 Found 371 hits with Last Name = 'luesch' and Initial = 'h'
Found 371 hits with Last Name = 'luesch' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human AVPR2 by PathHunter beta-arrestin assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115546
BindingDB Entry DOI: 10.7270/Q2F193D1 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50400214
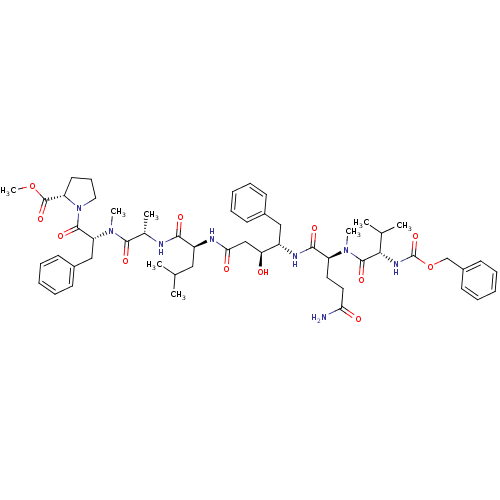
(CHEMBL2181022)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C55H76N8O12/c1-34(2)29-41(49(67)57-36(5)51(69)62(7)44(31-38-21-14-10-15-22-38)52(70)63-28-18-25-43(63)54(72)74-8)58-47(66)32-45(64)40(30-37-19-12-9-13-20-37)59-50(68)42(26-27-46(56)65)61(6)53(71)48(35(3)4)60-55(73)75-33-39-23-16-11-17-24-39/h9-17,19-24,34-36,40-45,48,64H,18,25-33H2,1-8H3,(H2,56,65)(H,57,67)(H,58,66)(H,59,68)(H,60,73)/t36-,40-,41-,42-,43-,44+,45-,48-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0783 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin E using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins f... |
Bioorg Med Chem 24: 3276-82 (2016)
Article DOI: 10.1016/j.bmc.2016.04.062
BindingDB Entry DOI: 10.7270/Q2QZ2CWR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of secreted cathepsin D in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50101331
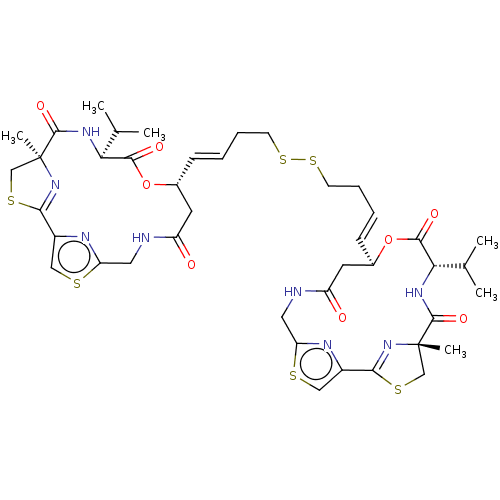
(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of secreted cathepsin E in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50003017

(CHEMBL3234202)Show SMILES [H][C@@]12CSC(=N1)[C@@H](C)[C@@H](O)C[C@H](C)C[C@H](OC(=O)[C@]1([H])CCCN1C(=O)[C@]([H])([C@@H](C)CC)N(C)C(=O)[C@H](C)N(C)C(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC2)C(C)(C)C |r,c:4| Show InChI InChI=1S/C44H69N5O8S/c1-12-27(3)38-42(54)49-21-13-14-34(49)43(55)57-36(44(6,7)8)23-26(2)22-35(50)28(4)39-45-31(25-58-39)17-20-37(51)46-33(24-30-15-18-32(56-11)19-16-30)41(53)47(9)29(5)40(52)48(38)10/h15-16,18-19,26-29,31,33-36,38,50H,12-14,17,20-25H2,1-11H3,(H,46,51)/t26-,27-,28-,29-,31+,33-,34-,35-,36-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-A production in human HCT116 cells after 12 hrs by alphaLISA assay |
J Med Chem 57: 3011-29 (2014)
Article DOI: 10.1021/jm4019965
BindingDB Entry DOI: 10.7270/Q2154JKD |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50400213

