 Found 520 hits with Last Name = 'lorrain' and Initial = 'ds'
Found 520 hits with Last Name = 'lorrain' and Initial = 'ds' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50549891

(CHEMBL4790083)Show SMILES O=C(CCS(=O)(=O)c1cccc2ncsc12)N1CC2CCC(C1)N2c1ccc(cn1)C#N |TLB:1:16:23:19.20| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic receptor M1 expressed in CHO-K1 cell membranes assessed as inhibition constant incubated fo... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50549891

(CHEMBL4790083)Show SMILES O=C(CCS(=O)(=O)c1cccc2ncsc12)N1CC2CCC(C1)N2c1ccc(cn1)C#N |TLB:1:16:23:19.20| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic receptor M3 expressed in CHO-K1 cell membranes assessed as inhibition constant incubated fo... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50549891

(CHEMBL4790083)Show SMILES O=C(CCS(=O)(=O)c1cccc2ncsc12)N1CC2CCC(C1)N2c1ccc(cn1)C#N |TLB:1:16:23:19.20| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic receptor M4 expressed in CHO-K1 cell membranes assessed as inhibition constant incubated fo... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50549891

(CHEMBL4790083)Show SMILES O=C(CCS(=O)(=O)c1cccc2ncsc12)N1CC2CCC(C1)N2c1ccc(cn1)C#N |TLB:1:16:23:19.20| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]NMS from human recombinant muscarinic receptor M2 expressed in CHO-K1 cell membranes assessed as inhibition constant incubated fo... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296979
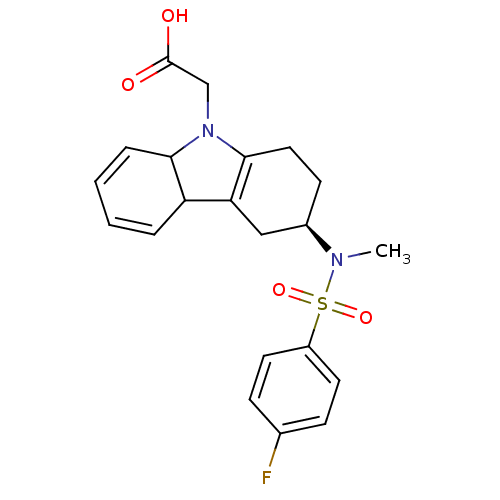
(2-((3R)-3-(4-fluoro-N-methylphenylsulfonamido)-3,4...)Show SMILES CN([C@@H]1CCC2=C(C1)C1C=CC=CC1N2CC(O)=O)S(=O)(=O)c1ccc(F)cc1 |r,c:5,10,12| Show InChI InChI=1S/C21H23FN2O4S/c1-23(29(27,28)16-9-6-14(22)7-10-16)15-8-11-20-18(12-15)17-4-2-3-5-19(17)24(20)13-21(25)26/h2-7,9-10,15,17,19H,8,11-13H2,1H3,(H,25,26)/t15-,17?,19?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human prostaglandin D2 receptor |
Bioorg Med Chem Lett 19: 4647-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.085
BindingDB Entry DOI: 10.7270/Q25T3KHB |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50335958
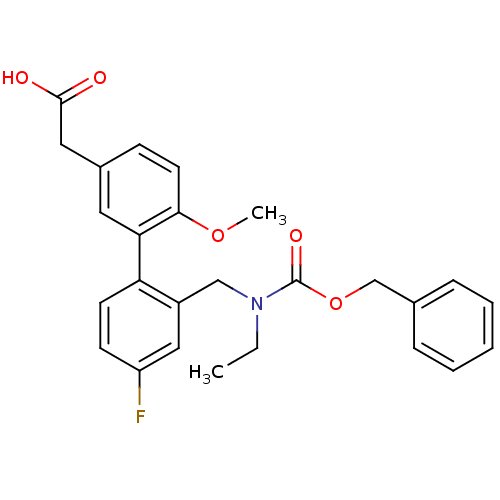
(2-(2'-(((benzyloxycarbonyl)(ethyl)amino)methyl)-4'...)Show SMILES CCN(Cc1cc(F)ccc1-c1cc(CC(O)=O)ccc1OC)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H26FNO5/c1-3-28(26(31)33-17-18-7-5-4-6-8-18)16-20-15-21(27)10-11-22(20)23-13-19(14-25(29)30)9-12-24(23)32-2/h4-13,15H,3,14,16-17H2,1-2H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in 293 cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304887
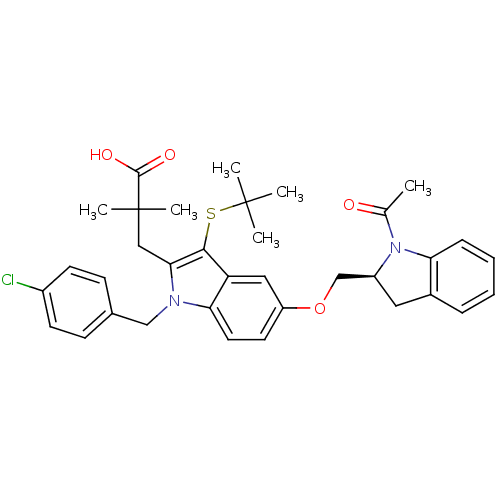
((S)-3-(5-((1-acetylindolin-2-yl)methoxy)-3-(tert-b...)Show SMILES CC(=O)N1[C@H](COc2ccc3n(Cc4ccc(Cl)cc4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)Cc2ccccc12 |r| Show InChI InChI=1S/C35H39ClN2O4S/c1-22(39)38-26(17-24-9-7-8-10-29(24)38)21-42-27-15-16-30-28(18-27)32(43-34(2,3)4)31(19-35(5,6)33(40)41)37(30)20-23-11-13-25(36)14-12-23/h7-16,18,26H,17,19-21H2,1-6H3,(H,40,41)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50549891

