 Found 6494 hits with Last Name = 'ma' and Initial = 'x'
Found 6494 hits with Last Name = 'ma' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198754

(CHEMBL3924888)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C33H48N8O5/c1-20-16-23(42)17-21(2)24(20)19-28-32(46)39-26(13-7-9-15-37-33(35)36)29(43)40-27(18-22-10-4-3-5-11-22)31(45)38-25(30(44)41-28)12-6-8-14-34/h3-5,10-11,16-17,25-28,42H,6-9,12-15,18-19,34H2,1-2H3,(H,38,45)(H,39,46)(H,40,43)(H,41,44)(H4,35,36,37)/t25-,26+,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198760
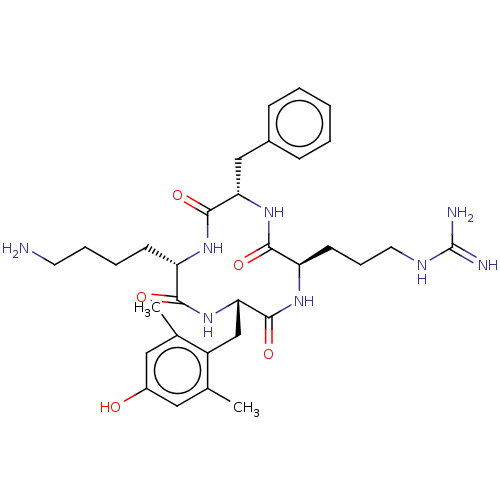
(CHEMBL3897031)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C32H46N8O5/c1-19-15-22(41)16-20(2)23(19)18-27-31(45)38-25(12-8-14-36-32(34)35)29(43)39-26(17-21-9-4-3-5-10-21)30(44)37-24(28(42)40-27)11-6-7-13-33/h3-5,9-10,15-16,24-27,41H,6-8,11-14,17-18,33H2,1-2H3,(H,37,44)(H,38,45)(H,39,43)(H,40,42)(H4,34,35,36)/t24-,25+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198758

(CHEMBL3908315)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)C(CCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C31H44N8O5/c1-18-14-21(40)15-19(2)22(18)17-26-30(44)37-24(11-7-13-35-31(33)34)28(42)38-25(16-20-8-4-3-5-9-20)29(43)36-23(10-6-12-32)27(41)39-26/h3-5,8-9,14-15,23-26,40H,6-7,10-13,16-17,32H2,1-2H3,(H,36,43)(H,37,44)(H,38,42)(H,39,41)(H4,33,34,35)/t23?,24-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50463772
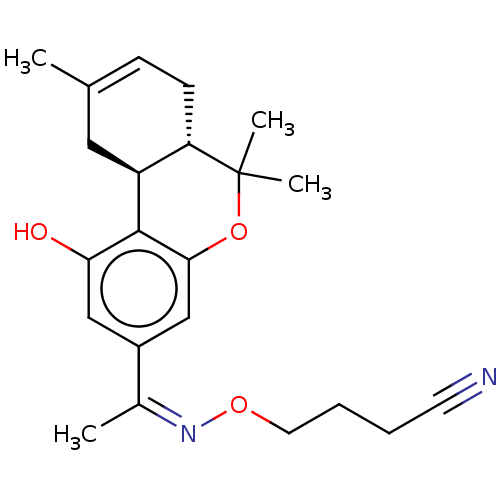
(CHEMBL4239190)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N/OCCCC#N |r,c:4| Show InChI InChI=1S/C22H28N2O3/c1-14-7-8-18-17(11-14)21-19(25)12-16(13-20(21)27-22(18,3)4)15(2)24-26-10-6-5-9-23/h7,12-13,17-18,25H,5-6,8,10-11H2,1-4H3/b24-15-/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant mouse Cb2 receptor expressed in HEK293 cells |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198755
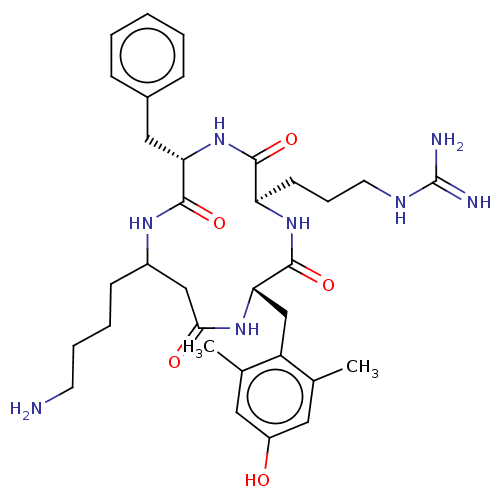
(CHEMBL3979449)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)CC(CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C33H48N8O5/c1-20-15-24(42)16-21(2)25(20)19-28-32(46)40-26(12-8-14-37-33(35)36)30(44)41-27(17-22-9-4-3-5-10-22)31(45)38-23(11-6-7-13-34)18-29(43)39-28/h3-5,9-10,15-16,23,26-28,42H,6-8,11-14,17-19,34H2,1-2H3,(H,38,45)(H,39,43)(H,40,46)(H,41,44)(H4,35,36,37)/t23?,26-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198757

