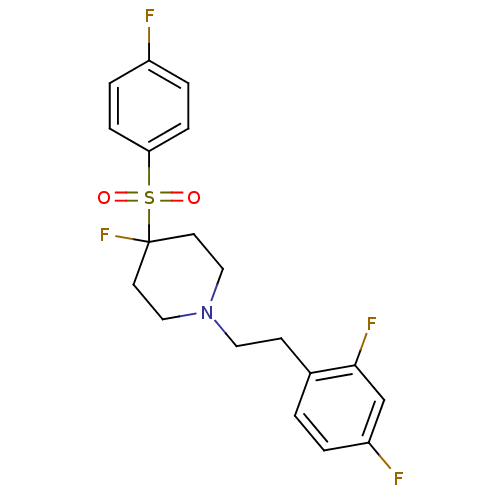
(1-(2,4-difluorophenethyl)-4-fluoro-4-(4-fluorophen...)Show SMILES Fc1ccc(cc1)S(=O)(=O)C1(F)CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H19F4NO2S/c20-15-3-5-17(6-4-15)27(25,26)19(23)8-11-24(12-9-19)10-7-14-1-2-16(21)13-18(14)22/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM388301
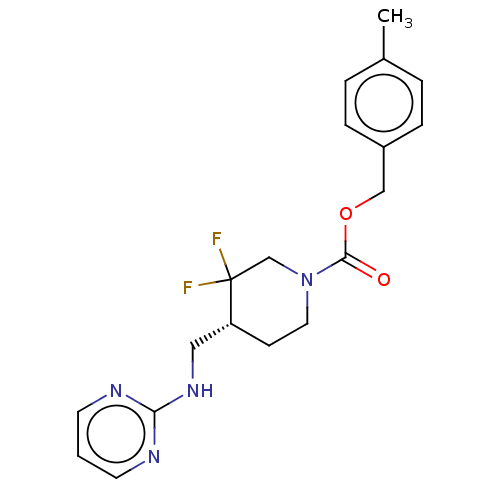
(US10294230, Compound E1-22.2 | US10584127, Compoun...)Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ncccn3)C(F)(F)C2)cc1 |r| Show InChI InChI=1S/C19H22F2N4O2/c1-14-3-5-15(6-4-14)12-27-18(26)25-10-7-16(19(20,21)13-25)11-24-17-22-8-2-9-23-17/h2-6,8-9,16H,7,10-13H2,1H3,(H,22,23,24)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.413 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617024
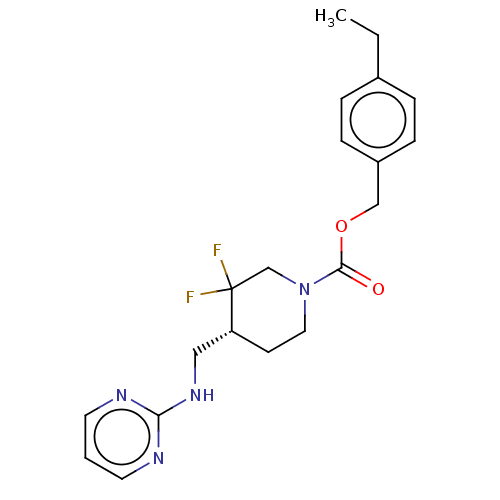
(US11752155, Compound E1-22.5B')Show SMILES CCc1ccc(COC(=O)N2CC[C@H](CNc3ncccn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205790
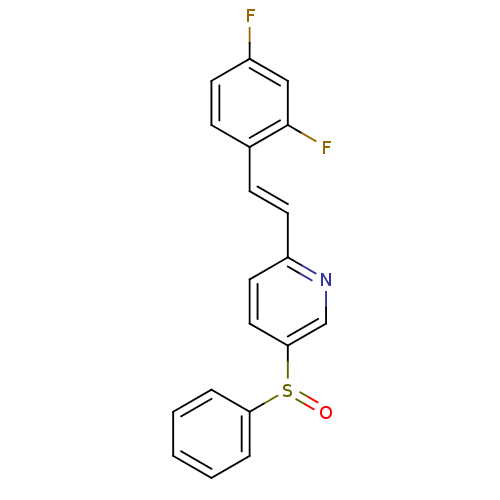
((E)-2-(2,4-difluorostyryl)-5-(phenylsulfinyl)pyrid...)Show InChI InChI=1S/C19H13F2NOS/c20-15-8-6-14(19(21)12-15)7-9-16-10-11-18(13-22-16)24(23)17-4-2-1-3-5-17/h1-13H/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205790