(CHEMBL4790083)Show SMILES O=C(CCS(=O)(=O)c1cccc2ncsc12)N1CC2CCC(C1)N2c1ccc(cn1)C#N |TLB:1:16:23:19.20| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant muscarinic receptor M1 expressed in CHO-K1 cells assessed as EC80 acetylcholine-induced calcium flux incubat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50549891

(CHEMBL4790083)Show SMILES O=C(CCS(=O)(=O)c1cccc2ncsc12)N1CC2CCC(C1)N2c1ccc(cn1)C#N |TLB:1:16:23:19.20| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant muscarinic receptor M1 expressed in CHO-K1 cells assessed as EC80 acetylcholine-induced calcium flux incubat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304898

((S)-3-(5-((1-acetylindolin-2-yl)methoxy)-3-(tert-b...)Show SMILES CC(=O)N1[C@H](COc2ccc3n(Cc4ccc(cc4)-c4ccc(F)cn4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)Cc2ccccc12 |r| Show InChI InChI=1S/C40H42FN3O4S/c1-25(45)44-30(19-28-9-7-8-10-34(28)44)24-48-31-16-18-35-32(20-31)37(49-39(2,3)4)36(21-40(5,6)38(46)47)43(35)23-26-11-13-27(14-12-26)33-17-15-29(41)22-42-33/h7-18,20,22,30H,19,21,23-24H2,1-6H3,(H,46,47)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50335954

(CHEMBL1668898 | Sodium [2'-[(cyclopropanecarbonyl-...)Show SMILES CCOc1ccc(cn1)-c1ccc(c(CN(CC)C(=O)C2CC2)c1)-c1cc(CC([O-])=O)ccc1OC Show InChI InChI=1S/C29H32N2O5/c1-4-31(29(34)20-7-8-20)18-23-16-21(22-10-13-27(30-17-22)36-5-2)9-11-24(23)25-14-19(15-28(32)33)6-12-26(25)35-3/h6,9-14,16-17,20H,4-5,7-8,15,18H2,1-3H3,(H,32,33)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape change after 4 hrs by flow cytometry |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357310
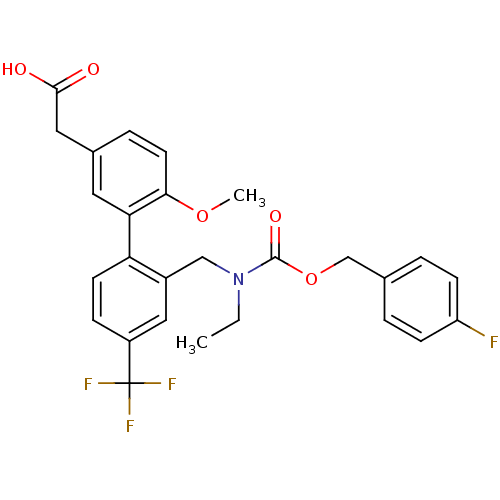
(CHEMBL1916710)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1ccc(F)cc1 Show InChI InChI=1S/C27H25F4NO5/c1-3-32(26(35)37-16-17-4-8-21(28)9-5-17)15-19-14-20(27(29,30)31)7-10-22(19)23-12-18(13-25(33)34)6-11-24(23)36-2/h4-12,14H,3,13,15-16H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50322851
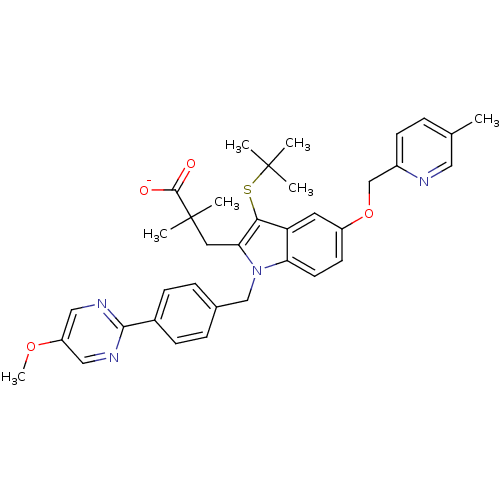
(CHEMBL1210423 | sodium 3-(3-(tert-butylthio)-1-(4-...)Show SMILES COc1cnc(nc1)-c1ccc(Cn2c(CC(C)(C)C([O-])=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C36H40N4O4S/c1-23-8-13-26(37-18-23)22-44-27-14-15-30-29(16-27)32(45-35(2,3)4)31(17-36(5,6)34(41)42)40(30)21-24-9-11-25(12-10-24)33-38-19-28(43-7)20-39-33/h8-16,18-20H,17,21-22H2,1-7H3,(H,41,42)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN by radioligand displacement assay |
Bioorg Med Chem Lett 20: 4598-601 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.011
BindingDB Entry DOI: 10.7270/Q2F76CRR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50335954

(CHEMBL1668898 | Sodium [2'-[(cyclopropanecarbonyl-...)Show SMILES CCOc1ccc(cn1)-c1ccc(c(CN(CC)C(=O)C2CC2)c1)-c1cc(CC([O-])=O)ccc1OC Show InChI InChI=1S/C29H32N2O5/c1-4-31(29(34)20-7-8-20)18-23-16-21(22-10-13-27(30-17-22)36-5-2)9-11-24(23)25-14-19(15-28(32)33)6-12-26(25)35-3/h6,9-14,16-17,20H,4-5,7-8,15,18H2,1-3H3,(H,32,33)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by time-dependent inhibition assay |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357308