(CHEMBL363142)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24?,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50328671
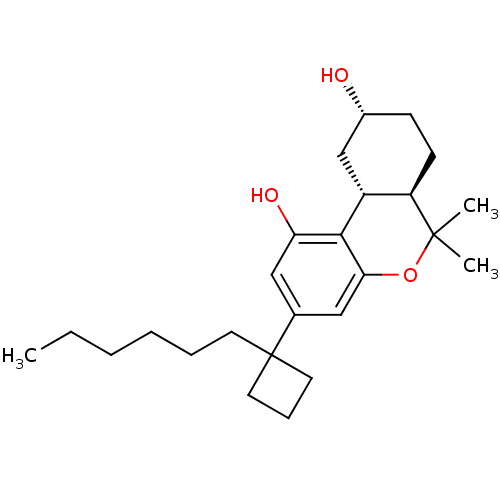
((6aR,9R,10aR)-3-(1-hexylcyclobutyl)-6,6-dimethyl-6...)Show SMILES CCCCCCC1(CCC1)c1cc(O)c2[C@@H]3C[C@H](O)CC[C@H]3C(C)(C)Oc2c1 |r| Show InChI InChI=1S/C25H38O3/c1-4-5-6-7-11-25(12-8-13-25)17-14-21(27)23-19-16-18(26)9-10-20(19)24(2,3)28-22(23)15-17/h14-15,18-20,26-27H,4-13,16H2,1-3H3/t18-,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22911

(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 420-6 (2001)
Article DOI: 10.1124/mol.59.3.420
BindingDB Entry DOI: 10.7270/Q26Q1VSR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50463772

(CHEMBL4239190)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N/OCCCC#N |r,c:4| Show InChI InChI=1S/C22H28N2O3/c1-14-7-8-18-17(11-14)21-19(25)12-16(13-20(21)27-22(18,3)4)15(2)24-26-10-6-5-9-23/h7,12-13,17-18,25H,5-6,8,10-11H2,1-4H3/b24-15-/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from rat brain Cb1 receptor |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50463775
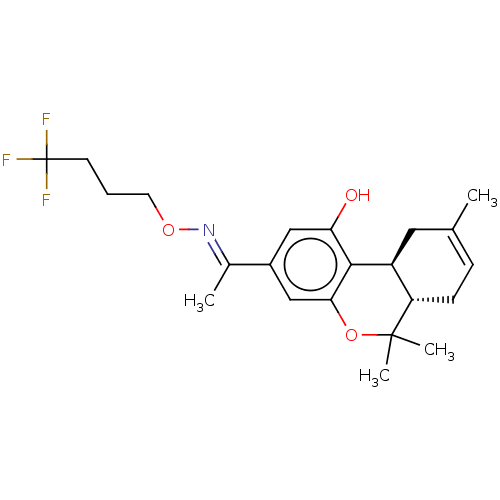
(CHEMBL4240671)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N\OCCCC(F)(F)F |r,c:4| Show InChI InChI=1S/C22H28F3NO3/c1-13-6-7-17-16(10-13)20-18(27)11-15(12-19(20)29-21(17,3)4)14(2)26-28-9-5-8-22(23,24)25/h6,11-12,16-17,27H,5,7-10H2,1-4H3/b26-14+/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from rat brain Cb1 receptor |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256911

(CHEMBL4095621)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C24H30ClN7O5/c1-13(30-23(37)19(32-24(27)28)11-15-4-8-17(33)9-5-15)22(36)29-12-20(34)31-18(21(26)35)10-14-2-6-16(25)7-3-14/h2-9,13,18-19,33H,10-12H2,1H3,(H2,26,35)(H,29,36)(H,30,37)(H,31,34)(H4,27,28,32)/t13-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603275
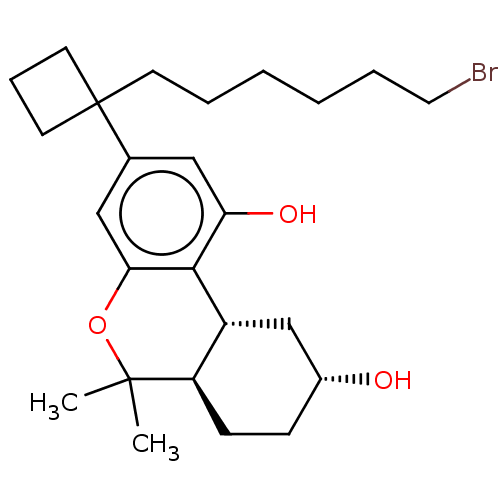
(CHEMBL5187249)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCCBr)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50463772

