

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50281617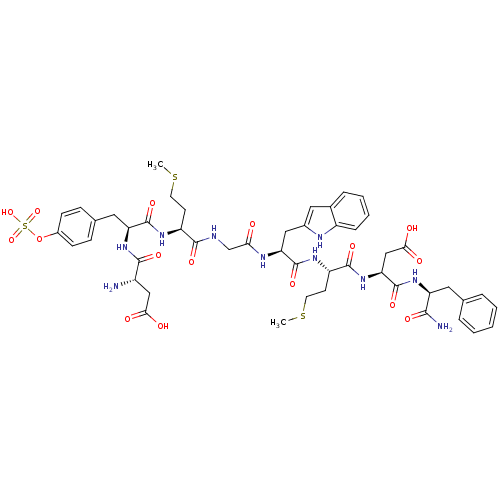 (Asp Tyr (OSO3H) Met Gly Trp Met Asp Phe | CHEMBL26...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | Bioorg Med Chem Lett 3: 667-670 (1993) Article DOI: 10.1016/S0960-894X(01)81250-6 BindingDB Entry DOI: 10.7270/Q2K937G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24341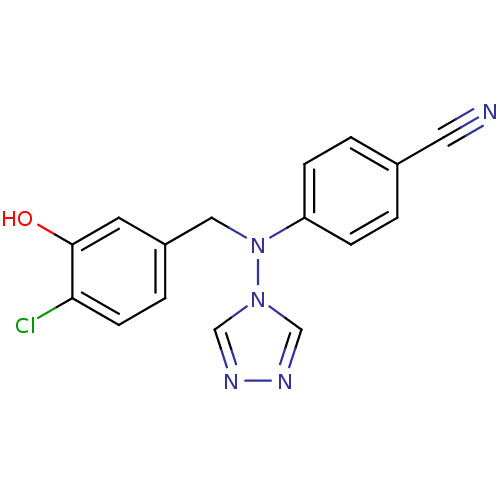 (4-{[(4-chloro-3-hydroxyphenyl)methyl](4H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50281617 (Asp Tyr (OSO3H) Met Gly Trp Met Asp Phe | CHEMBL26...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type B receptor in mouse cerebral cortex | Bioorg Med Chem Lett 3: 667-670 (1993) Article DOI: 10.1016/S0960-894X(01)81250-6 BindingDB Entry DOI: 10.7270/Q2K937G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24328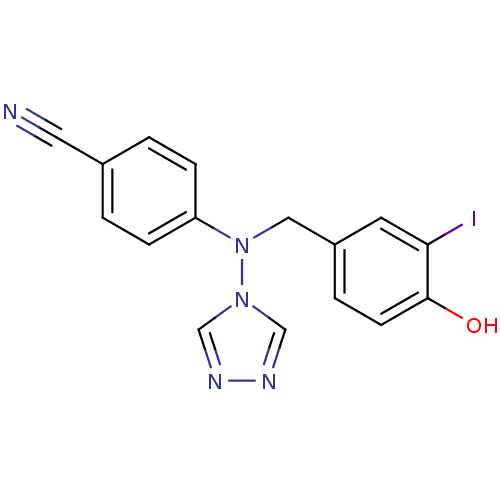 (4-{[(4-hydroxy-3-iodophenyl)methyl](4H-1,2,4-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24344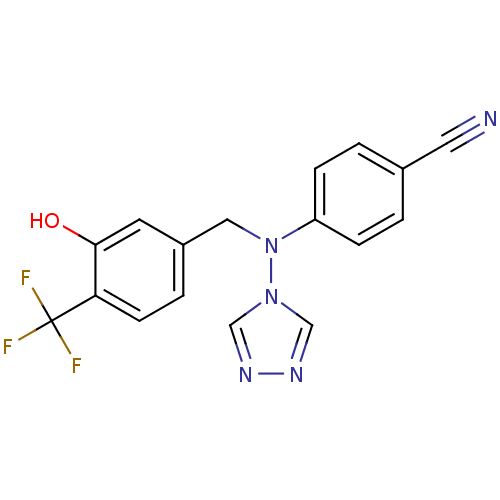 (4-({[3-hydroxy-4-(trifluoromethyl)phenyl]methyl}(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10016 (4-{[(4-bromophenyl)methyl](4H-1,2,4-triazol-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24342 (4-{[(4-bromo-3-hydroxyphenyl)methyl](4H-1,2,4-tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24332 (4-{[(3-chloro-4-hydroxy-5-methoxyphenyl)methyl](4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24340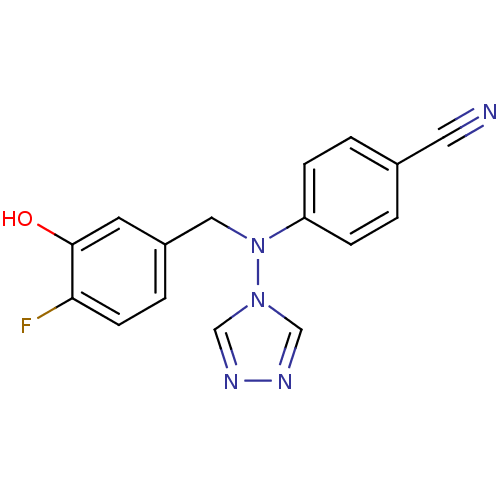 (4-{[(4-fluoro-3-hydroxyphenyl)methyl](4H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24335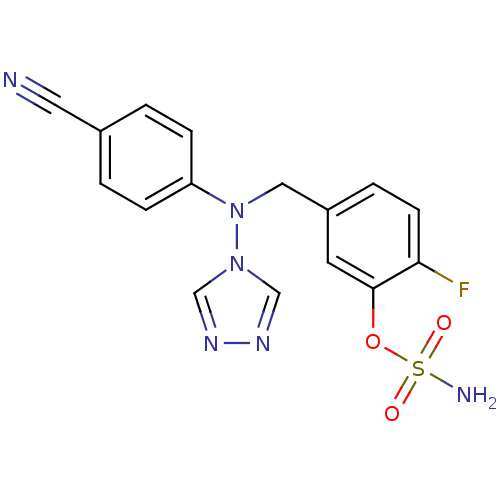 ((5-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10020 ((2-bromo-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24331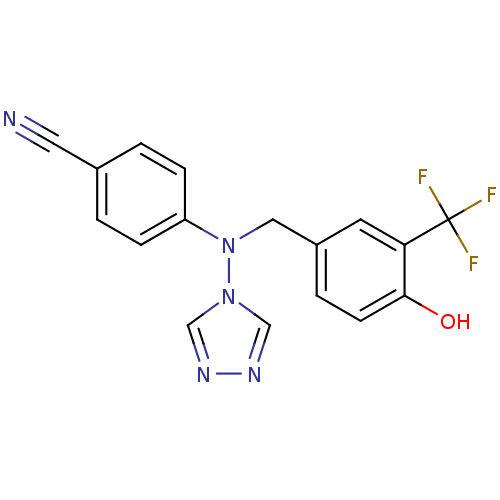 (4-({[4-hydroxy-3-(trifluoromethyl)phenyl]methyl}(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24336 ((2-chloro-5-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24327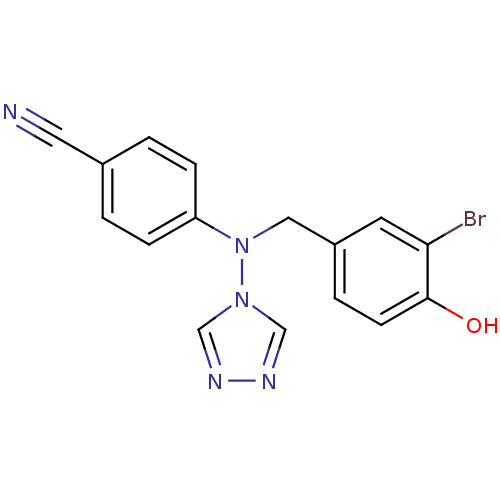 (4-{[(3-bromo-4-hydroxyphenyl)methyl](4H-1,2,4-tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24343 (4-{[(3-hydroxy-4-methoxyphenyl)methyl](4H-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24320 ((4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM13058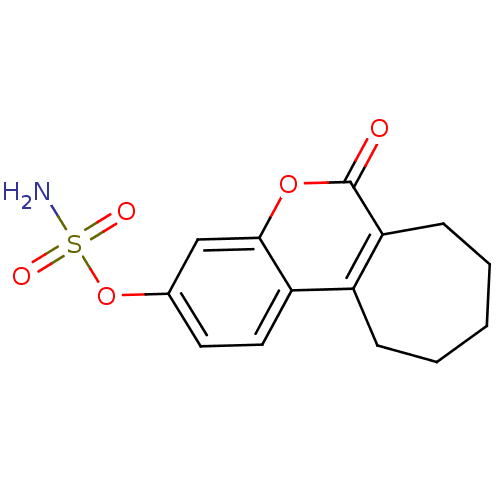 (6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10019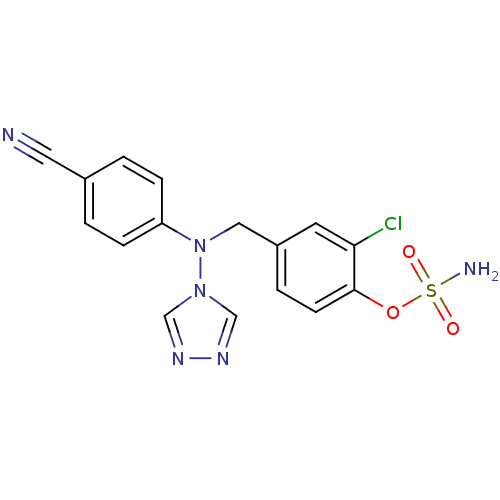 ((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24326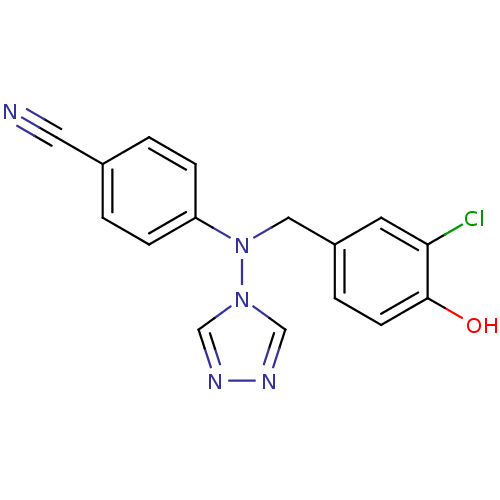 (4-{[(3-chloro-4-hydroxyphenyl)methyl](4H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24329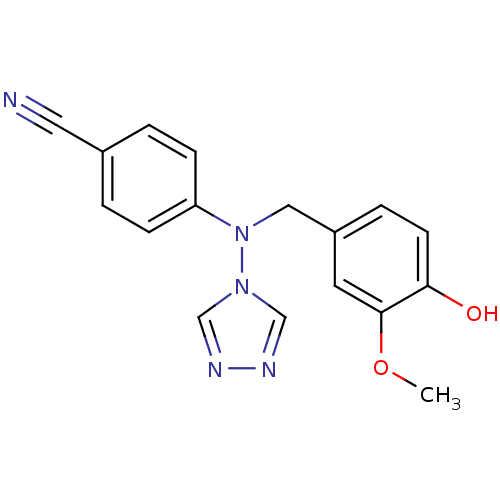 (4-{[(4-hydroxy-3-methoxyphenyl)methyl](4H-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24339 (4-{[(3-hydroxyphenyl)methyl](4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24323 ((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24325 (4-{[(3-fluoro-4-hydroxyphenyl)methyl](4H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50281619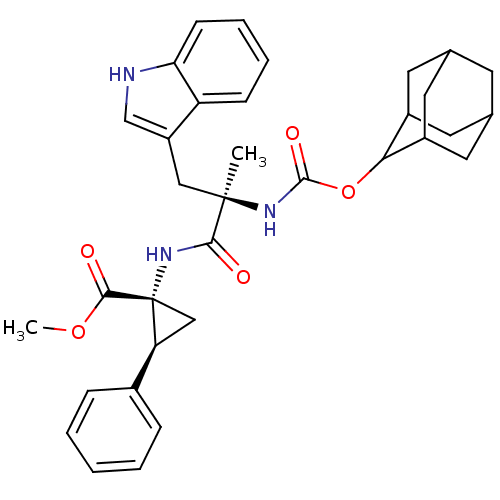 ((1R,2S)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type B receptor in mouse cerebral cortex | Bioorg Med Chem Lett 3: 667-670 (1993) Article DOI: 10.1016/S0960-894X(01)81250-6 BindingDB Entry DOI: 10.7270/Q2K937G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24337 ((2-bromo-5-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24333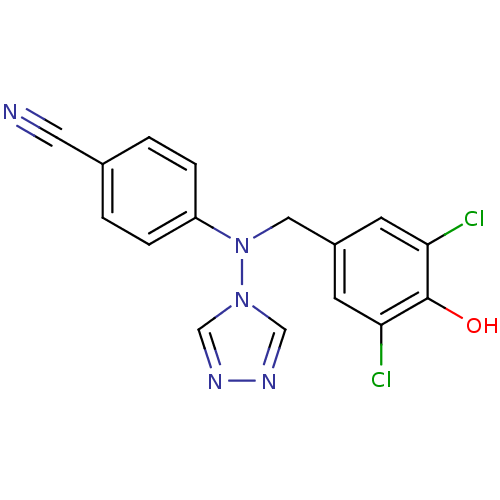 (4-{[(3,5-dichloro-4-hydroxyphenyl)methyl](4H-1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10018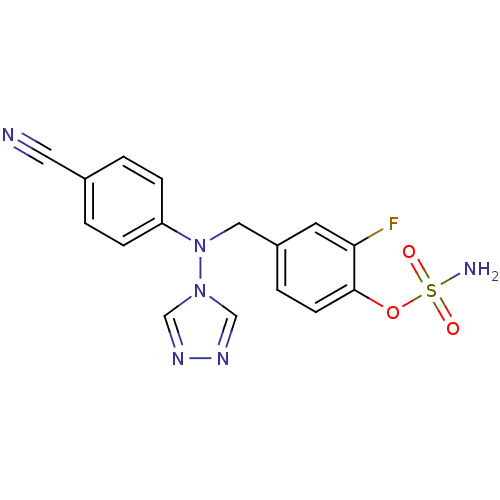 ((4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24338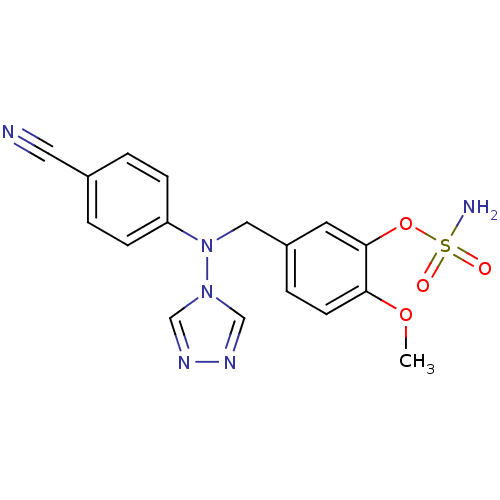 ((5-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50341860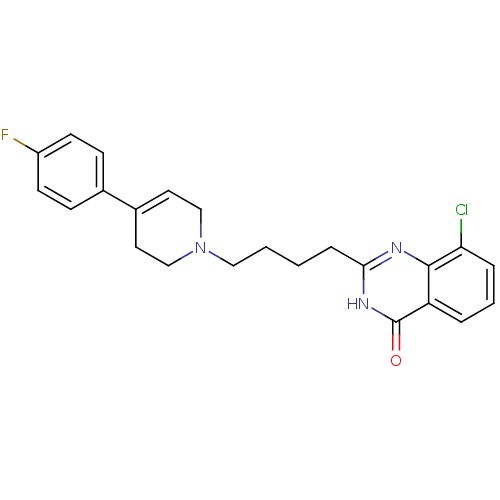 (8-chloro-2-(4-(4-(4-fluorophenyl)-5,6-dihydropyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of full length human PARP-1 after 10 mins by FlashPlate scintillation proximity assay | J Med Chem 54: 2049-59 (2011) Article DOI: 10.1021/jm1010918 BindingDB Entry DOI: 10.7270/Q24B31NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50171448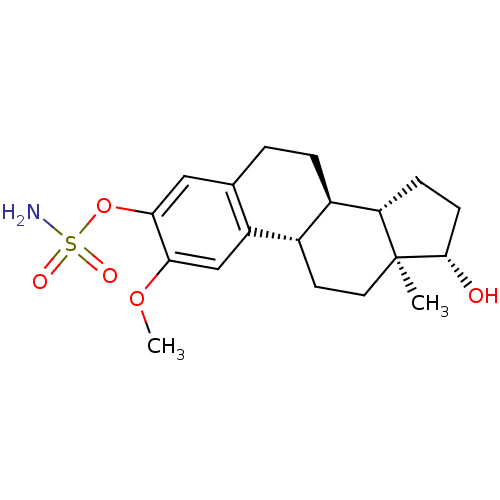 ((9BETA,14BETA,17BETA)-17-HYDROXY-2-METHOXYESTRA-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1 | J Med Chem 51: 1295-308 (2008) Article DOI: 10.1021/jm701319c BindingDB Entry DOI: 10.7270/Q2M0468S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM10019 ((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibitory concentration against steroid sulfatase in placental microsomes | J Med Chem 48: 5243-56 (2005) Article DOI: 10.1021/jm050066a BindingDB Entry DOI: 10.7270/Q2NZ875B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24324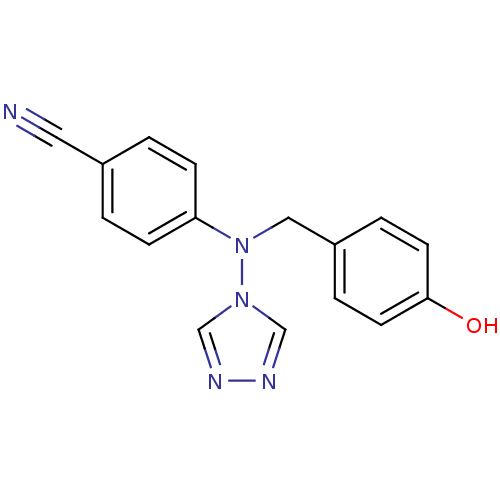 (4-{[(4-hydroxyphenyl)methyl](4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24330 (5-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281619 ((1R,2S)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | Bioorg