(CHEMBL1916707)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1cccc(Cl)c1 Show InChI InChI=1S/C27H25ClF3NO5/c1-3-32(26(35)37-16-18-5-4-6-21(28)11-18)15-19-14-20(27(29,30)31)8-9-22(19)23-12-17(13-25(33)34)7-10-24(23)36-2/h4-12,14H,3,13,15-16H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304903
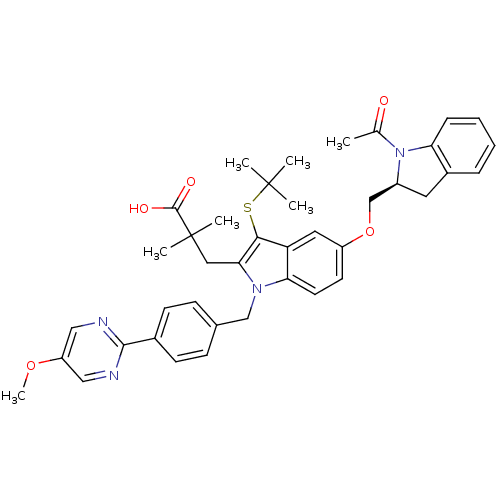
((S)-3-(5-((1-acetylindolin-2-yl)methoxy)-3-(tert-b...)Show SMILES COc1cnc(nc1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OC[C@@H]4Cc5ccccc5N4C(C)=O)ccc23)cc1 |r| Show InChI InChI=1S/C40H44N4O5S/c1-25(45)44-29(18-28-10-8-9-11-33(28)44)24-49-30-16-17-34-32(19-30)36(50-39(2,3)4)35(20-40(5,6)38(46)47)43(34)23-26-12-14-27(15-13-26)37-41-21-31(48-7)22-42-37/h8-17,19,21-22,29H,18,20,23-24H2,1-7H3,(H,46,47)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304899
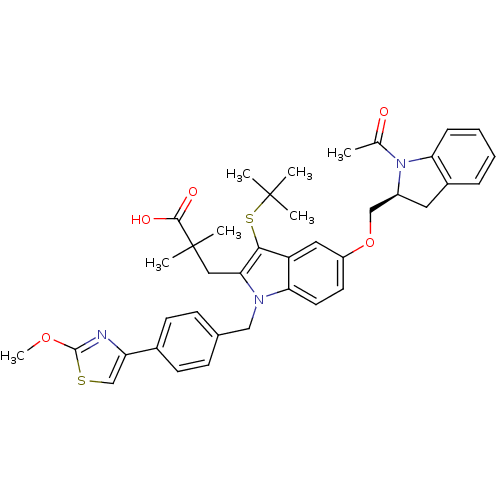
((S)-3-(5-((1-acetylindolin-2-yl)methoxy)-3-(tert-b...)Show SMILES COc1nc(cs1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OC[C@@H]4Cc5ccccc5N4C(C)=O)ccc23)cc1 |r| Show InChI InChI=1S/C39H43N3O5S2/c1-24(43)42-28(18-27-10-8-9-11-32(27)42)22-47-29-16-17-33-30(19-29)35(49-38(2,3)4)34(20-39(5,6)36(44)45)41(33)21-25-12-14-26(15-13-25)31-23-48-37(40-31)46-7/h8-17,19,23,28H,18,20-22H2,1-7H3,(H,44,45)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50013729

(CHEMBL3264919)Show SMILES CCn1c(CCCc2ccc(OC(C)(C)C(=O)NS(=O)(=O)c3ccccc3)cc2)nn(Cc2ccc(cc2)C(C)(C)C)c1=O Show InChI InChI=1S/C34H42N4O5S/c1-7-37-30(35-38(32(37)40)24-26-16-20-27(21-17-26)33(2,3)4)15-11-12-25-18-22-28(23-19-25)43-34(5,6)31(39)36-44(41,42)29-13-9-8-10-14-29/h8-10,13-14,16-23H,7,11-12,15,24H2,1-6H3,(H,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human PPAR-alpha assessed as inhibition of GW7647-induced effect after overnight incubation by cell-based luciferase reporter ... |
Bioorg Med Chem Lett 24: 2267-72 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.090
BindingDB Entry DOI: 10.7270/Q2GX4D33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50013730

