 Found 88 hits with Last Name = 'machulskis' and Initial = 'a'
Found 88 hits with Last Name = 'machulskis' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093259
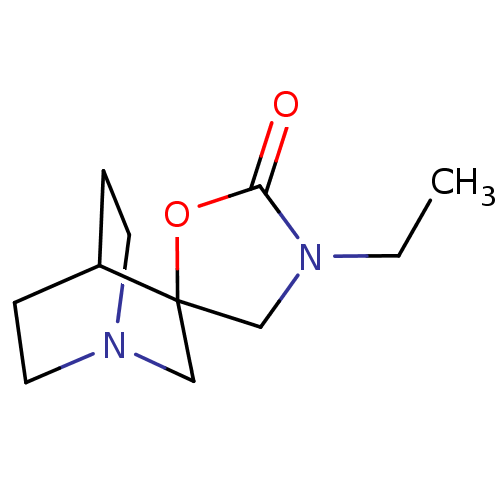
(3'-Ethylspiro[1-azabicyclo[2.2.2]octane-3,5'-oxazo...)Show SMILES CCN1CC2(CN3CCC2CC3)OC1=O |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(7.97,-7.56,;8.38,-6.07,;7.29,-4.98,;5.75,-4.98,;5.26,-3.5,;4.15,-4.09,;3.57,-2.87,;1.98,-3.39,;2.56,-2.35,;4.27,-1.93,;4.63,-.97,;3.57,-1.7,;6.52,-2.59,;7.77,-3.5,;9.25,-3.03,)| Show InChI InChI=1S/C11H18N2O2/c1-2-13-8-11(15-10(13)14)7-12-5-3-9(11)4-6-12/h9H,2-8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093253
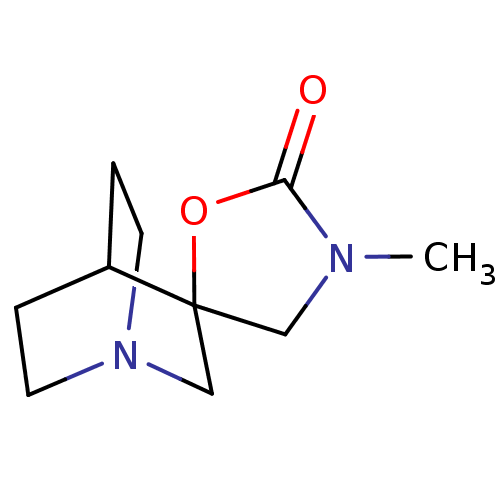
((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093251
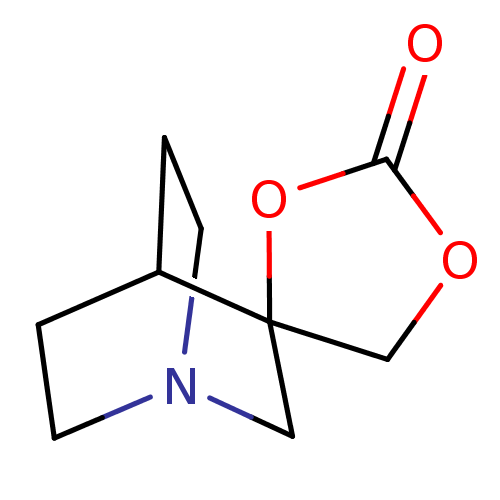
(CHEMBL338698 | Spiro[1-azabicyclo[2.2.2]octane-3,5...)Show SMILES O=C1OCC2(CN3CCC2CC3)O1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(9.83,-3.22,;8.26,-3.72,;7.75,-5.29,;6.11,-5.29,;5.59,-3.72,;4.41,-4.35,;3.8,-3.05,;2.1,-3.6,;2.73,-2.49,;4.54,-2.05,;4.92,-1.03,;3.8,-1.8,;6.93,-2.75,)| Show InChI InChI=1S/C9H13NO3/c11-8-12-6-9(13-8)5-10-3-1-7(9)2-4-10/h7H,1-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093259