((E)-2-(2,4-difluorostyryl)-5-(phenylsulfinyl)pyrid...)Show InChI InChI=1S/C19H13F2NOS/c20-15-8-6-14(19(21)12-15)7-9-16-10-11-18(13-22-16)24(23)17-4-2-1-3-5-17/h1-13H/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM388302
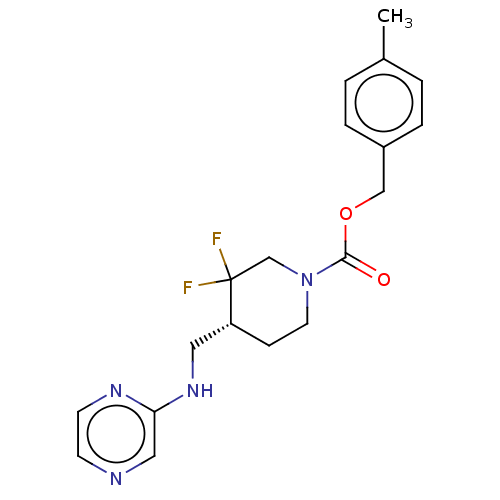
(US10294230, Compound E1-21.2 | US10584127, Compoun...)Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3cnccn3)C(F)(F)C2)cc1 |r| Show InChI InChI=1S/C19H22F2N4O2/c1-14-2-4-15(5-3-14)12-27-18(26)25-9-6-16(19(20,21)13-25)10-24-17-11-22-7-8-23-17/h2-5,7-8,11,16H,6,9-10,12-13H2,1H3,(H,23,24)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.716 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617047
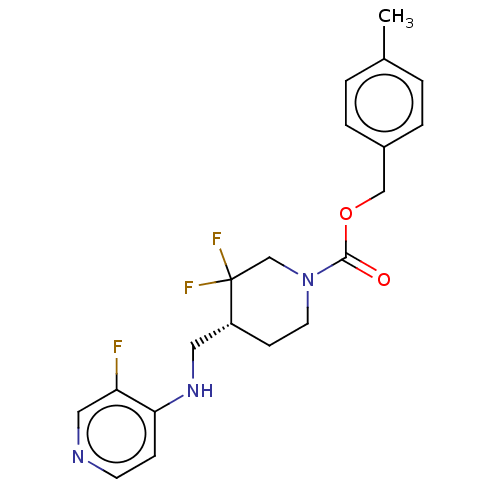
(US11752155, Compound E1-38.8B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ccncc3F)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617030
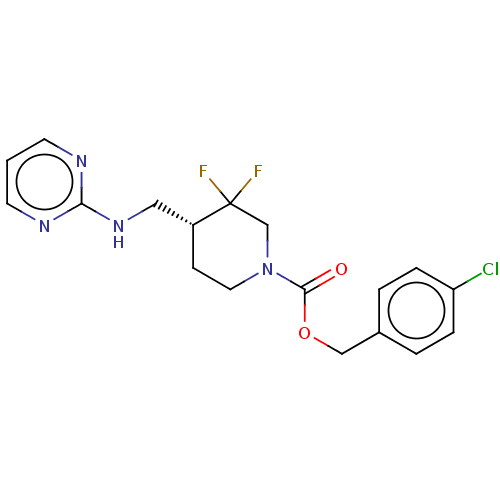
(US11752155, Compound E1-22.3B')Show SMILES FC1(F)CN(CC[C@@H]1CNc1ncccn1)C(=O)OCc1ccc(Cl)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM287603
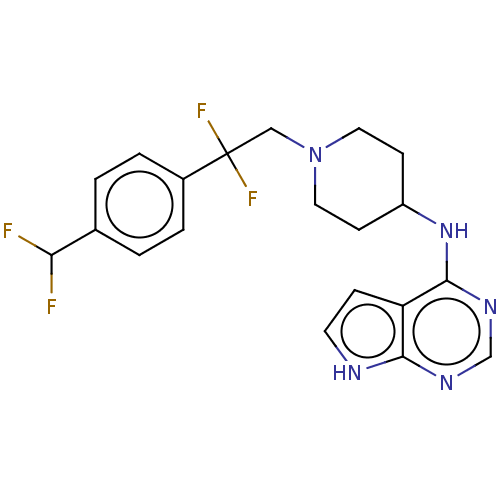
(BDBM393497 | BDBM413611 | BDBM617007 | US9567341, ...)Show SMILES FC(F)c1ccc(cc1)C(F)(F)CN1CCC(CC1)Nc1ncnc2[nH]ccc12 Show InChI InChI=1S/C20H21F4N5/c21-17(22)13-1-3-14(4-2-13)20(23,24)11-29-9-6-15(7-10-29)28-19-16-5-8-25-18(16)26-12-27-19/h1-5,8,12,15,17H,6-7,9-11H2,(H2,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.838 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371214
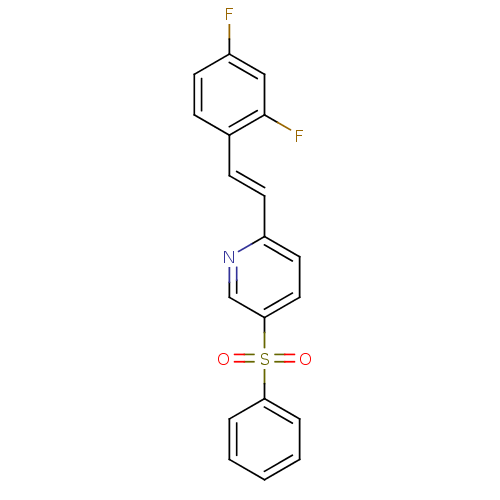
(CHEMBL1162962)Show SMILES Fc1ccc(\C=C\c2ccc(cn2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H13F2NO2S/c20-15-8-6-14(19(21)12-15)7-9-16-10-11-18(13-22-16)25(23,24)17-4-2-1-3-5-17/h1-13H/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617032