(CHEMBL3264920)Show SMILES CCn1c(CCCc2ccc(OC(C)(C)C(=O)NS(=O)(=O)c3ccc(C)cc3)cc2)nn(Cc2ccc(cc2)C(C)(C)C)c1=O Show InChI InChI=1S/C35H44N4O5S/c1-8-38-31(36-39(33(38)41)24-27-14-18-28(19-15-27)34(3,4)5)11-9-10-26-16-20-29(21-17-26)44-35(6,7)32(40)37-45(42,43)30-22-12-25(2)13-23-30/h12-23H,8-11,24H2,1-7H3,(H,37,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human PPAR-alpha assessed as inhibition of GW7647-induced effect after overnight incubation by cell-based luciferase reporter ... |
Bioorg Med Chem Lett 24: 2267-72 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.090
BindingDB Entry DOI: 10.7270/Q2GX4D33 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50335957
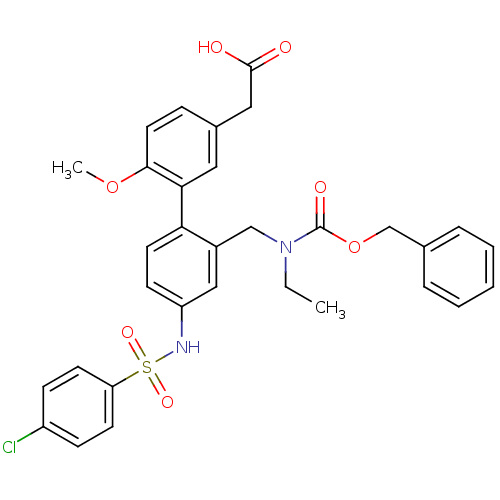
(2-(2'-(((benzyloxycarbonyl)(ethyl)amino)methyl)-4'...)Show SMILES CCN(Cc1cc(NS(=O)(=O)c2ccc(Cl)cc2)ccc1-c1cc(CC(O)=O)ccc1OC)C(=O)OCc1ccccc1 Show InChI InChI=1S/C32H31ClN2O7S/c1-3-35(32(38)42-21-22-7-5-4-6-8-22)20-24-19-26(34-43(39,40)27-13-10-25(33)11-14-27)12-15-28(24)29-17-23(18-31(36)37)9-16-30(29)41-2/h4-17,19,34H,3,18,20-21H2,1-2H3,(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in 293 cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50013734
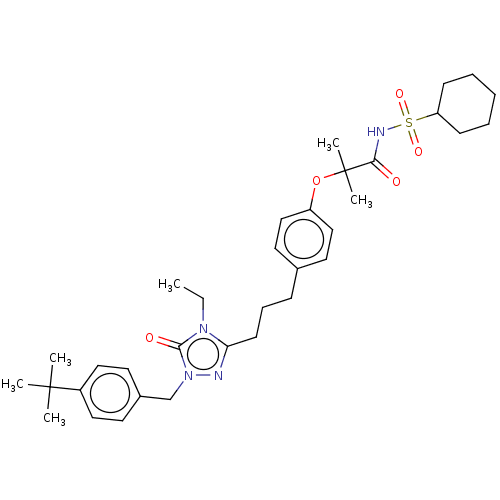
(CHEMBL3264924)Show SMILES CCn1c(CCCc2ccc(OC(C)(C)C(=O)NS(=O)(=O)C3CCCCC3)cc2)nn(Cc2ccc(cc2)C(C)(C)C)c1=O Show InChI InChI=1S/C34H48N4O5S/c1-7-37-30(35-38(32(37)40)24-26-16-20-27(21-17-26)33(2,3)4)15-11-12-25-18-22-28(23-19-25)43-34(5,6)31(39)36-44(41,42)29-13-9-8-10-14-29/h16-23,29H,7-15,24H2,1-6H3,(H,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human PPAR-alpha assessed as inhibition of GW7647-induced effect after overnight incubation by cell-based luciferase reporter ... |
Bioorg Med Chem Lett 24: 2267-72 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.090
BindingDB Entry DOI: 10.7270/Q2GX4D33 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357307

(CHEMBL1916706)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1ccccc1Cl Show InChI InChI=1S/C27H25ClF3NO5/c1-3-32(26(35)37-16-18-6-4-5-7-23(18)28)15-19-14-20(27(29,30)31)9-10-21(19)22-12-17(13-25(33)34)8-11-24(22)36-2/h4-12,14H,3,13,15-16H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357311

(CHEMBL1916711)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1cc(F)cc(F)c1 Show InChI InChI=1S/C27H24F5NO5/c1-3-33(26(36)38-15-17-8-20(28)13-21(29)9-17)14-18-12-19(27(30,31)32)5-6-22(18)23-10-16(11-25(34)35)4-7-24(23)37-2/h4-10,12-13H,3,11,14-15H2,1-2H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357289

(CHEMBL1916708)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1ccc(Cl)cc1 Show InChI InChI=1S/C27H25ClF3NO5/c1-3-32(26(35)37-16-17-4-8-21(28)9-5-17)15-19-14-20(27(29,30)31)7-10-22(19)23-12-18(13-25(33)34)6-11-24(23)36-2/h4-12,14H,3,13,15-16H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304902
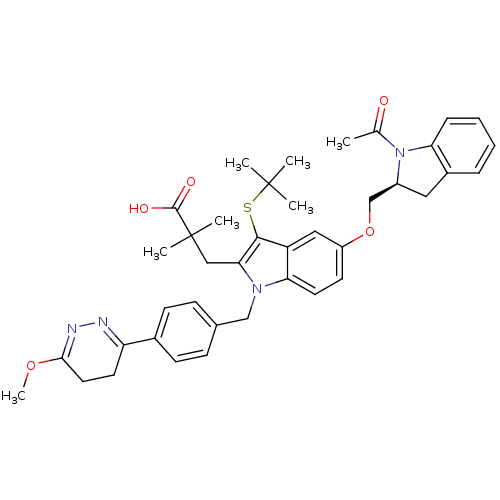
((S)-3-(5-((1-acetylindolin-2-yl)methoxy)-3-(tert-b...)Show SMILES COC1=NN=C(CC1)c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OC[C@@H]4Cc5ccccc5N4C(C)=O)ccc23)cc1 |r,c:4,t:2| Show InChI InChI=1S/C40H46N4O5S/c1-25(45)44-29(20-28-10-8-9-11-33(28)44)24-49-30-16-18-34-31(21-30)37(50-39(2,3)4)35(22-40(5,6)38(46)47)43(34)23-26-12-14-27(15-13-26)32-17-19-36(48-7)42-41-32/h8-16,18,21,29H,17,19-20,22-24H2,1-7H3,(H,46,47)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304901
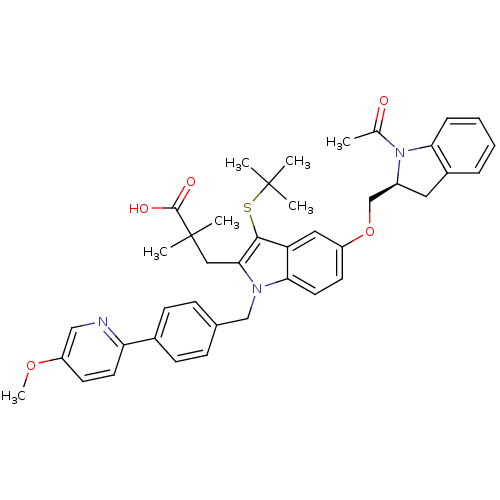
((S)-3-(5-((1-acetylindolin-2-yl)methoxy)-3-(tert-b...)Show SMILES COc1ccc(nc1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OC[C@@H]4Cc5ccccc5N4C(C)=O)ccc23)cc1 |r| Show InChI InChI=1S/C41H45N3O5S/c1-26(45)44-30(20-29-10-8-9-11-35(29)44)25-49-31-17-19-36-33(21-31)38(50-40(2,3)4)37(22-41(5,6)39(46)47)43(36)24-27-12-14-28(15-13-27)34-18-16-32(48-7)23-42-34/h8-19,21,23,30H,20,22,24-25H2,1-7H3,(H,46,47)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304882