(3'-Ethylspiro[1-azabicyclo[2.2.2]octane-3,5'-oxazo...)Show SMILES CCN1CC2(CN3CCC2CC3)OC1=O |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(7.97,-7.56,;8.38,-6.07,;7.29,-4.98,;5.75,-4.98,;5.26,-3.5,;4.15,-4.09,;3.57,-2.87,;1.98,-3.39,;2.56,-2.35,;4.27,-1.93,;4.63,-.97,;3.57,-1.7,;6.52,-2.59,;7.77,-3.5,;9.25,-3.03,)| Show InChI InChI=1S/C11H18N2O2/c1-2-13-8-11(15-10(13)14)7-12-5-3-9(11)4-6-12/h9H,2-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50006245
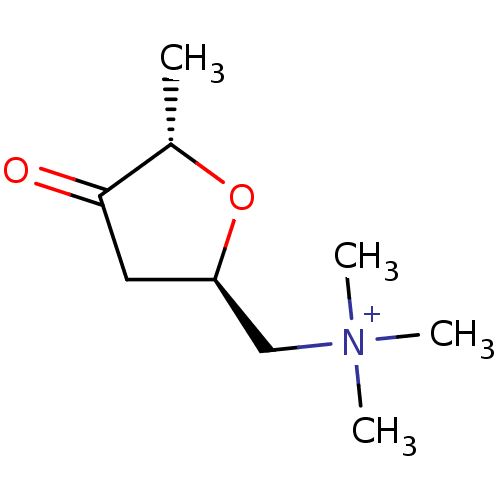
(CHEMBL59587 | Trimethyl-(5-methyl-4-oxo-tetrahydro...)Show InChI InChI=1S/C9H18NO2/c1-7-9(11)5-8(12-7)6-10(2,3)4/h7-8H,5-6H2,1-4H3/q+1/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50470758
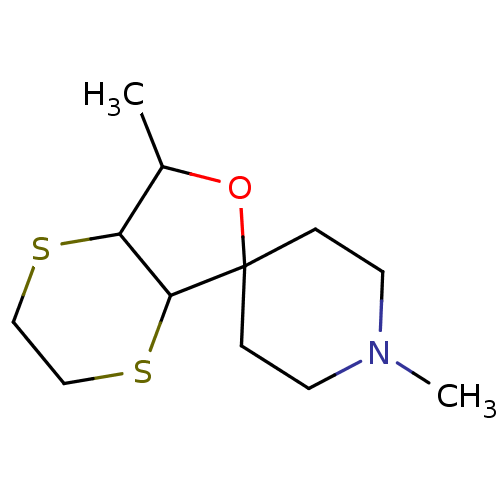
(CHEMBL1794941)Show SMILES OC(=O)C(O)=O.OC(=O)\C=C/C(O)=O.CC1OC2(CCN(C)CC2)C2SCCSC12 Show InChI InChI=1S/C12H21NOS2.C4H4O4.C2H2O4/c1-9-10-11(16-8-7-15-10)12(14-9)3-5-13(2)6-4-12;5-3(6)1-2-4(7)8;3-1(4)2(5)6/h9-11H,3-8H2,1-2H3;1-2H,(H,5,6)(H,7,8);(H,3,4)(H,5,6)/b;2-1-; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50470759
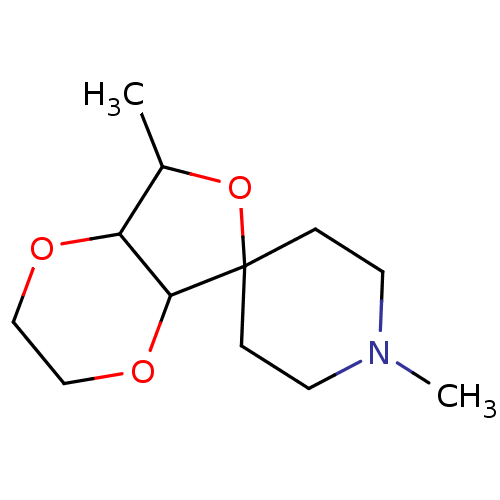
(CHEMBL1794940)Show SMILES OC(=O)C(O)=O.OC(=O)\C=C/C(O)=O.CC1OC2(CCN(C)CC2)C2OCCOC12 Show InChI InChI=1S/C12H21NO3.C4H4O4.C2H2O4/c1-9-10-11(15-8-7-14-10)12(16-9)3-5-13(2)6-4-12;5-3(6)1-2-4(7)8;3-1(4)2(5)6/h9-11H,3-8H2,1-2H3;1-2H,(H,5,6)(H,7,8);(H,3,4)(H,5,6)/b;2-1-; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50470766
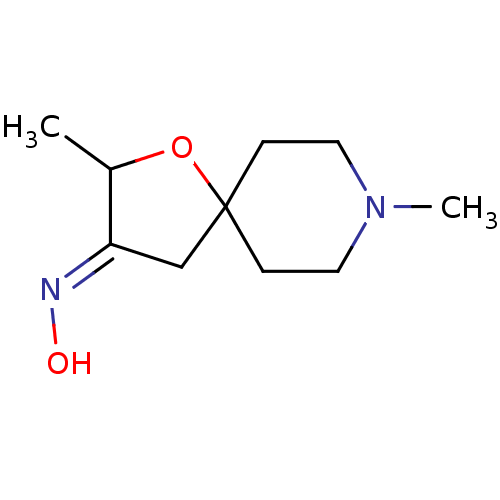
(CHEMBL40032)Show InChI InChI=1S/C10H18N2O2/c1-8-9(11-13)7-10(14-8)3-5-12(2)6-4-10/h8,13H,3-7H2,1-2H3/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093251