(US11752155, Compound E1-22.6B')Show SMILES FC(F)c1ccc(COC(=O)N2CC[C@H](CNc3ncccn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM388303
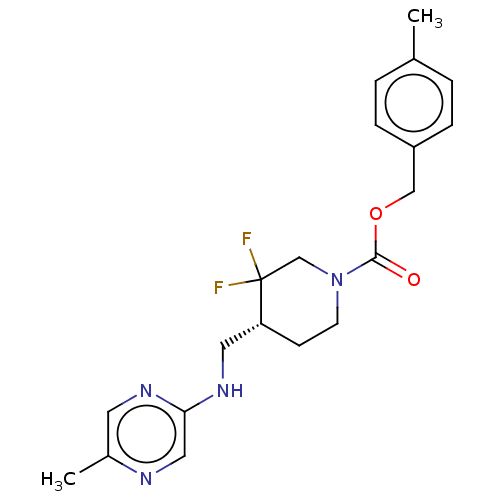
(US10294230, Compound E1-21.26 | US10584127, Compou...)Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3cnc(C)cn3)C(F)(F)C2)cc1 |r| Show InChI InChI=1S/C20H24F2N4O2/c1-14-3-5-16(6-4-14)12-28-19(27)26-8-7-17(20(21,22)13-26)10-25-18-11-23-15(2)9-24-18/h3-6,9,11,17H,7-8,10,12-13H2,1-2H3,(H,24,25)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617031

(US11752155, Compound E1-22.4B')Show SMILES Fc1ccc(COC(=O)N2CC[C@H](CNc3ncccn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617055
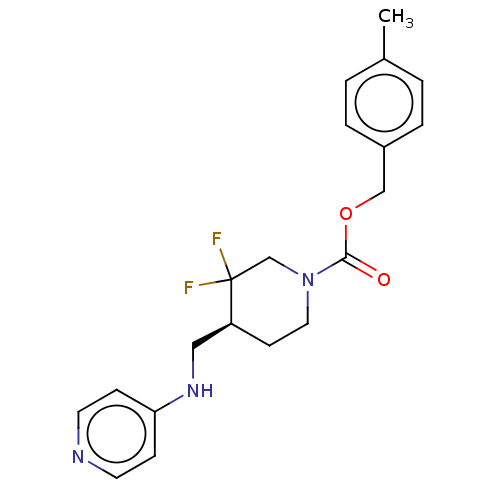
(US11752155, Compound E2-38.2B')Show SMILES Cc1ccc(COC(=O)N2CC[C@@H](CNc3ccncc3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617034

