

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM22113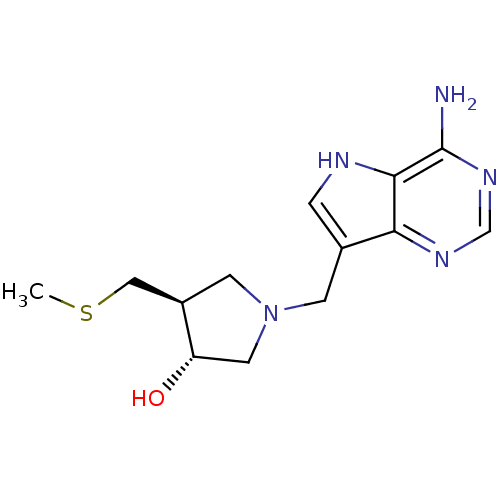 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate assessed as inhibition... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate by xanthine oxidase co... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151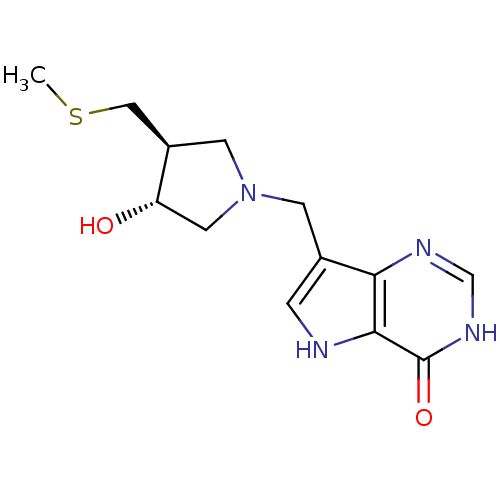 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex ... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-in... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM50116357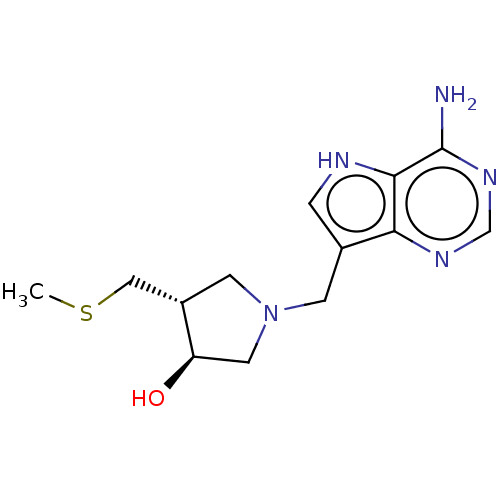 (CHEMBL3604360) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate by xanthine oxidase co... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50116358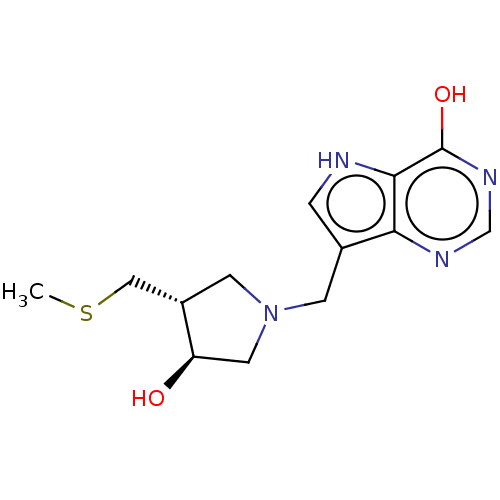 (CHEMBL3604359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex ... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50116358 (CHEMBL3604359) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-in... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50116358 (CHEMBL3604359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50116358 (CHEMBL3604359) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50116357 (CHEMBL3604360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50281297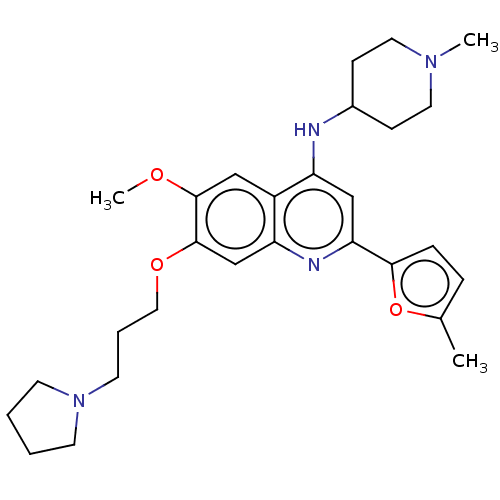 (CHEMBL4170114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of G9a (unknown origin) using biotinylated histone monomethyl-H3K9 peptide by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01609 BindingDB Entry DOI: 