(CHEMBL338698 | Spiro[1-azabicyclo[2.2.2]octane-3,5...)Show SMILES O=C1OCC2(CN3CCC2CC3)O1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(9.83,-3.22,;8.26,-3.72,;7.75,-5.29,;6.11,-5.29,;5.59,-3.72,;4.41,-4.35,;3.8,-3.05,;2.1,-3.6,;2.73,-2.49,;4.54,-2.05,;4.92,-1.03,;3.8,-1.8,;6.93,-2.75,)| Show InChI InChI=1S/C9H13NO3/c11-8-12-6-9(13-8)5-10-3-1-7(9)2-4-10/h7H,1-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50284961
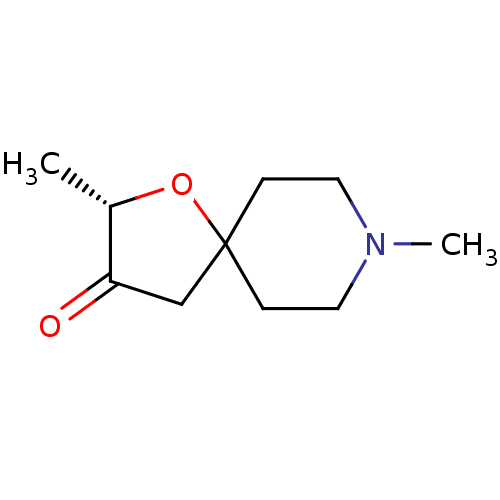
((S)-2,8-Dimethyl-1-oxa-8-aza-spiro[4.5]decan-3-one...)Show InChI InChI=1S/C10H17NO2/c1-8-9(12)7-10(13-8)3-5-11(2)6-4-10/h8H,3-7H2,1-2H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its affinity towards Muscarinic acetylcholine receptor M2, using [3H]- N-methyl-scopolamine, a radioligand displacement as... |
Bioorg Med Chem Lett 5: 1813-1818 (1995)
Article DOI: 10.1016/0960-894X(95)00301-9
BindingDB Entry DOI: 10.7270/Q27M07WJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50470770
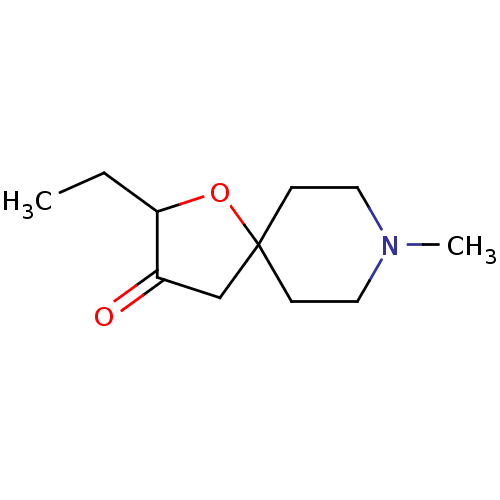
(CHEMBL40063)Show InChI InChI=1S/C11H19NO2/c1-3-10-9(13)8-11(14-10)4-6-12(2)7-5-11/h10H,3-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50034651
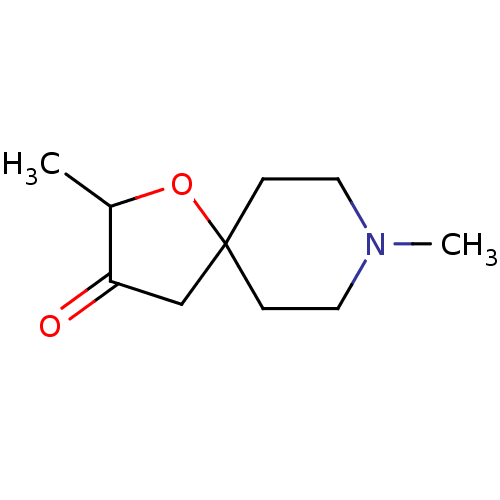
(2,8-Dimethyl-1-oxa-8-aza-spiro[4.5]decan-3-one | C...)Show InChI InChI=1S/C10H17NO2/c1-8-9(12)7-10(13-8)3-5-11(2)6-4-10/h8H,3-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50008072
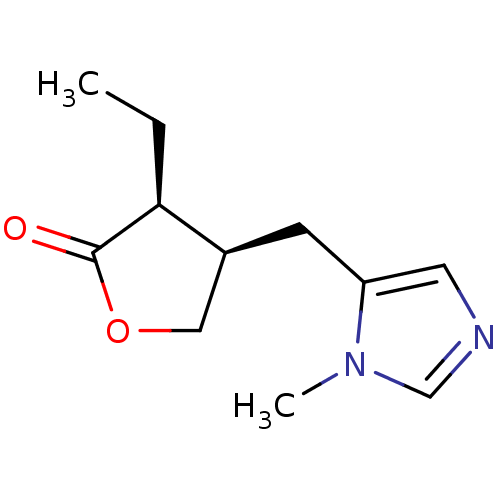
((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...)Show InChI InChI=1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its affinity towards Muscarinic acetylcholine receptor M1, using [3H]- pirenzepine, a radioligand displacement assay in ra... |
Bioorg Med Chem Lett 5: 1813-1818 (1995)
Article DOI: 10.1016/0960-894X(95)00301-9
BindingDB Entry DOI: 10.7270/Q27M07WJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50008072