(CHEMBL2181024)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C |r| Show InChI InChI=1S/C52H78N8O12/c1-31(2)27-37(45(64)54-33(5)47(66)59(10)40(29-35-21-16-13-17-22-35)48(67)60-26-18-23-39(60)50(69)71-11)55-43(63)30-41(61)36(28-34-19-14-12-15-20-34)56-46(65)38(24-25-42(53)62)58(9)49(68)44(32(3)4)57-51(70)72-52(6,7)8/h12-17,19-22,31-33,36-41,44,61H,18,23-30H2,1-11H3,(H2,53,62)(H,54,64)(H,55,63)(H,56,65)(H,57,70)/t33-,36-,37-,38-,39-,40+,41-,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.173 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50101330
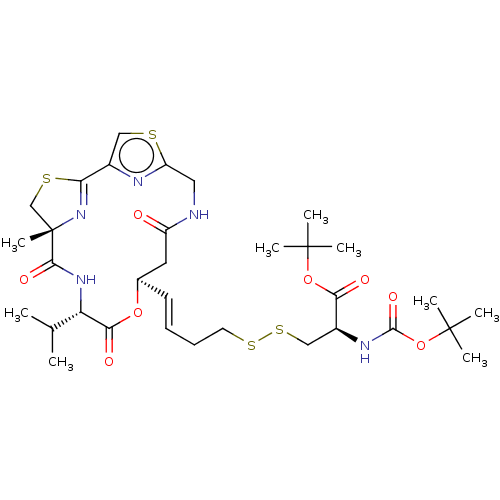
(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50101331