Med Chem Lett 3: 667-670 (1993) Article DOI: 10.1016/S0960-894X(01)81250-6 BindingDB Entry DOI: 10.7270/Q2K937G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24322 ((2-cyano-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50237104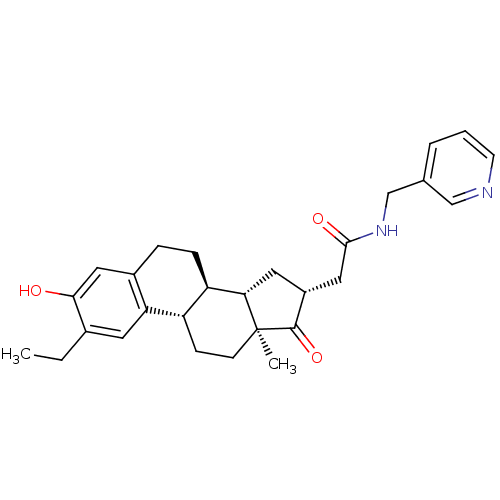 (2-((8R,9S,13S,14S,16R)-2-ethyl-3-hydroxy-13-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibitory activity against 17 beta hydroxysteroid dehydrogenase type 1 in T47D cells | J Med Chem 48: 2759-62 (2005) Article DOI: 10.1021/jm049045r BindingDB Entry DOI: 10.7270/Q2BG2PSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50341859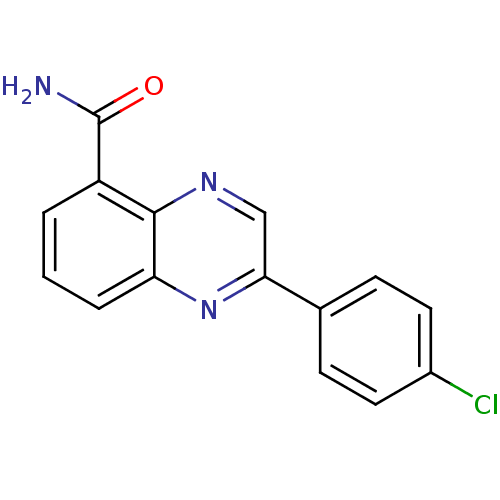 (2-(4-chlorophenyl)quinoxaline-5-carboxamide | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of full length human PARP-1 after 10 mins by FlashPlate scintillation proximity assay | J Med Chem 54: 2049-59 (2011) Article DOI: 10.1021/jm1010918 BindingDB Entry DOI: 10.7270/Q24B31NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50171448 ((9BETA,14BETA,17BETA)-17-HYDROXY-2-METHOXYESTRA-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibitory concentration against steroid sulfatase in placental microsomes | J Med Chem 48: 5243-56 (2005) Article DOI: 10.1021/jm050066a