(CHEMBL4239190)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N/OCCCC#N |r,c:4| Show InChI InChI=1S/C22H28N2O3/c1-14-7-8-18-17(11-14)21-19(25)12-16(13-20(21)27-22(18,3)4)15(2)24-26-10-6-5-9-23/h7,12-13,17-18,25H,5-6,8,10-11H2,1-4H3/b24-15-/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human Cb2 receptor expressed in HEK293 cells |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 420-6 (2001)
Article DOI: 10.1124/mol.59.3.420
BindingDB Entry DOI: 10.7270/Q26Q1VSR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256914
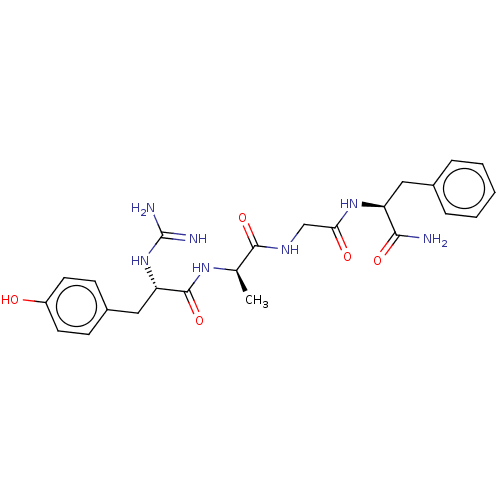
(CHEMBL4061665)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C24H31N7O5/c1-14(29-23(36)19(31-24(26)27)12-16-7-9-17(32)10-8-16)22(35)28-13-20(33)30-18(21(25)34)11-15-5-3-2-4-6-15/h2-10,14,18-19,32H,11-13H2,1H3,(H2,25,34)(H,28,35)(H,29,36)(H,30,33)(H4,26,27,31)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603264
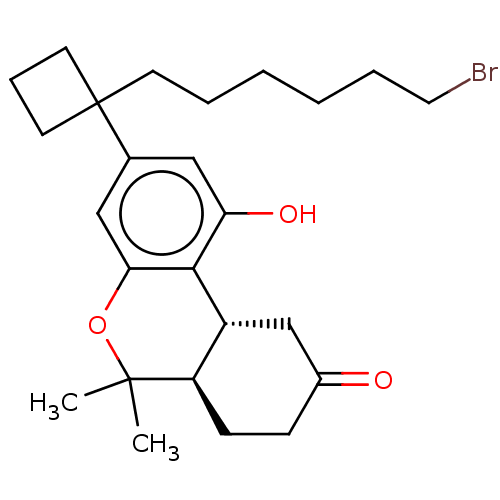
(CHEMBL5206676)Show SMILES [H][C@@]12CC(=O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCCBr)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50463785
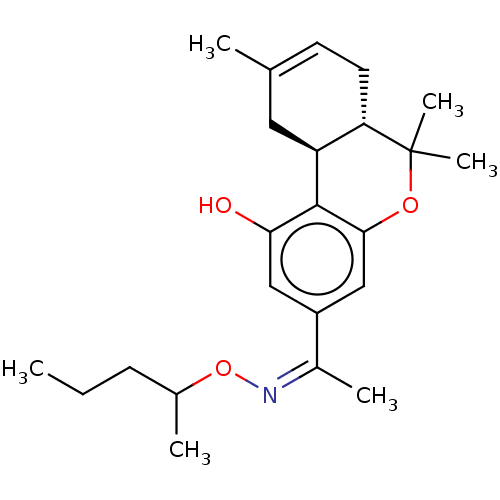
(CHEMBL4238778)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N/OC(C)CCC |r,c:4| Show InChI InChI=1S/C23H33NO3/c1-7-8-15(3)27-24-16(4)17-12-20(25)22-18-11-14(2)9-10-19(18)23(5,6)26-21(22)13-17/h9,12-13,15,18-19,25H,7-8,10-11H2,1-6H3/b24-16-/t15?,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from rat brain Cb1 receptor |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50463775