(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055512
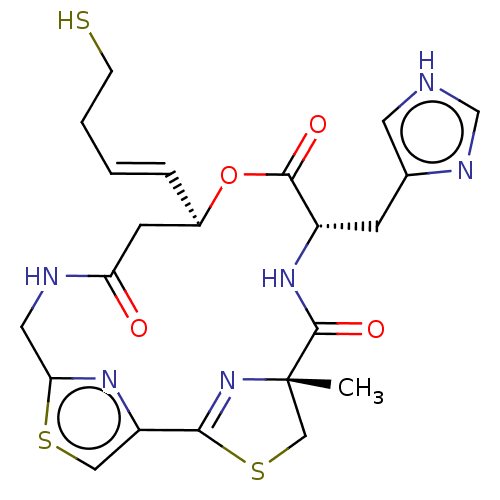
(CHEMBL3317812)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3c[nH]cn3)NC2=O)\C=C\CCS)n1 |r,c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Kallikrein-7
(Homo sapiens (Human)) | BDBM50612151
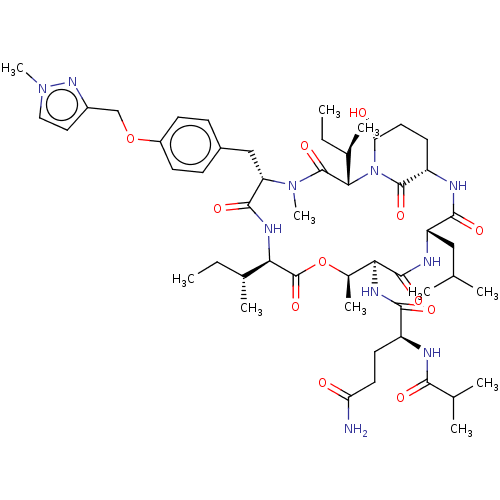
(CHEMBL5287216)Show SMILES [H][C@@]12CC[C@@H](O)N([C@@H]([C@H](C)CC)C(=O)N(C)[C@@H](Cc3ccc(OCc4ccn(C)n4)cc3)C(=O)N[C@H]([C@H](C)CC)C(=O)O[C@H](C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N1)C2=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055513
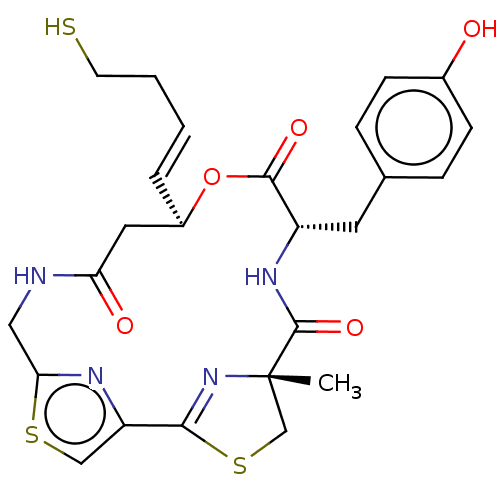
(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055514
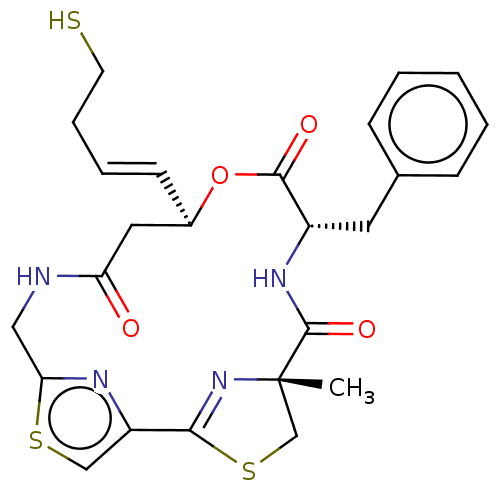
(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50002930
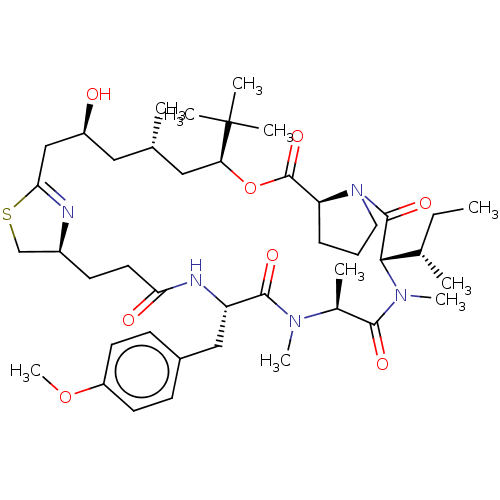
(CHEMBL3234200)Show SMILES [H][C@@]12CSC(C[C@@H](O)C[C@H](C)C[C@H](OC(=O)[C@]3([H])CCCN3C(=O)[C@]([H])([C@@H](C)CC)N(C)C(=O)[C@H](C)N(C)C(=O)[C@H](Cc3ccc(OC)cc3)NC(=O)CC1)C(C)(C)C)=N2 |r,c:61| Show InChI InChI=1S/C43H67N5O8S/c1-11-27(3)38-41(53)48-20-12-13-34(48)42(54)56-35(43(5,6)7)22-26(2)21-31(49)24-37-44-30(25-57-37)16-19-36(50)45-33(23-29-14-17-32(55-10)18-15-29)40(52)46(8)28(4)39(51)47(38)9/h14-15,17-18,26-28,30-31,33-35,38,49H,11-13,16,19-25H2,1-10H3,(H,45,50)/t26-,27-,28-,30-,31-,33-,34-,35-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-A production in human HCT116 cells after 12 hrs by alphaLISA assay |
J Med Chem 57: 3011-29 (2014)
Article DOI: 10.1021/jm4019965
BindingDB Entry DOI: 10.7270/Q2154JKD |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50101331