((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...)Show InChI InChI=1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093260
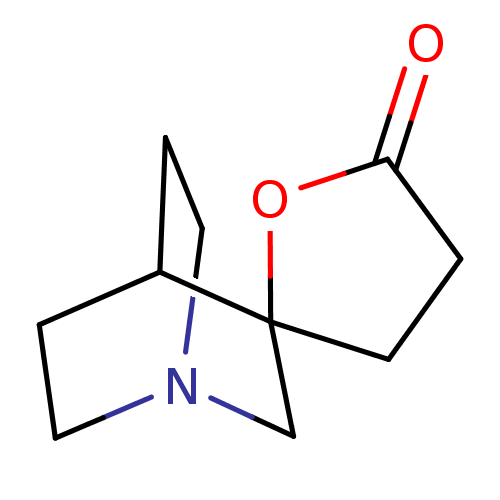
(CHEMBL133295 | Spiro[1-azabicyclo[2.2.2]octane-3,5...)Show SMILES O=C1CCC2(CN3CCC2CC3)O1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(9.2,-3.01,;7.73,-3.48,;7.26,-4.95,;5.72,-4.95,;5.23,-3.48,;4.13,-4.07,;3.55,-2.85,;1.97,-3.37,;2.55,-2.33,;4.25,-1.92,;4.6,-.96,;3.55,-1.69,;6.49,-2.57,)| Show InChI InChI=1S/C10H15NO2/c12-9-1-4-10(13-9)7-11-5-2-8(10)3-6-11/h8H,1-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50034651

(2,8-Dimethyl-1-oxa-8-aza-spiro[4.5]decan-3-one | C...)Show InChI InChI=1S/C10H17NO2/c1-8-9(12)7-10(13-8)3-5-11(2)6-4-10/h8H,3-7H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its affinity towards Muscarinic acetylcholine receptor M1, using [3H]- pirenzepine, a radioligand displacement assay in ra... |
Bioorg Med Chem Lett 5: 1813-1818 (1995)
Article DOI: 10.1016/0960-894X(95)00301-9
BindingDB Entry DOI: 10.7270/Q27M07WJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50034651

(2,8-Dimethyl-1-oxa-8-aza-spiro[4.5]decan-3-one | C...)Show InChI InChI=1S/C10H17NO2/c1-8-9(12)7-10(13-8)3-5-11(2)6-4-10/h8H,3-7H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093257

(CHEMBL131574 | Spiro[1-azabicyclo[2.2.2]octane-3,4...)Show SMILES O=C1CC2(CN1)CN1CCC2CC1 |TLB:4:3:11.12:9.8,THB:2:3:11.12:9.8,(9.19,-3.01,;7.72,-3.48,;6.48,-2.57,;5.23,-3.48,;5.71,-4.95,;7.25,-4.95,;4.13,-4.06,;3.55,-2.85,;1.97,-3.36,;2.55,-2.33,;4.24,-1.92,;4.6,-.96,;3.55,-1.69,)| Show InChI InChI=1S/C10H16N2O/c13-9-5-10(6-11-9)7-12-3-1-8(10)2-4-12/h8H,1-7H2,(H,11,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50284961