(CHEMBL4240671)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N\OCCCC(F)(F)F |r,c:4| Show InChI InChI=1S/C22H28F3NO3/c1-13-6-7-17-16(10-13)20-18(27)11-15(12-19(20)29-21(17,3)4)14(2)26-28-9-5-8-22(23,24)25/h6,11-12,16-17,27H,5,7-10H2,1-4H3/b26-14+/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant mouse Cb2 receptor expressed in HEK293 cells |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22530
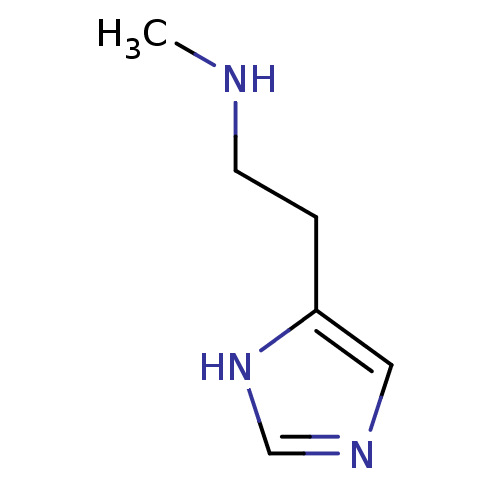
(N(alpha)-Methylhistamine | N-alpha-methylhistamine...)Show InChI InChI=1S/C6H11N3/c1-7-3-2-6-4-8-5-9-6/h4-5,7H,2-3H2,1H3,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 420-6 (2001)
Article DOI: 10.1124/mol.59.3.420
BindingDB Entry DOI: 10.7270/Q26Q1VSR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603277
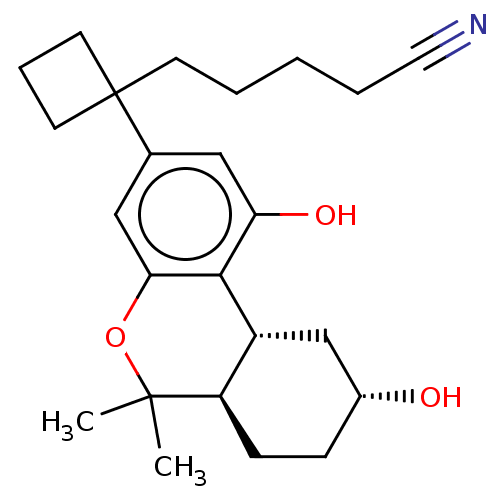
(CHEMBL5194920)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCC#N)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603266
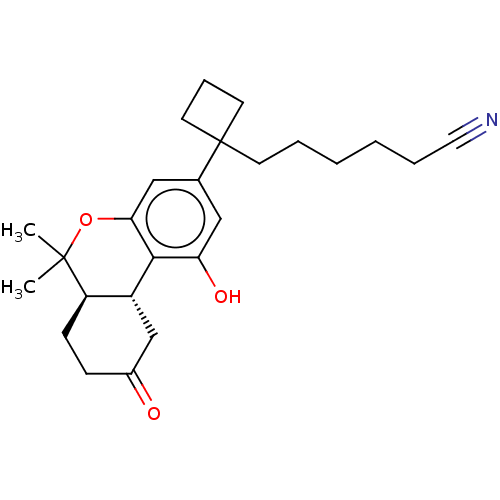
(CHEMBL5203712)Show SMILES [H][C@@]12CC(=O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCC#N)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603279
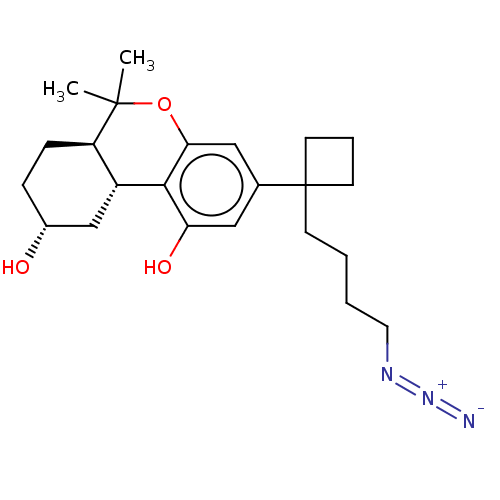
(CHEMBL5203974)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCN=[N+]=[N-])CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 420-6 (2001)
Article DOI: 10.1124/mol.59.3.420
BindingDB Entry DOI: 10.7270/Q26Q1VSR |
More data for this
Ligand-Target Pair | |
Aurora kinase A-interacting protein
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603286
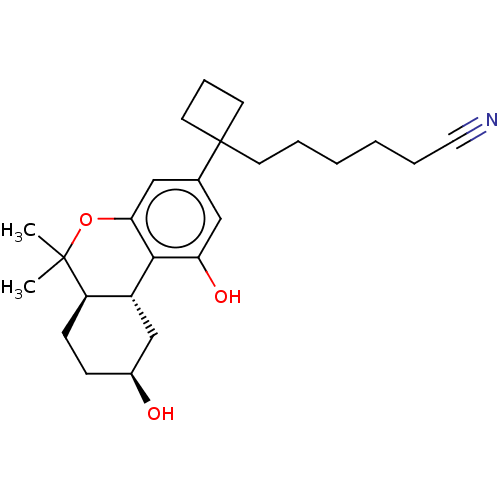
(CHEMBL5183405)Show SMILES [H][C@@]12C[C@@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCC#N)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603268
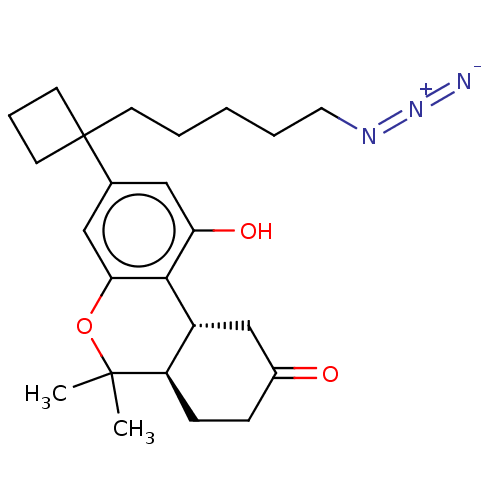
(CHEMBL5206273)Show SMILES [H][C@@]12CC(=O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCN=[N+]=[N-])CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603278
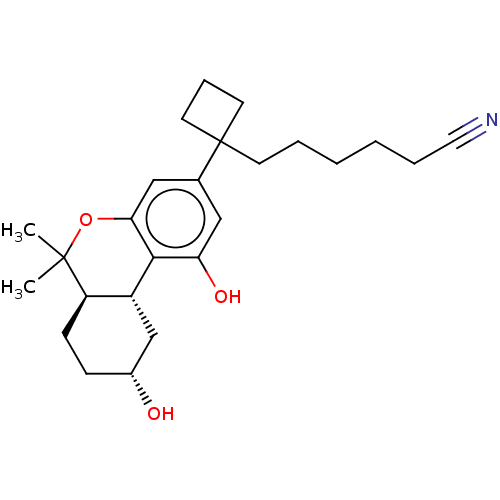
(CHEMBL5176554)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCC#N)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50463776
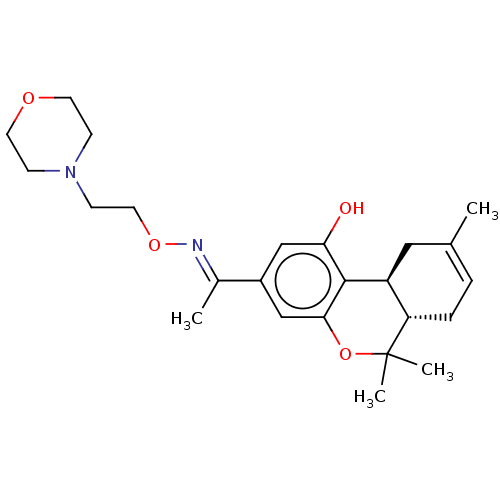
(CHEMBL4246445)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N\OCCN1CCOCC1 |r,c:4| Show InChI InChI=1S/C24H34N2O4/c1-16-5-6-20-19(13-16)23-21(27)14-18(15-22(23)30-24(20,3)4)17(2)25-29-12-9-26-7-10-28-11-8-26/h5,14-15,19-20,27H,6-13H2,1-4H3/b25-17+/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human Cb2 receptor expressed in HEK293 cells |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 420-6 (2001)
Article DOI: 10.1124/mol.59.3.420
BindingDB Entry DOI: 10.7270/Q26Q1VSR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50198754