(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50101331

(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50002918
(Apratoxin S4)Show SMILES [H][C@]12CSC(=N1)[C@@H](C)[C@@H](O)C[C@H](C)C[C@H](OC(=O)[C@]1([H])CCCN1C(=O)[C@]([H])([C@@H](C)CC)N(C)C(=O)[C@H](C)N(C)C(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC2)C(C)(C)C |r,c:4| Show InChI InChI=1S/C44H69N5O8S/c1-12-27(3)38-42(54)49-21-13-14-34(49)43(55)57-36(44(6,7)8)23-26(2)22-35(50)28(4)39-45-31(25-58-39)17-20-37(51)46-33(24-30-15-18-32(56-11)19-16-30)41(53)47(9)29(5)40(52)48(38)10/h15-16,18-19,26-29,31,33-36,38,50H,12-14,17,20-25H2,1-11H3,(H,46,51)/t26-,27-,28-,29-,31-,33-,34-,35-,36-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-A production in human HCT116 cells after 12 hrs by alphaLISA assay |
J Med Chem 57: 3011-29 (2014)
Article DOI: 10.1021/jm4019965
BindingDB Entry DOI: 10.7270/Q2154JKD |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins followe... |
Bioorg Med Chem 24: 3276-82 (2016)
Article DOI: 10.1016/j.bmc.2016.04.062
BindingDB Entry DOI: 10.7270/Q2QZ2CWR |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302109

(CHEMBL568553 | Grassystatin B)Show SMILES CC[C@H](NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C59H97N9O16/c1-17-38(55(77)67(15)43(28-37-22-19-18-20-23-37)56(78)68-25-21-24-42(68)57(79)82-16)61-53(75)47(36(12)69)65-46(72)30-44(70)39(26-31(2)3)62-52(74)41(29-45(60)71)63-51(73)40(27-32(4)5)64-54(76)49(34(8)9)83-59(81)50(35(10)11)84-58(80)48(33(6)7)66(13)14/h18-20,22-23,31-36,38-44,47-50,69-70H,17,21,24-30H2,1-16H3,(H2,60,71)(H,61,75)(H,62,74)(H,63,73)(H,64,76)(H,65,72)/t36-,38+,39+,40+,41+,42+,43-,44+,47+,48+,49-,50+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.354 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055513

(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302109

(CHEMBL568553 | Grassystatin B)Show SMILES CC[C@H](NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C59H97N9O16/c1-17-38(55(77)67(15)43(28-37-22-19-18-20-23-37)56(78)68-25-21-24-42(68)57(79)82-16)61-53(75)47(36(12)69)65-46(72)30-44(70)39(26-31(2)3)62-52(74)41(29-45(60)71)63-51(73)40(27-32(4)5)64-54(76)49(34(8)9)83-59(81)50(35(10)11)84-58(80)48(33(6)7)66(13)14/h18-20,22-23,31-36,38-44,47-50,69-70H,17,21,24-30H2,1-16H3,(H2,60,71)(H,61,75)(H,62,74)(H,63,73)(H,64,76)(H,65,72)/t36-,38+,39+,40+,41+,42+,43-,44+,47+,48+,49-,50+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50098414

(CHEMBL3593247)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:11| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length C-terminal His/FLAG-tagged human HDAC1 expressed in baculovirus infected Sf9 insect cells using BPS HDAC substr... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50101330

(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.446 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor A
(Homo sapiens (Human)) | BDBM50002932
(CHEMBL3234201)Show SMILES [H][C@]12CSC(=N1)C(C)(C)[C@@H](O)C[C@H](C)C[C@H](OC(=O)[C@]1([H])CCCN1C(=O)[C@]([H])([C@@H](C)CC)N(C)C(=O)[C@H](C)N(C)C(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC2)C(C)(C)C |r,c:4| Show InChI InChI=1S/C45H71N5O8S/c1-13-28(3)38-41(55)50-22-14-15-34(50)42(56)58-36(44(5,6)7)24-27(2)23-35(51)45(8,9)43-46-31(26-59-43)18-21-37(52)47-33(25-30-16-19-32(57-12)20-17-30)40(54)48(10)29(4)39(53)49(38)11/h16-17,19-20,27-29,31,33-36,38,51H,13-15,18,21-26H2,1-12H3,(H,47,52)/t27-,28-,29-,31-,33-,34-,35-,36-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-A production in human HCT116 cells after 12 hrs by alphaLISA assay |
J Med Chem 57: 3011-29 (2014)
Article DOI: 10.1021/jm4019965
BindingDB Entry DOI: 10.7270/Q2154JKD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50101331