(US11752155, Compound E1-23.2B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ccccn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617026
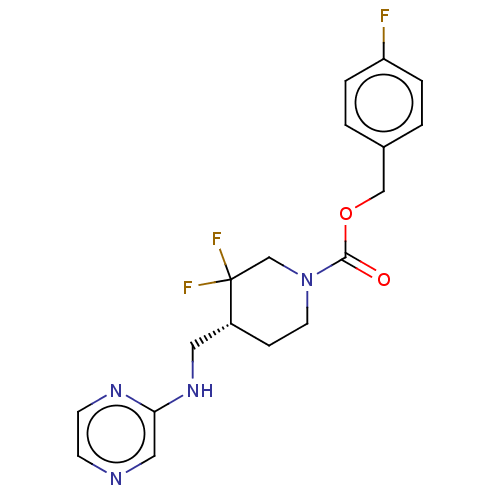
(US11752155, Compound E1-21.4B')Show SMILES Fc1ccc(COC(=O)N2CC[C@H](CNc3cnccn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM435146

(US10584127, Compound E1-8.2 | US11136328, Compound...)Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ncc4cn[nH]c4n3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM287602

(US10420768, Compound C-178 | US11752155, Compound ...)Show SMILES FC(F)(F)c1ccc(cc1)C(F)(F)CN1CCC(CC1)Nc1ncnc2[nH]ccc12 Show InChI InChI=1S/C20H20F5N5/c21-19(22,13-1-3-14(4-2-13)20(23,24)25)11-30-9-6-15(7-10-30)29-18-16-5-8-26-17(16)27-12-28-18/h1-5,8,12,15H,6-7,9-11H2,(H2,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617049
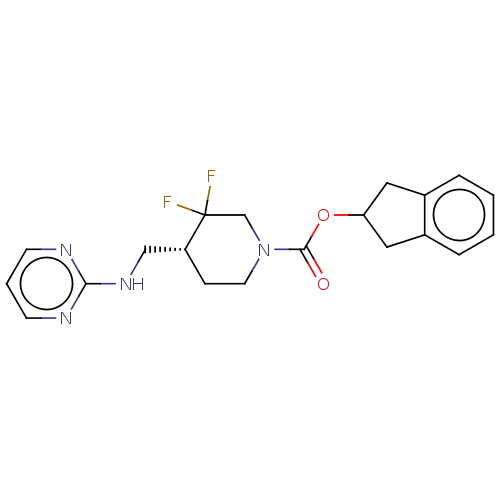
(US11752155, Compound III-E1-22.1B')Show SMILES FC1(F)CN(CC[C@@H]1CNc1ncccn1)C(=O)OC1Cc2ccccc2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617033

(US11752155, Compound E1-22.28B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ncc(F)cn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205801
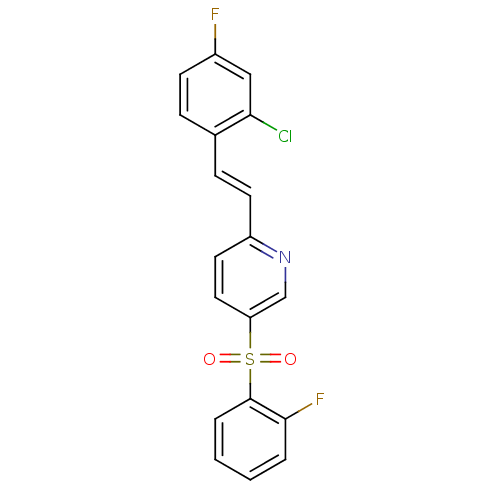
((E)-2-(2-chloro-4-fluorostyryl)-5-(2-fluorophenyls...)Show SMILES Fc1ccc(\C=C\c2ccc(cn2)S(=O)(=O)c2ccccc2F)c(Cl)c1 Show InChI InChI=1S/C19H12ClF2NO2S/c20-17-11-14(21)7-5-13(17)6-8-15-9-10-16(12-23-15)26(24,25)19-4-2-1-3-18(19)22/h1-12H/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617038