(CHEMBL3924888)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C33H48N8O5/c1-20-16-23(42)17-21(2)24(20)19-28-32(46)39-26(13-7-9-15-37-33(35)36)29(43)40-27(18-22-10-4-3-5-11-22)31(45)38-25(30(44)41-28)12-6-8-14-34/h3-5,10-11,16-17,25-28,42H,6-9,12-15,18-19,34H2,1-2H3,(H,38,45)(H,39,46)(H,40,43)(H,41,44)(H4,35,36,37)/t25-,26+,27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50287936
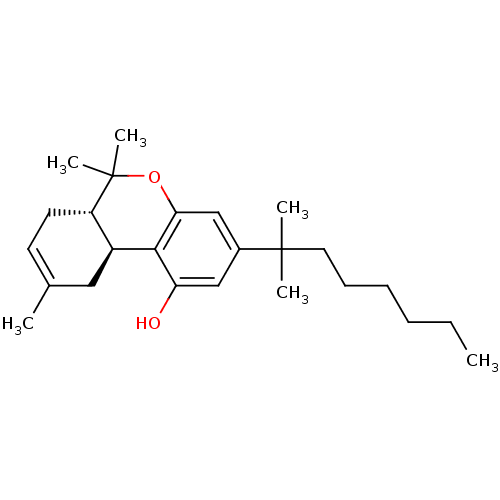
((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-6,6,9-trimethyl...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@H]3CC(C)=CC[C@@H]3C(C)(C)Oc2c1 |c:17| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h11,15-16,19-20,26H,7-10,12-14H2,1-6H3/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from rat brain Cb1 receptor |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50198755

(CHEMBL3979449)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)CC(CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C33H48N8O5/c1-20-15-24(42)16-21(2)25(20)19-28-32(46)40-26(12-8-14-37-33(35)36)30(44)41-27(17-22-9-4-3-5-10-22)31(45)38-23(11-6-7-13-34)18-29(43)39-28/h3-5,9-10,15-16,23,26-28,42H,6-8,11-14,17-19,34H2,1-2H3,(H,38,45)(H,39,43)(H,40,46)(H,41,44)(H4,35,36,37)/t23?,26-,27+,28+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.786 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198754