(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50101330

(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50283681
(CHEMBL4163078)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)OC(=O)[C@H](Cc1ccccc1)N(C)C)C(=O)N[C@@H](C)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C59H91N9O13/c1-14-37(6)51(54(74)61-38(7)55(75)67(12)46(32-40-22-17-15-18-23-40)57(77)68-29-21-26-45(68)58(78)80-13)64-50(71)34-48(69)42(30-35(2)3)62-53(73)44(27-28-49(60)70)66(11)56(76)43(31-36(4)5)63-52(72)39(8)81-59(79)47(65(9)10)33-41-24-19-16-20-25-41/h15-20,22-25,35-39,42-48,51,69H,14,21,26-34H2,1-13H3,(H2,60,70)(H,61,74)(H,62,73)(H,63,72)(H,64,71)/t37-,38-,39-,42-,43-,44-,45-,46+,47-,48-,51-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50098414

(CHEMBL3593247)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:11| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17+,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC10 (1 to 481 residues) expressed in baculovirus infected Sf9 insect cells using B... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50101330

(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055515

(CHEMBL3317818)Show SMILES CC(C)(C)OC(=O)n1cnc(C[C@@H]2NC(=O)[C@]3(C)CSC(=N3)c3csc(CNC(=O)C[C@H](OC2=O)\C=C\CCS)n3)c1 |r,c:20| Show InChI InChI=1S/C27H34N6O6S3/c1-26(2,3)39-25(37)33-12-16(29-15-33)9-18-23(35)38-17(7-5-6-8-40)10-20(34)28-11-21-30-19(13-41-21)22-32-27(4,14-42-22)24(36)31-18/h5,7,12-13,15,17-18,40H,6,8-11,14H2,1-4H3,(H,28,34)(H,31,36)/b7-5+/t17-,18+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50055514

(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.693 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50098414

(CHEMBL3593247)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:11| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of full length human C-terminal His-tagged HDAC3/N-terminal GST-tagged NCOR2 (395 to 489 residues) expressed in baculovirus infected Sf9 i... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50400214

(CHEMBL2181022)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C55H76N8O12/c1-34(2)29-41(49(67)57-36(5)51(69)62(7)44(31-38-21-14-10-15-22-38)52(70)63-28-18-25-43(63)54(72)74-8)58-47(66)32-45(64)40(30-37-19-12-9-13-20-37)59-50(68)42(26-27-46(56)65)61(6)53(71)48(35(3)4)60-55(73)75-33-39-23-16-11-17-24-39/h9-17,19-24,34-36,40-45,48,64H,18,25-33H2,1-8H3,(H2,56,65)(H,57,67)(H,58,66)(H,59,68)(H,60,73)/t36-,40-,41-,42-,43-,44+,45-,48-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50612149

(CHEMBL5284383)Show SMILES [H][C@@]12CC[C@@H](O)N([C@@H](Cc3ccccc3)C(=O)N(C)[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](C(C)C)C(=O)O[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)C\C(C)=C\Cl)[C@@H](C)CC)C(=O)N\C(=C/C)C(=O)N1)C2=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302107
(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302107

(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E using MCA-KPILFFRLK(Dnp)-D-R-NH2 as substrate incubated for 10 to 15 mins prior to substrate addition measured every 5 min ... |
Bioorg Med Chem 20: 4774-80 (2012)
Article DOI: 10.1016/j.bmc.2012.05.077
BindingDB Entry DOI: 10.7270/Q2JH3N8B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data