(US11752155, Compound E1-24.2B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3cccnn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617041
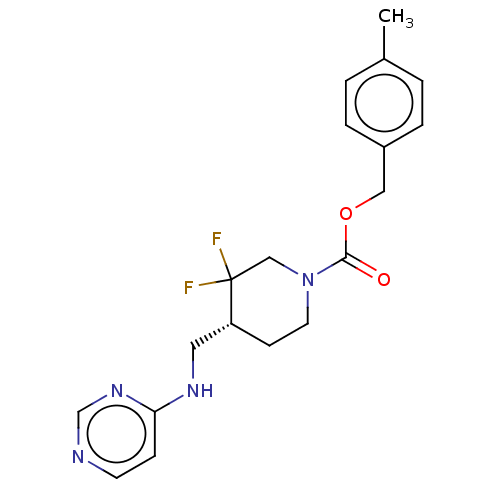
(US11752155, Compound E1-37.2B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ccncn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617021
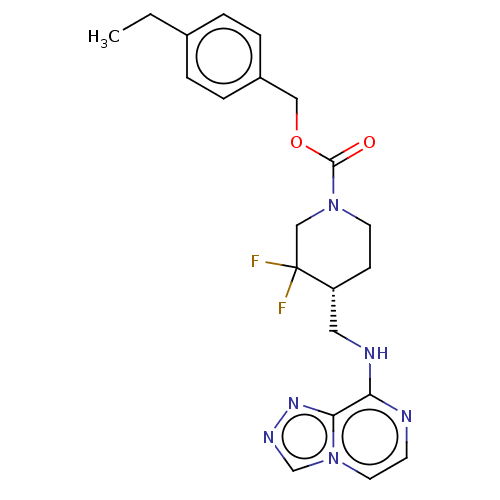
(US11752155, Compound E1-1.5B')Show SMILES CCc1ccc(COC(=O)N2CC[C@H](CNc3nccn4cnnc34)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617025

(US11752155, Compound E1-22.30B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ncc(cn3)C#N)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205798
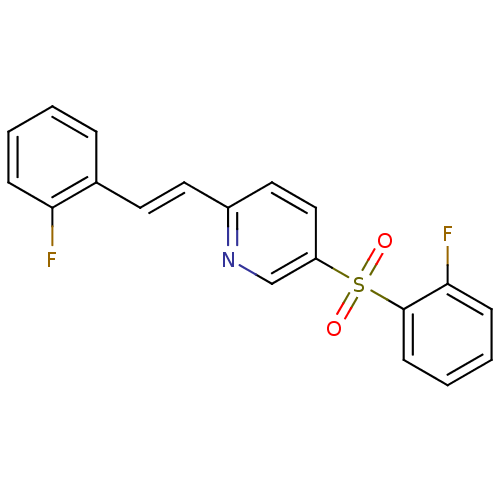
((E)-2-(2-fluorostyryl)-5-(2-fluorophenylsulfonyl)p...)Show InChI InChI=1S/C19H13F2NO2S/c20-17-6-2-1-5-14(17)9-10-15-11-12-16(13-22-15)25(23,24)19-8-4-3-7-18(19)21/h1-13H/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205793
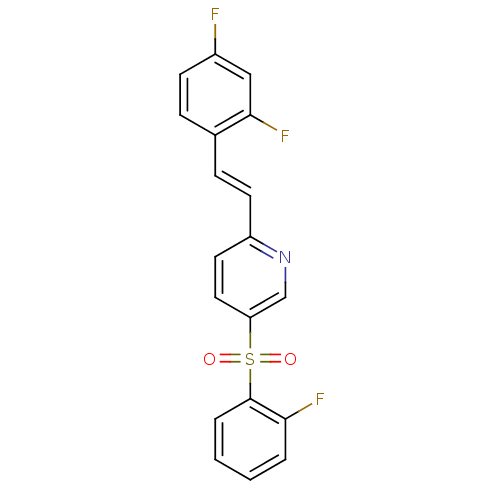
((E)-2-(2,4-difluorostyryl)-5-(2-fluorophenylsulfon...)Show SMILES Fc1ccc(\C=C\c2ccc(cn2)S(=O)(=O)c2ccccc2F)c(F)c1 Show InChI InChI=1S/C19H12F3NO2S/c20-14-7-5-13(18(22)11-14)6-8-15-9-10-16(12-23-15)26(24,25)19-4-2-1-3-17(19)21/h1-12H/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617050
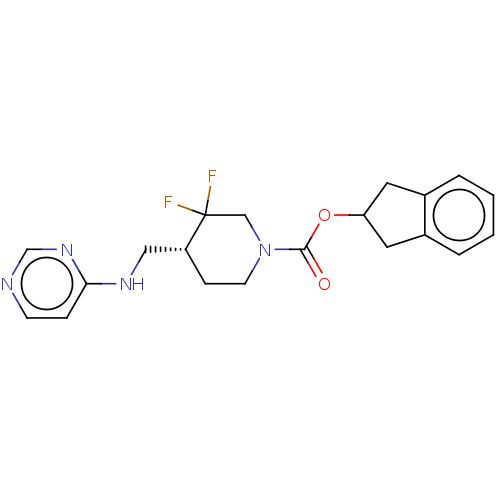
(US11752155, Compound III-E1-37.1B')Show SMILES FC1(F)CN(CC[C@@H]1CNc1ccncn1)C(=O)OC1Cc2ccccc2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617022