(CHEMBL3924888)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C33H48N8O5/c1-20-16-23(42)17-21(2)24(20)19-28-32(46)39-26(13-7-9-15-37-33(35)36)29(43)40-27(18-22-10-4-3-5-11-22)31(45)38-25(30(44)41-28)12-6-8-14-34/h3-5,10-11,16-17,25-28,42H,6-9,12-15,18-19,34H2,1-2H3,(H,38,45)(H,39,46)(H,40,43)(H,41,44)(H4,35,36,37)/t25-,26+,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.807 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DSLET from DOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50328664
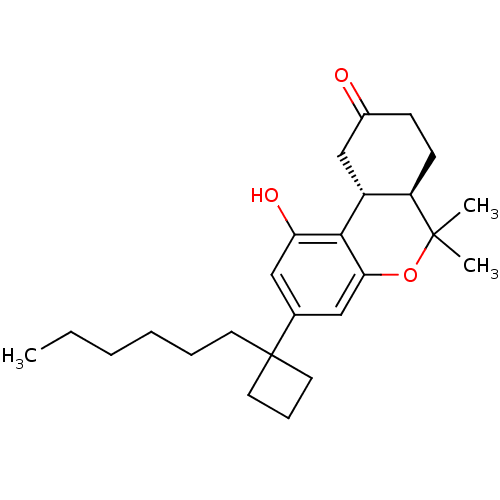
((6aR,10aR)-3-(1-Hexyl-cyclobut-1-yl)-6,6a,7,8,10,1...)Show SMILES CCCCCCC1(CCC1)c1cc(O)c2[C@@H]3CC(=O)CC[C@H]3C(C)(C)Oc2c1 |r| Show InChI InChI=1S/C25H36O3/c1-4-5-6-7-11-25(12-8-13-25)17-14-21(27)23-19-16-18(26)9-10-20(19)24(2,3)28-22(23)15-17/h14-15,19-20,27H,4-13,16H2,1-3H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50463786
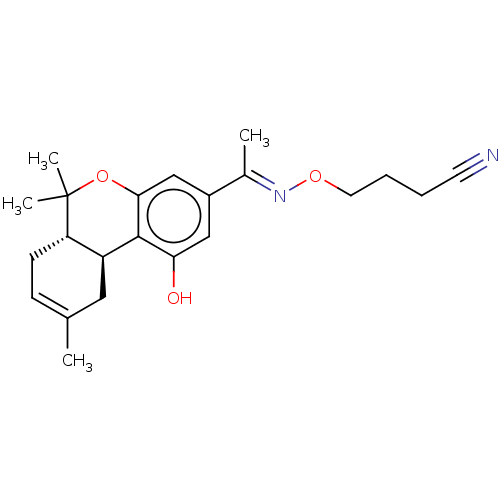
(CHEMBL4249273)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N\OCCCC#N |r,c:4| Show InChI InChI=1S/C22H28N2O3/c1-14-7-8-18-17(11-14)21-19(25)12-16(13-20(21)27-22(18,3)4)15(2)24-26-10-6-5-9-23/h7,12-13,17-18,25H,5-6,8,10-11H2,1-4H3/b24-15+/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from rat brain Cb1 receptor |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50057015
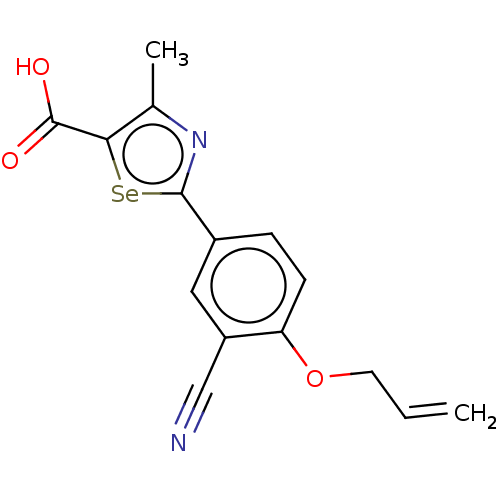
(CHEMBL3331617)Show InChI InChI=1S/C15H12N2O3Se/c1-3-6-20-12-5-4-10(7-11(12)8-16)14-17-9(2)13(21-14)15(18)19/h3-5,7H,1,6H2,2H3,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine xanthine oxidase assessed as inhibition of uric acid formation by Lineweaver-Burk plot |
Eur J Med Chem 85: 508-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.014
BindingDB Entry DOI: 10.7270/Q2HX1F9C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603288