((S)-2,8-Dimethyl-1-oxa-8-aza-spiro[4.5]decan-3-one...)Show InChI InChI=1S/C10H17NO2/c1-8-9(12)7-10(13-8)3-5-11(2)6-4-10/h8H,3-7H2,1-2H3/t8-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its affinity towards Muscarinic acetylcholine receptor M1, using [3H]- pirenzepine, a radioligand displacement assay in ra... |
Bioorg Med Chem Lett 5: 1813-1818 (1995)
Article DOI: 10.1016/0960-894X(95)00301-9
BindingDB Entry DOI: 10.7270/Q27M07WJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093256
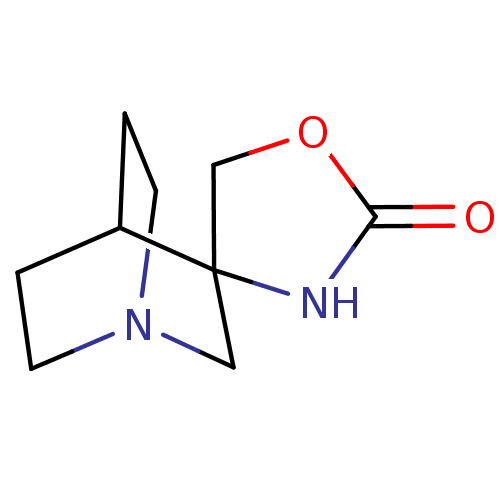
(CHEMBL130205 | Spiro[1-azabicyclo[2.2.2]octane-3,4...)Show SMILES O=C1NC2(CO1)CN1CCC2CC1 |TLB:4:3:11.12:9.8,THB:2:3:11.12:9.8,(24.72,-6.48,;23.15,-7,;21.81,-6.02,;20.48,-7,;20.98,-8.57,;22.63,-8.57,;19.28,-7.62,;18.67,-6.32,;16.97,-6.87,;17.6,-5.76,;19.41,-5.32,;19.8,-4.3,;18.67,-5.07,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-9(6-13-8)5-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288143
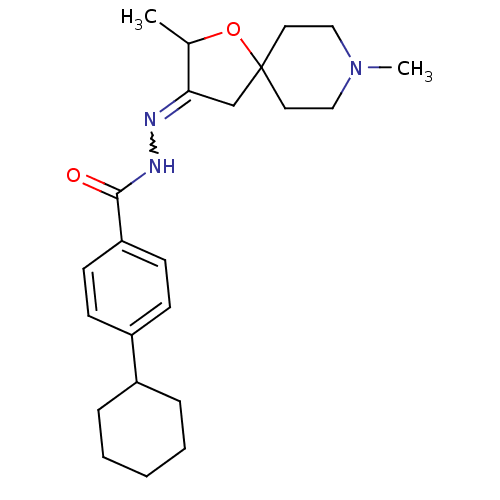
(4-Cyclohexyl-benzoic acid [2,8-dimethyl-1-oxa-8-az...)Show SMILES CC1OC2(CC1=NNC(=O)c1ccc(cc1)C1CCCCC1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C23H33N3O2/c1-17-21(16-23(28-17)12-14-26(2)15-13-23)24-25-22(27)20-10-8-19(9-11-20)18-6-4-3-5-7-18/h8-11,17-18H,3-7,12-16H2,1-2H3,(H,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50034651

(2,8-Dimethyl-1-oxa-8-aza-spiro[4.5]decan-3-one | C...)Show InChI InChI=1S/C10H17NO2/c1-8-9(12)7-10(13-8)3-5-11(2)6-4-10/h8H,3-7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its affinity towards Muscarinic acetylcholine receptor M2, using [3H]- N-methyl-scopolamine, a radioligand displacement as... |
Bioorg Med Chem Lett 5: 1813-1818 (1995)
Article DOI: 10.1016/0960-894X(95)00301-9
BindingDB Entry DOI: 10.7270/Q27M07WJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM46858
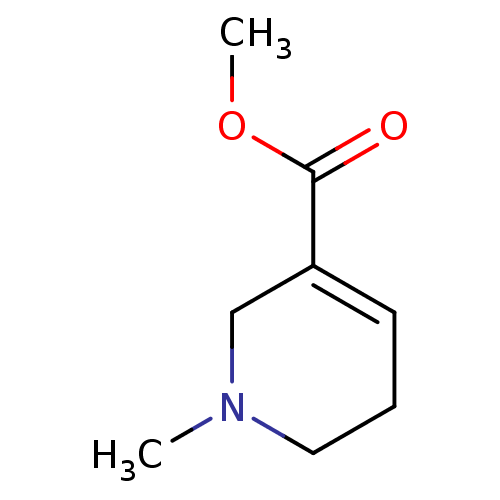
(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288159
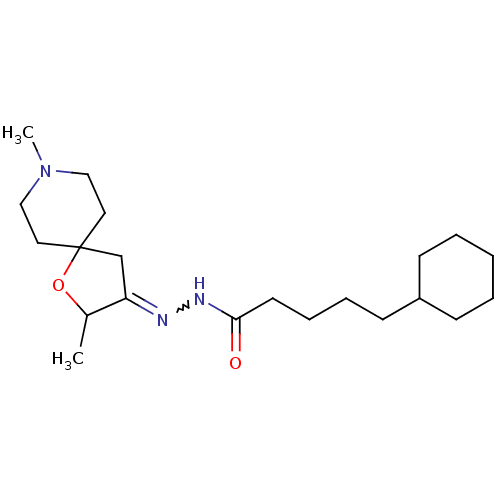
(5-Cyclohexyl-pentanoic acid [2,8-dimethyl-1-oxa-8-...)Show SMILES CC1OC2(CC1=NNC(=O)CCCCC1CCCCC1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C21H37N3O2/c1-17-19(16-21(26-17)12-14-24(2)15-13-21)22-23-20(25)11-7-6-10-18-8-4-3-5-9-18/h17-18H,3-16H2,1-2H3,(H,23,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50034625