(US11752155, Compound E1-22.26B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ncc(C)cn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205783
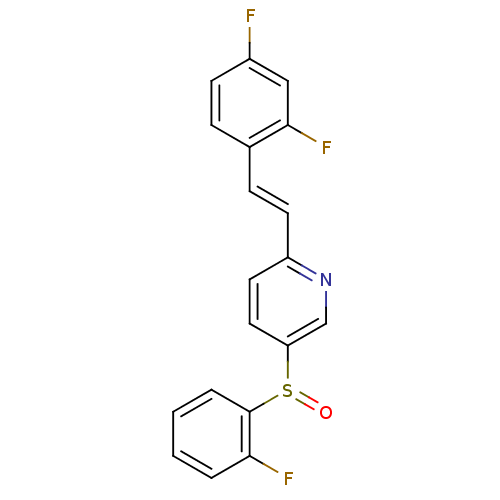
((E)-2-(2,4-difluorostyryl)-5-(2-fluorophenylsulfin...)Show SMILES Fc1ccc(\C=C\c2ccc(cn2)S(=O)c2ccccc2F)c(F)c1 Show InChI InChI=1S/C19H12F3NOS/c20-14-7-5-13(18(22)11-14)6-8-15-9-10-16(12-23-15)25(24)19-4-2-1-3-17(19)21/h1-12H/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205799
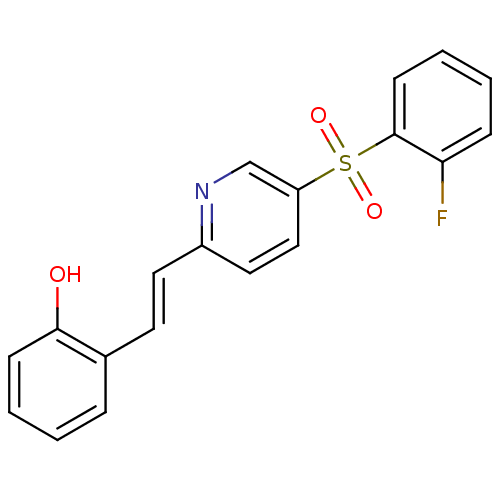
((E)-2-(2-(5-(2-fluorophenylsulfonyl)pyridin-2-yl)v...)Show InChI InChI=1S/C19H14FNO3S/c20-17-6-2-4-8-19(17)25(23,24)16-12-11-15(21-13-16)10-9-14-5-1-3-7-18(14)22/h1-13,22H/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617048
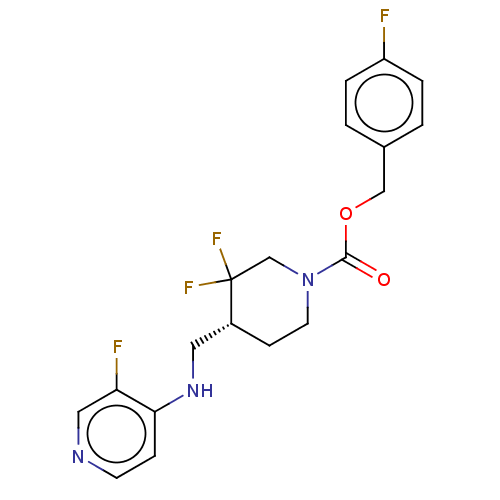
(US11752155, Compound E1-38.10B')Show SMILES Fc1ccc(COC(=O)N2CC[C@H](CNc3ccncc3F)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617043