((S)-3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-((1...)Show SMILES CCC(=O)N1CCC[C@H]1COc1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 |r| Show InChI InChI=1S/C32H41ClN2O4S/c1-7-28(36)34-16-8-9-23(34)20-39-24-14-15-26-25(17-24)29(40-31(2,3)4)27(18-32(5,6)30(37)38)35(26)19-21-10-12-22(33)13-11-21/h10-15,17,23H,7-9,16,18-20H2,1-6H3,(H,37,38)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296979

(2-((3R)-3-(4-fluoro-N-methylphenylsulfonamido)-3,4...)Show SMILES CN([C@@H]1CCC2=C(C1)C1C=CC=CC1N2CC(O)=O)S(=O)(=O)c1ccc(F)cc1 |r,c:5,10,12| Show InChI InChI=1S/C21H23FN2O4S/c1-23(29(27,28)16-9-6-14(22)7-10-16)15-8-11-20-18(12-15)17-4-2-3-5-19(17)24(20)13-21(25)26/h2-7,9-10,15,17,19H,8,11-13H2,1H3,(H,25,26)/t15-,17?,19?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human prostaglandin D2 receptor in presence of human serum albumin |
Bioorg Med Chem Lett 19: 4647-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.085
BindingDB Entry DOI: 10.7270/Q25T3KHB |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50335951
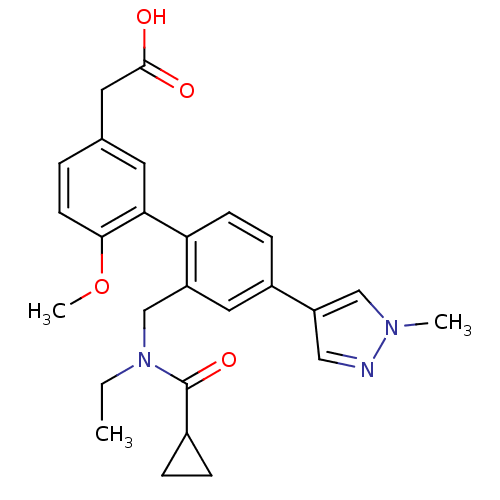
(2-(2'-((N-ethylcyclopropanecarboxamido)methyl)-6-m...)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)-c1cnn(C)c1)C(=O)C1CC1 Show InChI InChI=1S/C26H29N3O4/c1-4-29(26(32)18-6-7-18)16-20-13-19(21-14-27-28(2)15-21)8-9-22(20)23-11-17(12-25(30)31)5-10-24(23)33-3/h5,8-11,13-15,18H,4,6-7,12,16H2,1-3H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape change by flow cytometry |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357304
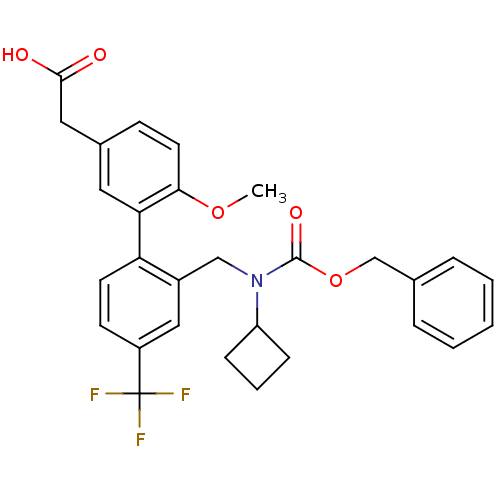
(CHEMBL1916702)Show SMILES COc1ccc(CC(O)=O)cc1-c1ccc(cc1CN(C1CCC1)C(=O)OCc1ccccc1)C(F)(F)F Show InChI InChI=1S/C29H28F3NO5/c1-37-26-13-10-20(15-27(34)35)14-25(26)24-12-11-22(29(30,31)32)16-21(24)17-33(23-8-5-9-23)28(36)38-18-19-6-3-2-4-7-19/h2-4,6-7,10-14,16,23H,5,8-9,15,17-18H2,1H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357303
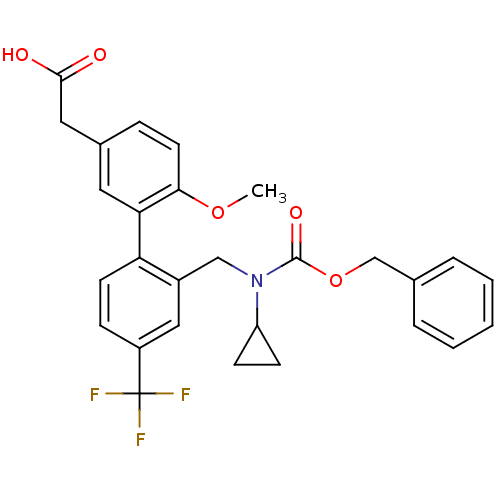
(CHEMBL1916701)Show SMILES COc1ccc(CC(O)=O)cc1-c1ccc(cc1CN(C1CC1)C(=O)OCc1ccccc1)C(F)(F)F Show InChI InChI=1S/C28H26F3NO5/c1-36-25-12-7-19(14-26(33)34)13-24(25)23-11-8-21(28(29,30)31)15-20(23)16-32(22-9-10-22)27(35)37-17-18-5-3-2-4-6-18/h2-8,11-13,15,22H,9-10,14,16-17H2,1H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357310