(Aceclidine | Acetic acid 1-aza-bicyclo[2.2.2]oct-3...)Show SMILES CC(=O)OC1CN2CCC1CC2 |(10.76,-5.27,;9.32,-4.51,;9.32,-2.9,;7.94,-5.34,;6.53,-4.61,;6.53,-3.08,;5.18,-2.29,;4.35,-3.24,;4.35,-4.51,;5.2,-5.39,;3.85,-4.62,;3.85,-3.08,)| Show InChI InChI=1S/C9H15NO2/c1-7(11)12-9-6-10-4-2-8(9)3-5-10/h8-9H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 8.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor from rat brain crude membrane, using [3H]-NMS (N-Methylscopolamine) as the radioligand. |
J Med Chem 38: 1558-70 (1995)
Article DOI: 10.1021/jm00009a016
BindingDB Entry DOI: 10.7270/Q24B3420 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288156

(CHEMBL81954 | Octanoic acid N'-[2,8-dimethyl-1-oxa...)Show SMILES CCCCCCCC(=O)N(C)N=C1CC2(CCN(C)CC2)OC1C |w:11.10| Show InChI InChI=1S/C19H35N3O2/c1-5-6-7-8-9-10-18(23)22(4)20-17-15-19(24-16(17)2)11-13-21(3)14-12-19/h16H,5-15H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288153

(CHEMBL420596 | Nonanoic acid [2,8-dimethyl-1-oxa-8...)Show SMILES CCCCCCCCC(=O)NN=C1CC2(CCN(C)CC2)OC1C |w:11.10| Show InChI InChI=1S/C19H35N3O2/c1-4-5-6-7-8-9-10-18(23)21-20-17-15-19(24-16(17)2)11-13-22(3)14-12-19/h16H,4-15H2,1-3H3,(H,21,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288167

(CHEMBL84985 | Octanoic acid [2,8-dimethyl-1-oxa-8-...)Show SMILES CCCCCCCC(=O)NN=C1CC2(CCN(C)CC2)OC1C |w:10.9| Show InChI InChI=1S/C18H33N3O2/c1-4-5-6-7-8-9-17(22)20-19-16-14-18(23-15(16)2)10-12-21(3)13-11-18/h15H,4-14H2,1-3H3,(H,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50008072

((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...)Show InChI InChI=1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its affinity towards Muscarinic acetylcholine receptor M2, using [3H]- N-methyl-scopolamine, a radioligand displacement as... |
Bioorg Med Chem Lett 5: 1813-1818 (1995)
Article DOI: 10.1016/0960-894X(95)00301-9
BindingDB Entry DOI: 10.7270/Q27M07WJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288163
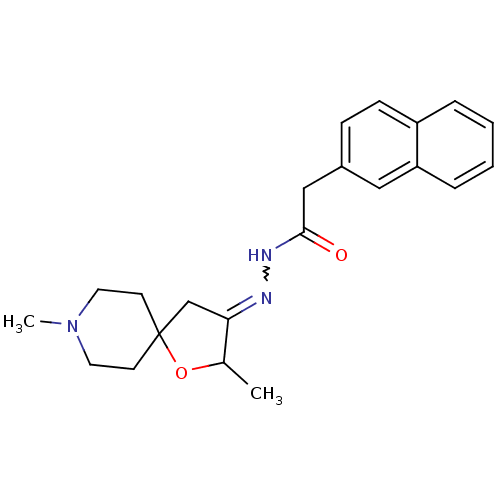
(CHEMBL84109 | Naphthalen-2-yl-acetic acid [2,8-dim...)Show SMILES CC1OC2(CC1=NNC(=O)Cc1ccc3ccccc3c1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C22H27N3O2/c1-16-20(15-22(27-16)9-11-25(2)12-10-22)23-24-21(26)14-17-7-8-18-5-3-4-6-19(18)13-17/h3-8,13,16H,9-12,14-15H2,1-2H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093252
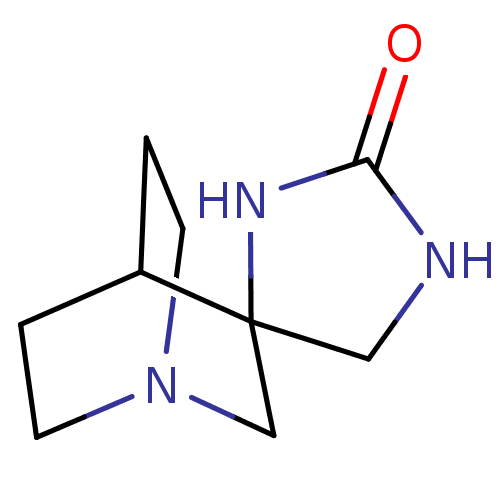
(CHEMBL422259 | Spiro[1-azabicyclo[2.2.2]octane-3,4...)Show SMILES O=C1NCC2(CN3CCC2CC3)N1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(24.72,-6.48,;23.15,-7,;22.63,-8.57,;20.98,-8.57,;20.48,-7,;19.28,-7.62,;18.67,-6.32,;16.97,-6.87,;17.6,-5.76,;19.41,-5.32,;19.8,-4.3,;18.67,-5.07,;21.81,-6.02,)| Show InChI InChI=1S/C9H15N3O/c13-8-10-5-9(11-8)6-12-3-1-7(9)2-4-12/h7H,1-6H2,(H2,10,11,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093258