(US11752155, Compound E1-37.6B')Show SMILES FC(F)c1ccc(COC(=O)N2CC[C@H](CNc3ccncn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205784

((E)-2-(4-fluorostyryl)-5-(2-fluorophenylsulfinyl)p...)Show InChI InChI=1S/C19H13F2NOS/c20-15-8-5-14(6-9-15)7-10-16-11-12-17(13-22-16)24(23)19-4-2-1-3-18(19)21/h1-13H/b10-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617042

(US11752155, Compound E1-37.4B')Show SMILES Fc1ccc(COC(=O)N2CC[C@H](CNc3ccncn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617040

(US11752155, Compound E1-24.6B')Show SMILES FC(F)c1ccc(COC(=O)N2CC[C@H](CNc3cccnn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM388294

(US10294230, Compound E1-1.2 | US10584127, Compound...)Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3nccn4cnnc34)C(F)(F)C2)cc1 |r| Show InChI InChI=1S/C20H22F2N6O2/c1-14-2-4-15(5-3-14)11-30-19(29)27-8-6-16(20(21,22)12-27)10-24-17-18-26-25-13-28(18)9-7-23-17/h2-5,7,9,13,16H,6,8,10-12H2,1H3,(H,23,24)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205791
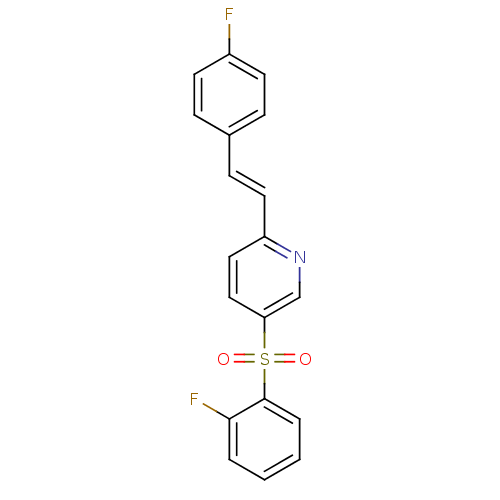
((E)-2-(4-fluorostyryl)-5-(2-fluorophenylsulfonyl)p...)Show SMILES Fc1ccc(\C=C\c2ccc(cn2)S(=O)(=O)c2ccccc2F)cc1 Show InChI InChI=1S/C19H13F2NO2S/c20-15-8-5-14(6-9-15)7-10-16-11-12-17(13-22-16)25(23,24)19-4-2-1-3-18(19)21/h1-13H/b10-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617037

(US11752155, Compound E1-23.26B')Show SMILES Cc1ccc(COC(=O)N2CC[C@H](CNc3ccc(C)cn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205786
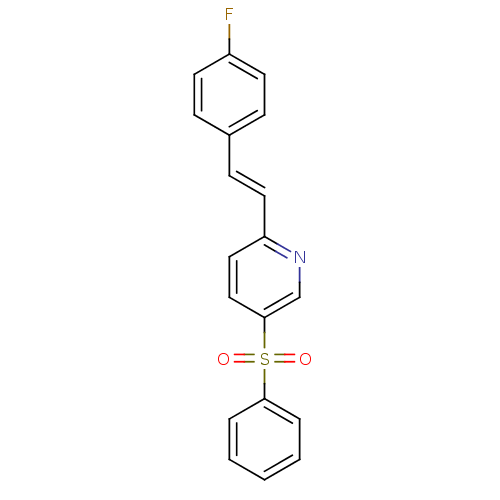
((E)-2-(4-fluorostyryl)-5-(phenylsulfonyl)pyridine ...)Show SMILES Fc1ccc(\C=C\c2ccc(cn2)S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C19H14FNO2S/c20-16-9-6-15(7-10-16)8-11-17-12-13-19(14-21-17)24(22,23)18-4-2-1-3-5-18/h1-14H/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205788
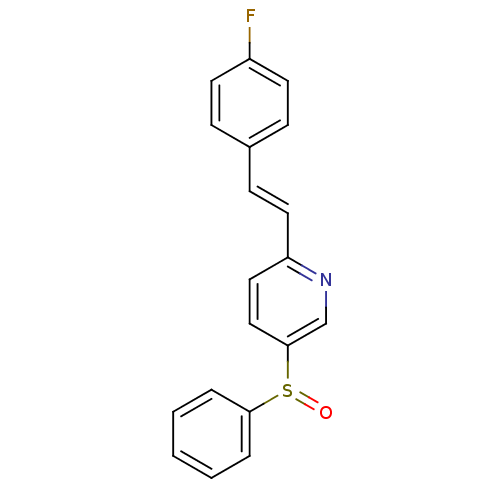
((E)-2-(4-fluorostyryl)-5-(phenylsulfinyl)pyridine ...)Show InChI InChI=1S/C19H14FNOS/c20-16-9-6-15(7-10-16)8-11-17-12-13-19(14-21-17)23(22)18-4-2-1-3-5-18/h1-14H/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM617036