(CHEMBL5178005)Show SMILES [H][C@@]12C[C@@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCN=[N+]=[N-])CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603284
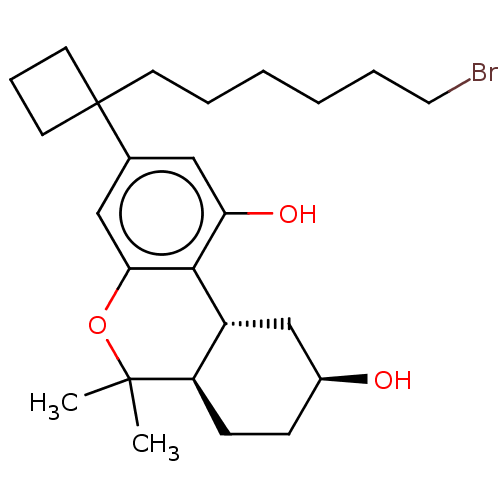
(CHEMBL5178943)Show SMILES [H][C@@]12C[C@@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCCBr)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198752
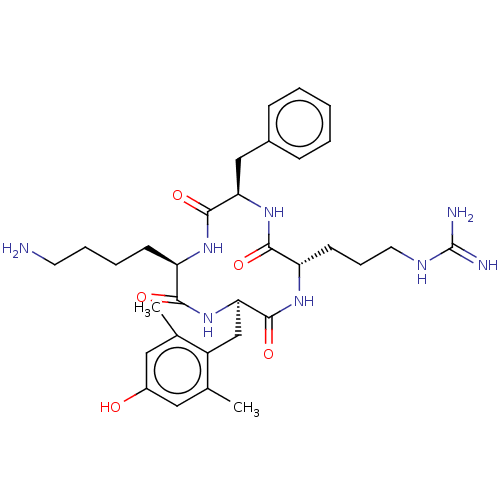
(CHEMBL3976694)Show SMILES Cc1cc(O)cc(C)c1C[C@H]1NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C32H46N8O5/c1-19-15-22(41)16-20(2)23(19)18-27-31(45)38-25(12-8-14-36-32(34)35)29(43)39-26(17-21-9-4-3-5-10-21)30(44)37-24(28(42)40-27)11-6-7-13-33/h3-5,9-10,15-16,24-27,41H,6-8,11-14,17-18,33H2,1-2H3,(H,37,44)(H,38,45)(H,39,43)(H,40,42)(H4,34,35,36)/t24-,25+,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603283
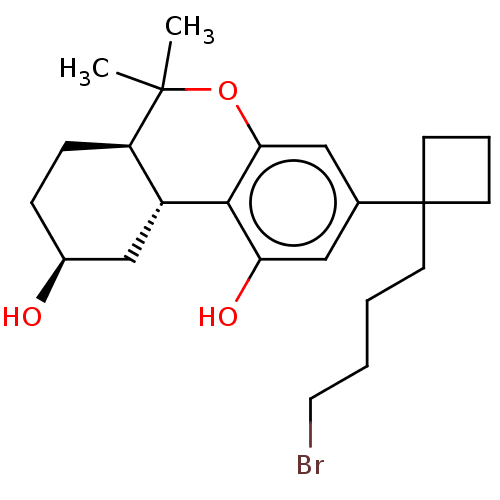
(CHEMBL5191435)Show SMILES [H][C@@]12C[C@@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCBr)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603281
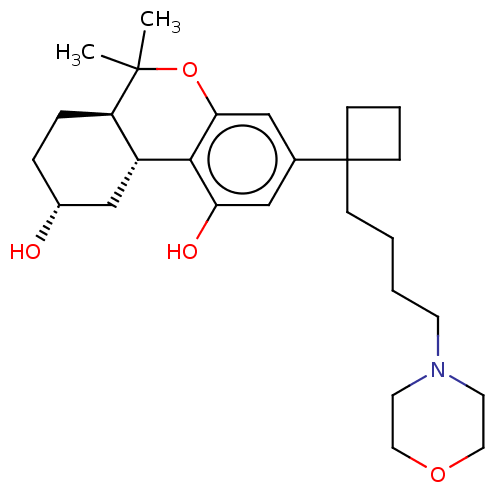
(CHEMBL5187812)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCN2CCOCC2)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50563846

(CHEMBL4787010)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc(CN3CCC4(CC3)C=Cc3ccccc43)c(c2)C(F)(F)F)cc1 |r,c:26| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-TAK-875 from human recombinant full-length GPR40 expressed in human HEK293 cell membrane incubated for 2 hrs by solid scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b00892
BindingDB Entry DOI: 10.7270/Q2GH9NPC |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM108220
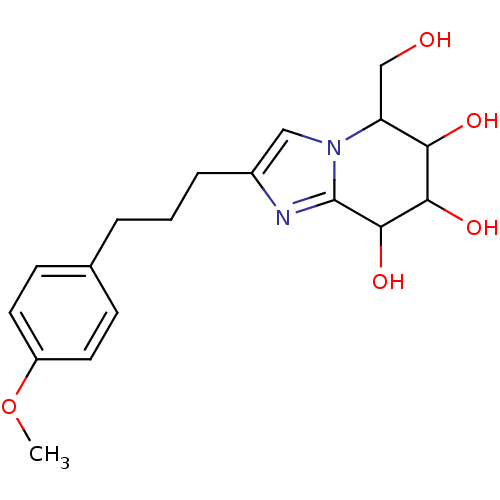
(5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...)Show InChI InChI=1S/C18H24N2O5/c1-25-13-7-5-11(6-8-13)3-2-4-12-9-20-14(10-21)15(22)16(23)17(24)18(20)19-12/h5-9,14-17,21-24H,2-4,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University
| Assay Description
To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... |
Chembiochem 14: 1239-47 (2013)
Article DOI: 10.1002/cbic.201300197
BindingDB Entry DOI: 10.7270/Q2NS0SHP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603274
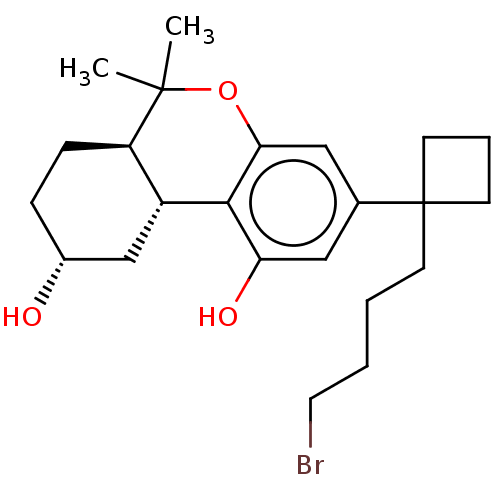
(CHEMBL5203428)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCBr)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50463777
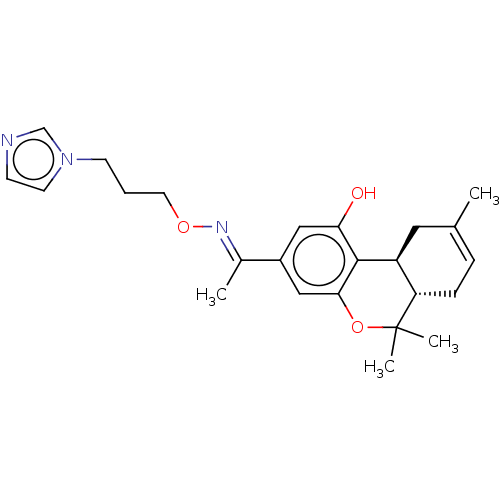
(CHEMBL4238224)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N\OCCCn1ccnc1 |r,c:4| Show InChI InChI=1S/C24H31N3O3/c1-16-6-7-20-19(12-16)23-21(28)13-18(14-22(23)30-24(20,3)4)17(2)26-29-11-5-9-27-10-8-25-15-27/h6,8,10,13-15,19-20,28H,5,7,9,11-12H2,1-4H3/b26-17+/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from rat brain Cb1 receptor |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256915