(3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,4'-2H-t...)Show SMILES CN1C(=O)NCC11CN2CCC1CC2 |TLB:5:6:12.13:10.9,THB:1:6:12.13:10.9,(20.42,-4.1,;20.42,-5.64,;21.67,-6.55,;23.15,-6.07,;21.19,-8.03,;19.64,-8.03,;19.18,-6.55,;18.05,-7.14,;17.48,-5.92,;15.89,-6.44,;16.48,-5.39,;18.17,-4.98,;18.54,-4.03,;17.48,-4.75,)| Show InChI InChI=1S/C10H17N3O/c1-12-9(14)11-6-10(12)7-13-4-2-8(10)3-5-13/h8H,2-7H2,1H3,(H,11,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093252

(CHEMBL422259 | Spiro[1-azabicyclo[2.2.2]octane-3,4...)Show SMILES O=C1NCC2(CN3CCC2CC3)N1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(24.72,-6.48,;23.15,-7,;22.63,-8.57,;20.98,-8.57,;20.48,-7,;19.28,-7.62,;18.67,-6.32,;16.97,-6.87,;17.6,-5.76,;19.41,-5.32,;19.8,-4.3,;18.67,-5.07,;21.81,-6.02,)| Show InChI InChI=1S/C9H15N3O/c13-8-10-5-9(11-8)6-12-3-1-7(9)2-4-12/h7H,1-6H2,(H2,10,11,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288144
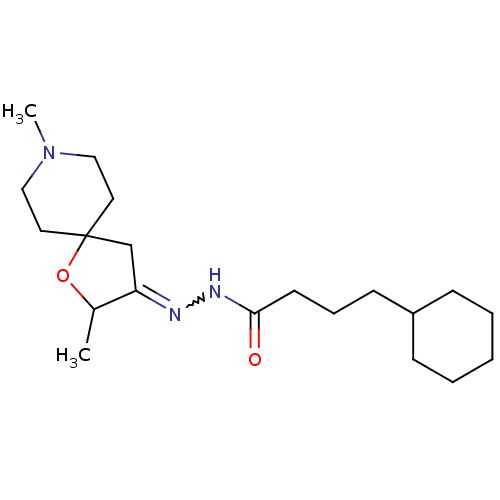
(4-Cyclohexyl-butyric acid [2,8-dimethyl-1-oxa-8-az...)Show SMILES CC1OC2(CC1=NNC(=O)CCCC1CCCCC1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C20H35N3O2/c1-16-18(15-20(25-16)11-13-23(2)14-12-20)21-22-19(24)10-6-9-17-7-4-3-5-8-17/h16-17H,3-15H2,1-2H3,(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288147
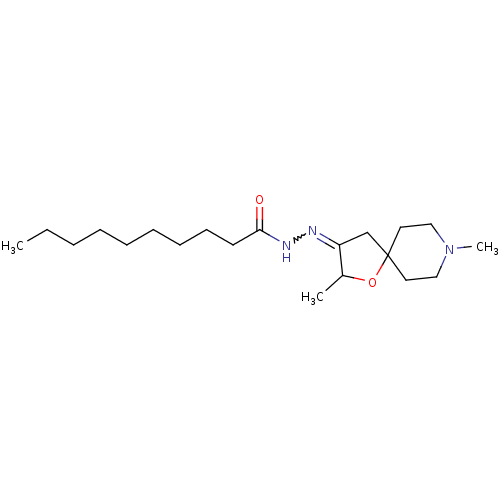
(CHEMBL314796 | Decanoic acid [2,8-dimethyl-1-oxa-8...)Show SMILES CCCCCCCCCC(=O)NN=C1CC2(CCN(C)CC2)OC1C |w:12.11| Show InChI InChI=1S/C20H37N3O2/c1-4-5-6-7-8-9-10-11-19(24)22-21-18-16-20(25-17(18)2)12-14-23(3)15-13-20/h17H,4-16H2,1-3H3,(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288158