(CHEMBL1916710)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1ccc(F)cc1 Show InChI InChI=1S/C27H25F4NO5/c1-3-32(26(35)37-16-17-4-8-21(28)9-5-17)15-19-14-20(27(29,30)31)7-10-22(19)23-12-18(13-25(33)34)6-11-24(23)36-2/h4-12,14H,3,13,15-16H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human prostanoid DP2 receptor in human whole bood assessed as eosinophil shape change preincubated for 15 mins before addition of PGD2 ... |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357309

(CHEMBL1916709)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C27H24Cl2F3NO5/c1-3-33(26(36)38-15-17-8-20(28)13-21(29)9-17)14-18-12-19(27(30,31)32)5-6-22(18)23-10-16(11-25(34)35)4-7-24(23)37-2/h4-10,12-13H,3,11,14-15H2,1-2H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357302

(CHEMBL1916699)Show SMILES COc1ccc(CC(O)=O)cc1-c1ccc(cc1CN(C)C(=O)OCc1ccccc1)C(F)(F)F Show InChI InChI=1S/C26H24F3NO5/c1-30(25(33)35-16-17-6-4-3-5-7-17)15-19-14-20(26(27,28)29)9-10-21(19)22-12-18(13-24(31)32)8-11-23(22)34-2/h3-12,14H,13,15-16H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357286
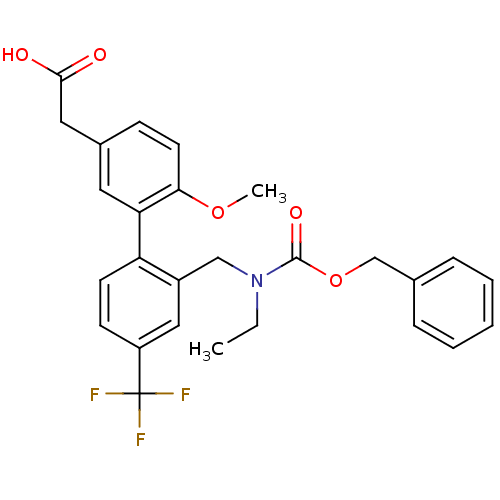
(CHEMBL1916700)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1ccccc1 Show InChI InChI=1S/C27H26F3NO5/c1-3-31(26(34)36-17-18-7-5-4-6-8-18)16-20-15-21(27(28,29)30)10-11-22(20)23-13-19(14-25(32)33)9-12-24(23)35-2/h4-13,15H,3,14,16-17H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from human prostanoid DP2 receptor expressed in human 293T cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304897
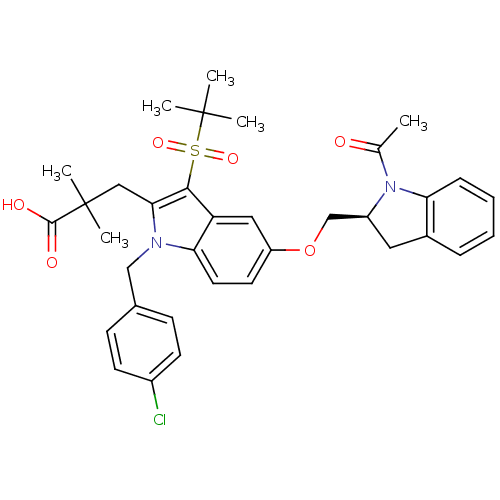
((S)-3-(5-((1-acetylindolin-2-yl)methoxy)-3-(tert-b...)Show SMILES CC(=O)N1[C@H](COc2ccc3n(Cc4ccc(Cl)cc4)c(CC(C)(C)C(O)=O)c(c3c2)S(=O)(=O)C(C)(C)C)Cc2ccccc12 |r| Show InChI InChI=1S/C35H39ClN2O6S/c1-22(39)38-26(17-24-9-7-8-10-29(24)38)21-44-27-15-16-30-28(18-27)32(45(42,43)34(2,3)4)31(19-35(5,6)33(40)41)37(30)20-23-11-13-25(36)14-12-23/h7-16,18,26H,17,19-21H2,1-6H3,(H,40,41)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50013735

(CHEMBL3264925)Show SMILES CCn1c(CCCc2ccc(OC(C)(C)C(=O)NS(=O)(=O)C3CCCC3)cc2)nn(Cc2ccc(cc2)C(C)(C)C)c1=O Show InChI InChI=1S/C33H46N4O5S/c1-7-36-29(34-37(31(36)39)23-25-15-19-26(20-16-25)32(2,3)4)14-10-11-24-17-21-27(22-18-24)42-33(5,6)30(38)35-43(40,41)28-12-8-9-13-28/h15-22,28H,7-14,23H2,1-6H3,(H,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human PPAR-alpha assessed as inhibition of GW7647-induced effect after overnight incubation by cell-based luciferase reporter ... |
Bioorg Med Chem Lett 24: 2267-72 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.090
BindingDB Entry DOI: 10.7270/Q2GX4D33 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304895

((S)-3-(5-((1-acetylindolin-2-yl)methoxy)-1-(4-chlo...)Show SMILES CC(=O)N1[C@H](COc2ccc3n(Cc4ccc(Cl)cc4)c(CC(C)(C)C(O)=O)c(CCC(C)(C)C)c3c2)Cc2ccccc12 |r| Show InChI InChI=1S/C37H43ClN2O4/c1-24(41)40-28(19-26-9-7-8-10-32(26)40)23-44-29-15-16-33-31(20-29)30(17-18-36(2,3)4)34(21-37(5,6)35(42)43)39(33)22-25-11-13-27(38)14-12-25/h7-16,20,28H,17-19,21-23H2,1-6H3,(H,42,43)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357311