(US11752155, Compound E1-23.4B')Show SMILES Fc1ccc(COC(=O)N2CC[C@H](CNc3ccccn3)C(F)(F)C2)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205785
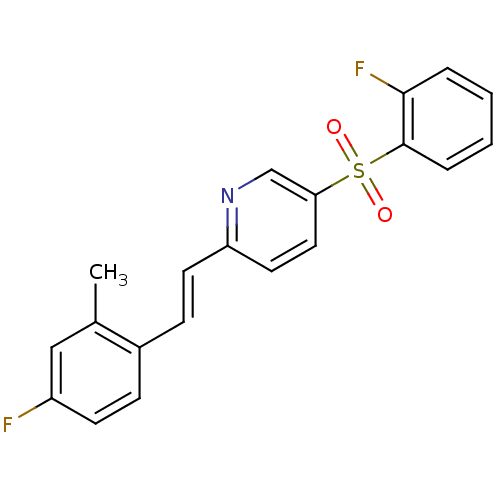
((E)-2-(4-fluoro-2-methylstyryl)-5-(2-fluorophenyls...)Show SMILES Cc1cc(F)ccc1\C=C\c1ccc(cn1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C20H15F2NO2S/c1-14-12-16(21)8-6-15(14)7-9-17-10-11-18(13-23-17)26(24,25)20-5-3-2-4-19(20)22/h2-13H,1H3/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM388299

(US10294230, Compound E1-1.3 | US10584127, Compound...)Show SMILES FC1(F)CN(CC[C@@H]1CNc1nccn2cnnc12)C(=O)OCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H19ClF2N6O2/c20-15-3-1-13(2-4-15)10-30-18(29)27-7-5-14(19(21,22)11-27)9-24-16-17-26-25-12-28(17)8-6-23-16/h1-4,6,8,12,14H,5,7,9-11H2,(H,23,24)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 8.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26T0RS5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205782
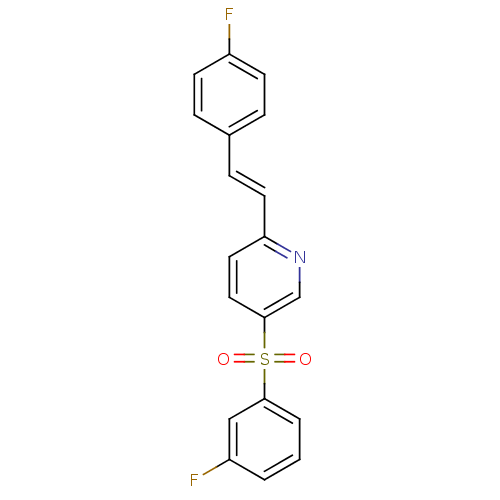
((E)-2-(4-fluorostyryl)-5-(3-fluorophenylsulfonyl)p...)Show SMILES Fc1ccc(\C=C\c2ccc(cn2)S(=O)(=O)c2cccc(F)c2)cc1 Show InChI InChI=1S/C19H13F2NO2S/c20-15-7-4-14(5-8-15)6-9-17-10-11-19(13-22-17)25(23,24)18-3-1-2-16(21)12-18/h1-13H/b9-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data