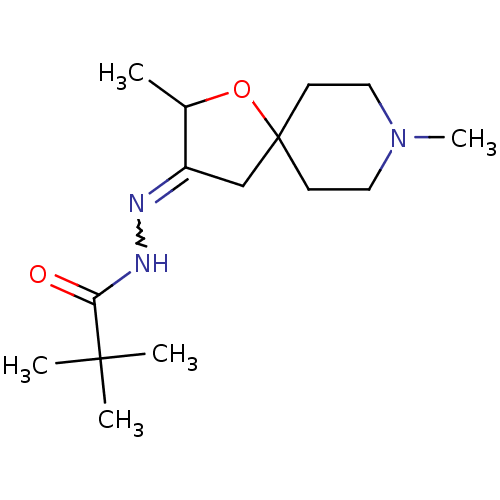
(2,2-Dimethyl-propionic acid [2,8-dimethyl-1-oxa-8-...)Show SMILES CC1OC2(CC1=NNC(=O)C(C)(C)C)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C15H27N3O2/c1-11-12(16-17-13(19)14(2,3)4)10-15(20-11)6-8-18(5)9-7-15/h11H,6-10H2,1-5H3,(H,17,19) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288161
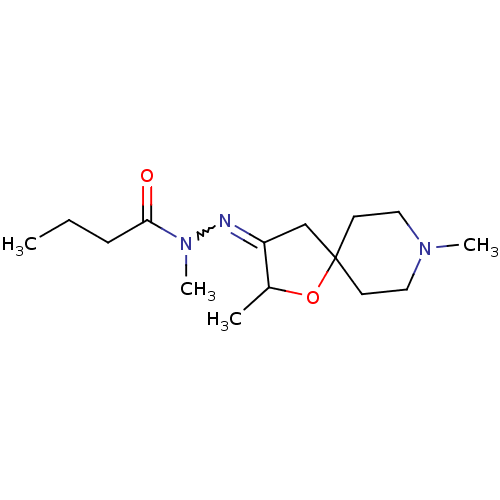
(Butyric acid N'-[2,8-dimethyl-1-oxa-8-aza-spiro[4....)Show InChI InChI=1S/C15H27N3O2/c1-5-6-14(19)18(4)16-13-11-15(20-12(13)2)7-9-17(3)10-8-15/h12H,5-11H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093254
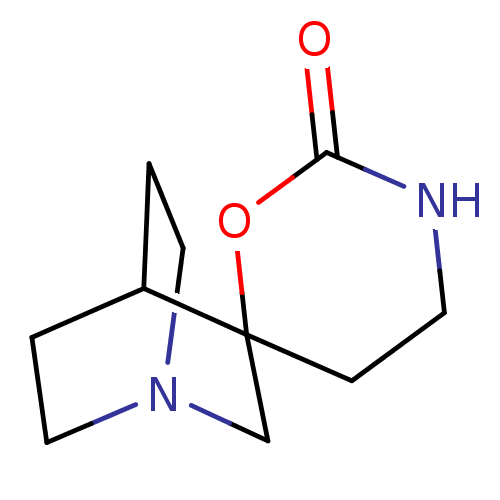
(CHEMBL336197 | Spiro[1-azabicyclo[2.2.2]octane-3,6...)Show SMILES O=C1NCCC2(CN3CCC2CC3)O1 |TLB:4:5:11.12:9.8,THB:13:5:11.12:9.8,(9.22,-3.02,;7.75,-3.49,;7.74,-5.03,;6.58,-5.81,;5.25,-5.03,;5.25,-3.49,;4.14,-4.08,;3.56,-2.86,;1.97,-3.37,;2.56,-2.34,;4.26,-1.93,;4.61,-.97,;3.56,-1.69,;6.5,-2.58,)| Show InChI InChI=1S/C10H16N2O2/c13-9-11-4-3-10(14-9)7-12-5-1-8(10)2-6-12/h8H,1-7H2,(H,11,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7; value range is 1000. |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor; value range is 1000. |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288157
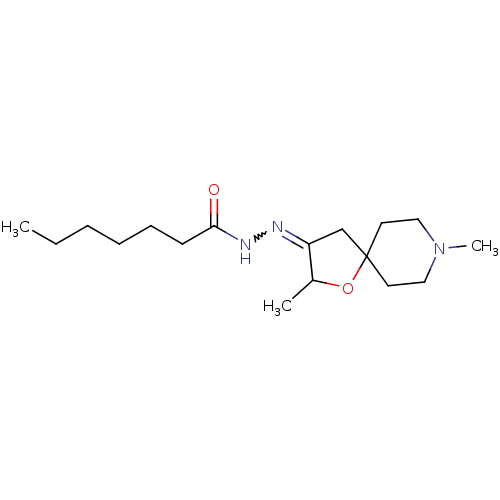
(CHEMBL314421 | Heptanoic acid [2,8-dimethyl-1-oxa-...)Show InChI InChI=1S/C17H31N3O2/c1-4-5-6-7-8-16(21)19-18-15-13-17(22-14(15)2)9-11-20(3)12-10-17/h14H,4-13H2,1-3H3,(H,19,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data