(CHEMBL1916711)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1cc(F)cc(F)c1 Show InChI InChI=1S/C27H24F5NO5/c1-3-33(26(36)38-15-17-8-20(28)13-21(29)9-17)14-18-12-19(27(30,31)32)5-6-22(18)23-10-16(11-25(34)35)4-7-24(23)37-2/h4-10,12-13H,3,11,14-15H2,1-2H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human prostanoid DP2 receptor in human whole bood assessed as eosinophil shape change preincubated for 15 mins before addition of PGD2 ... |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50335959

(2-(2'-(((benzyloxycarbonyl)(ethyl)amino)methyl)-6-...)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)S(C)(=O)=O)C(=O)OCc1ccccc1 Show InChI InChI=1S/C27H29NO7S/c1-4-28(27(31)35-18-19-8-6-5-7-9-19)17-21-16-22(36(3,32)33)11-12-23(21)24-14-20(15-26(29)30)10-13-25(24)34-2/h5-14,16H,4,15,17-18H2,1-3H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in 293 cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50335954

(CHEMBL1668898 | Sodium [2'-[(cyclopropanecarbonyl-...)Show SMILES CCOc1ccc(cn1)-c1ccc(c(CN(CC)C(=O)C2CC2)c1)-c1cc(CC([O-])=O)ccc1OC Show InChI InChI=1S/C29H32N2O5/c1-4-31(29(34)20-7-8-20)18-23-16-21(22-10-13-27(30-17-22)36-5-2)9-11-24(23)25-14-19(15-28(32)33)6-12-26(25)35-3/h6,9-14,16-17,20H,4-5,7-8,15,18H2,1-3H3,(H,32,33)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape change by flow cytometry |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357286

(CHEMBL1916700)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1ccccc1 Show InChI InChI=1S/C27H26F3NO5/c1-3-31(26(34)36-17-18-7-5-4-6-8-18)16-20-15-21(27(28,29)30)10-11-22(20)23-13-19(14-25(32)33)9-12-24(23)35-2/h4-13,15H,3,14,16-17H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human prostanoid DP2 receptor in human whole bood assessed as eosinophil shape change preincubated for 15 mins before addition of PGD2 ... |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50366775
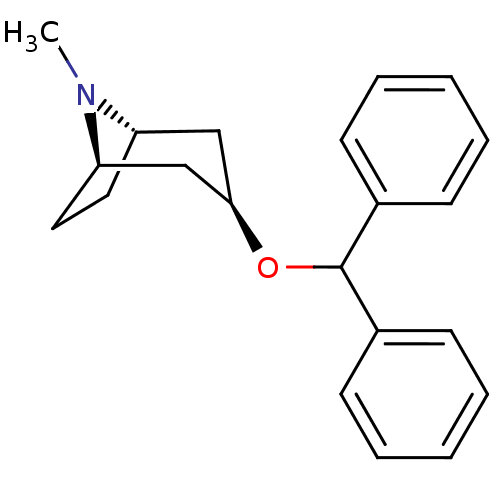
(BENZTROPINE | Benzatropine)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H25NO/c1-22-18-12-13-19(22)15-20(14-18)23-21(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18-21H,12-15H2,1H3/t18-,19+,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant muscarinic receptor M4 expressed in CHO-K1 cells assessed as EC80 acetylcholine-induced calcium flux incubat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50549901
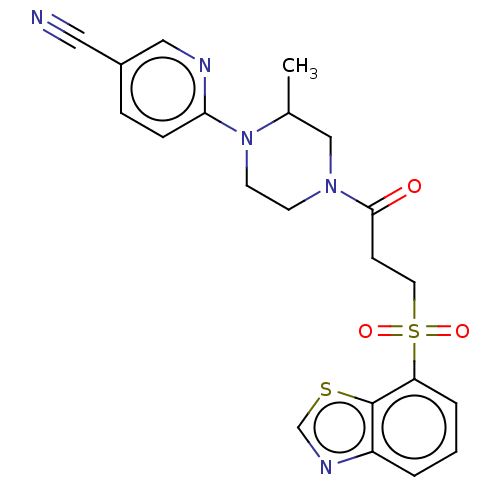
(CHEMBL4799006)Show SMILES CC1CN(CCN1c1ccc(cn1)C#N)C(=O)CCS(=O)(=O)c1cccc2ncsc12 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant muscarinic receptor M1 expressed in CHO-K1 cells assessed as EC80 acetylcholine-induced calcium flux incubat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50549908
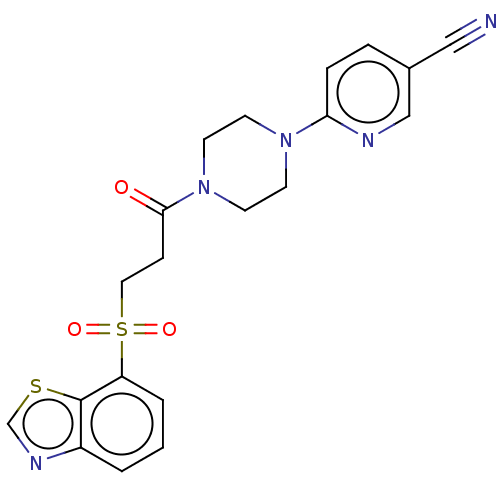
(CHEMBL4760610)Show SMILES O=C(CCS(=O)(=O)c1cccc2ncsc12)N1CCN(CC1)c1ccc(cn1)C#N | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant muscarinic receptor M1 expressed in CHO-K1 cells assessed as EC80 acetylcholine-induced calcium flux incubat... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00626
BindingDB Entry DOI: 10.7270/Q2445R41 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50304907
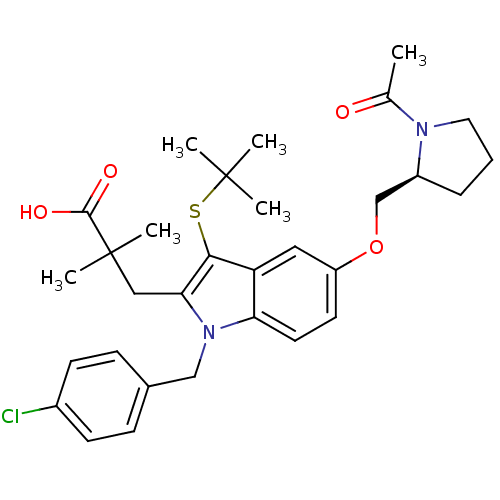
((S)-3-(5-((1-acetylpyrrolidin-2-yl)methoxy)-3-(ter...)Show SMILES CC(=O)N1CCC[C@H]1COc1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 |r| Show InChI InChI=1S/C31H39ClN2O4S/c1-20(35)33-15-7-8-23(33)19-38-24-13-14-26-25(16-24)28(39-30(2,3)4)27(17-31(5,6)29(36)37)34(26)18-21-9-11-22(32)12-10-21/h9-14,16,23H,7-8,15,17-19H2,1-6H3,(H,36,37)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to FLAP in human PMN derived membrane |
Bioorg Med Chem Lett 20: 213-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.131
BindingDB Entry DOI: 10.7270/Q2MW2H78 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50357289