(CHEMBL4068851)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C23H28ClN5O5/c1-13(28-23(34)18(25)10-14-4-8-17(30)9-5-14)22(33)27-12-20(31)29-19(21(26)32)11-15-2-6-16(24)7-3-15/h2-9,13,18-19,30H,10-12,25H2,1H3,(H2,26,32)(H,27,33)(H,28,34)(H,29,31)/t13-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50563845

(CHEMBL4793371)Show SMILES CC#C[C@@H](CC(O)=O)c1ccc(OCc2ccc(CN3CCC4(CC3)C=Cc3ccccc43)c(C)c2)cc1 |r,c:26| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-TAK-875 from human recombinant full-length GPR40 expressed in human HEK293 cell membrane incubated for 2 hrs by solid scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b00892
BindingDB Entry DOI: 10.7270/Q2GH9NPC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252861
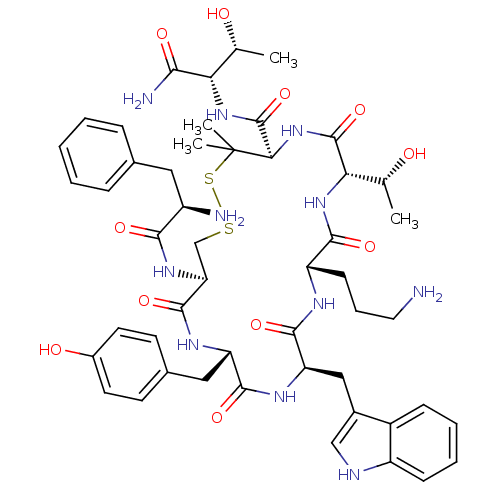
(CHEMBL507214 | H-D-Phe-c[Cys-Tyr-DTrp-Orn-Thr-Pen]...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C50H67N11O11S2/c1-26(62)39(42(53)65)59-49(72)41-50(3,4)74-73-25-38(58-43(66)33(52)21-28-11-6-5-7-12-28)47(70)56-36(22-29-16-18-31(64)19-17-29)45(68)57-37(23-30-24-54-34-14-9-8-13-32(30)34)46(69)55-35(15-10-20-51)44(67)60-40(27(2)63)48(71)61-41/h5-9,11-14,16-19,24,26-27,33,35-41,54,62-64H,10,15,20-23,25,51-52H2,1-4H3,(H2,53,65)(H,55,69)(H,56,70)(H,57,68)(H,58,66)(H,59,72)(H,60,67)(H,61,71)/t26-,27-,33-,35+,36+,37-,38+,39+,40+,41-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50463786

(CHEMBL4249273)Show SMILES [H][C@]12CC(C)=CC[C@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(\C)=N\OCCCC#N |r,c:4| Show InChI InChI=1S/C22H28N2O3/c1-14-7-8-18-17(11-14)21-19(25)12-16(13-20(21)27-22(18,3)4)15(2)24-26-10-6-5-9-23/h7,12-13,17-18,25H,5-6,8,10-11H2,1-4H3/b24-15+/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human Cb2 receptor expressed in HEK293 cells |
Bioorg Med Chem 26: 4963-4970 (2018)
Article DOI: 10.1016/j.bmc.2018.08.003
BindingDB Entry DOI: 10.7270/Q2BG2RNX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50174298
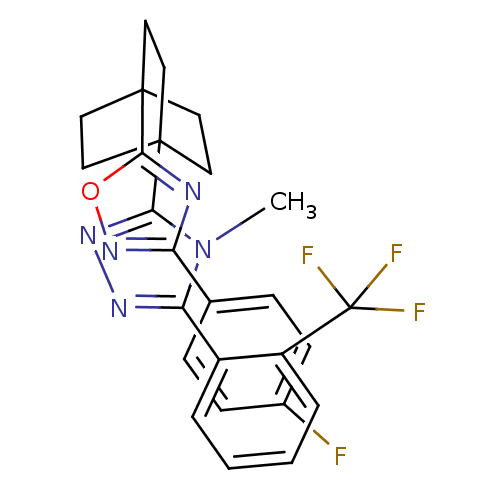
(3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data