10.7270/Q2GH9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50281297 (CHEMBL4170114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 382 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DNMT1 (unknown origin) using biotinylated histone monomethyl-H3K9 peptide by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01609 BindingDB Entry DOI: 10.7270/Q2GH9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50411202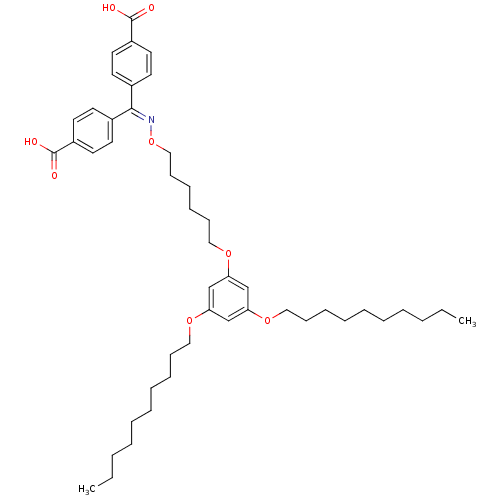 (CHEMBL216129) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University Curated by ChEMBL | Assay Description Inhibition of MM6 cell adhesion to recombinant E-selectin by flow chamber assay | J Med Chem 49: 5988-99 (2006) Article DOI: 10.1021/jm060468y BindingDB Entry DOI: 10.7270/Q21Z45NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50411201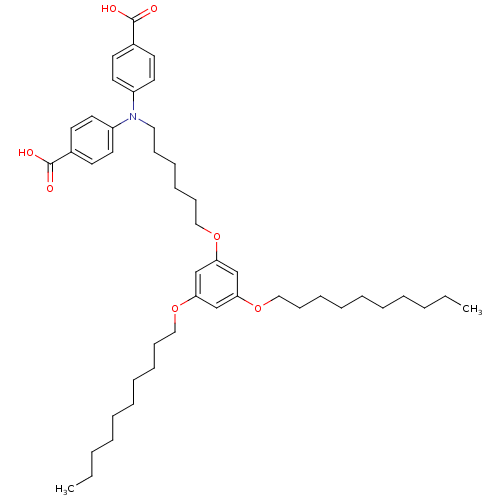 (CHEMBL217490) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University Curated by ChEMBL | Assay Description Inhibition of MM6 cell adhesion to recombinant E-selectin by flow chamber assay | J Med Chem 49: 5988-99 (2006) Article DOI: 10.1021/jm060468y BindingDB Entry DOI: 10.7270/Q21Z45NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50411200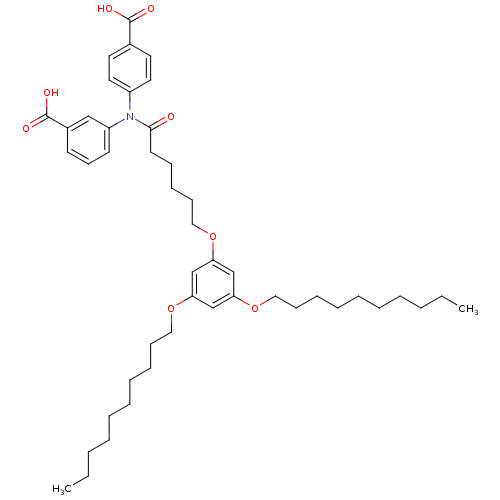 (CHEMBL385486) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University Curated by ChEMBL | Assay Description Inhibition of MM6 cell adhesion to recombinant E-selectin by flow chamber assay | J Med Chem 49: 5988-99 (2006) Article DOI: 10.1021/jm060468y BindingDB Entry DOI: 10.7270/Q21Z45NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||