(CHEMBL1916708)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)ccc1OC)C(F)(F)F)C(=O)OCc1ccc(Cl)cc1 Show InChI InChI=1S/C27H25ClF3NO5/c1-3-32(26(35)37-16-17-4-8-21(28)9-5-17)15-19-14-20(27(29,30)31)7-10-22(19)23-12-18(13-25(33)34)6-11-24(23)36-2/h4-12,14H,3,13,15-16H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human prostanoid DP2 receptor in human whole bood assessed as eosinophil shape change preincubated for 15 mins before addition of PGD2 ... |
Bioorg Med Chem Lett 21: 6608-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.024
BindingDB Entry DOI: 10.7270/Q2513ZM9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50335954

(CHEMBL1668898 | Sodium [2'-[(cyclopropanecarbonyl-...)Show SMILES CCOc1ccc(cn1)-c1ccc(c(CN(CC)C(=O)C2CC2)c1)-c1cc(CC([O-])=O)ccc1OC Show InChI InChI=1S/C29H32N2O5/c1-4-31(29(34)20-7-8-20)18-23-16-21(22-10-13-27(30-17-22)36-5-2)9-11-24(23)25-14-19(15-28(32)33)6-12-26(25)35-3/h6,9-14,16-17,20H,4-5,7-8,15,18H2,1-3H3,(H,32,33)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in 293 cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296980
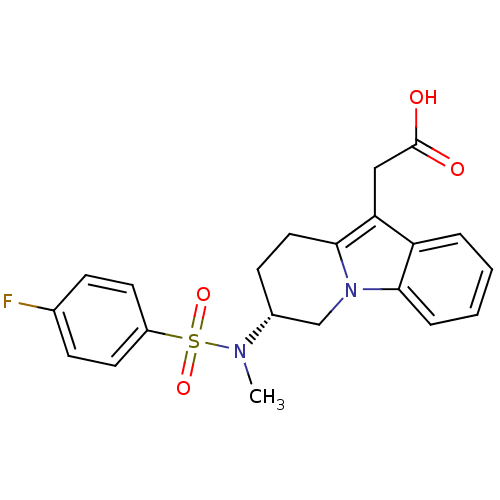
((R)-2-(7-(4-fluoro-N-methylphenylsulfonamido)-6,7,...)Show SMILES CN([C@@H]1CCc2c(CC(O)=O)c3ccccc3n2C1)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C21H21FN2O4S/c1-23(29(27,28)16-9-6-14(22)7-10-16)15-8-11-20-18(12-21(25)26)17-4-2-3-5-19(17)24(20)13-15/h2-7,9-10,15H,8,11-13H2,1H3,(H,25,26)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human prostaglandin D2 receptor |
Bioorg Med Chem Lett 19: 4647-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.085
BindingDB Entry DOI: 10.7270/Q25T3KHB |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296977
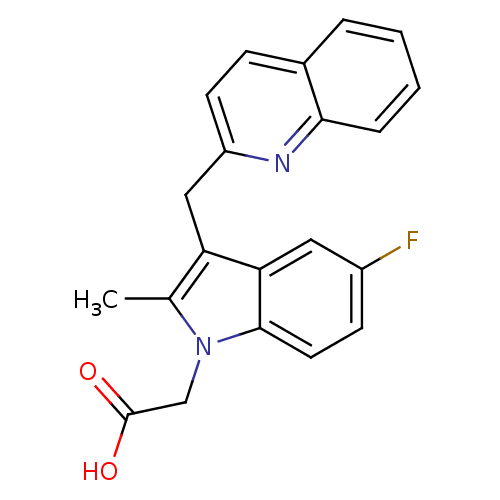
(2-(5-fluoro-2-methyl-3-(quinolin-2-ylmethyl)-1H-in...)Show InChI InChI=1S/C21H17FN2O2/c1-13-17(11-16-8-6-14-4-2-3-5-19(14)23-16)18-10-15(22)7-9-20(18)24(13)12-21(25)26/h2-10H,11-12H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human prostaglandin D2 receptor |
Bioorg Med Chem Lett 19: 4647-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.085
BindingDB Entry DOI: 10.7270/Q25T3KHB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data