BindingDB Entry DOI: 10.7270/Q2NZ875B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50370656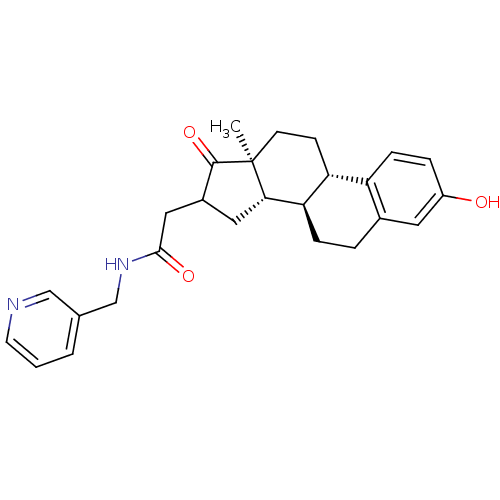 (CHEMBL1627749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibitory activity against 17 beta hydroxysteroid dehydrogenase type 1 in T47D cells | J Med Chem 48: 2759-62 (2005) Article DOI: 10.1021/jm049045r BindingDB Entry DOI: 10.7270/Q2BG2PSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50370656 (CHEMBL1627749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of 17-beta HSD1 in T47D cells | J Med Chem 49: 1325-45 (2006) Article DOI: 10.1021/jm050830t BindingDB Entry DOI: 10.7270/Q2C24X7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM10020 ((2-bromo-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50200936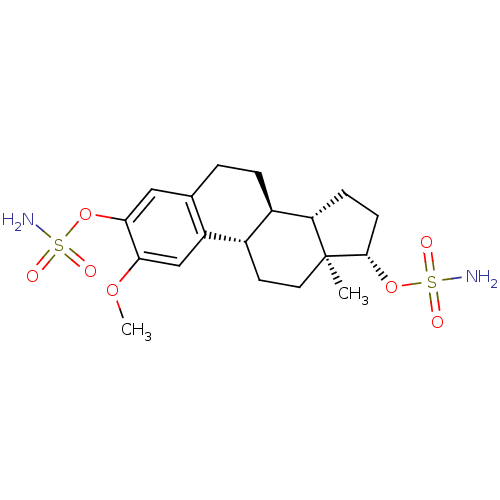 ((9BETA,13ALPHA,14BETA,17ALPHA)-2-METHOXYESTRA-1,3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1 | J Med Chem 51: 1295-308 (2008) Article DOI: 10.1021/jm701319c BindingDB Entry DOI: 10.7270/Q2M0468S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10021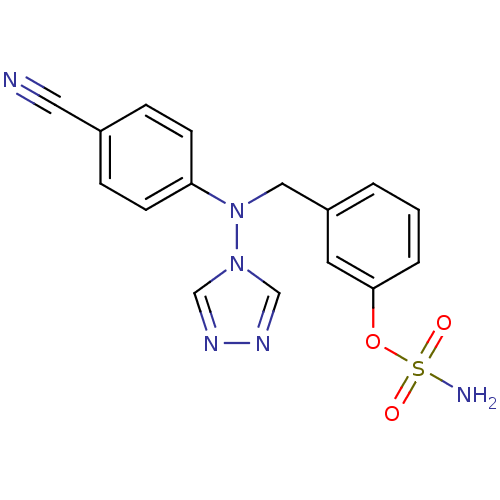 ((3-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM10018 ((4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50219531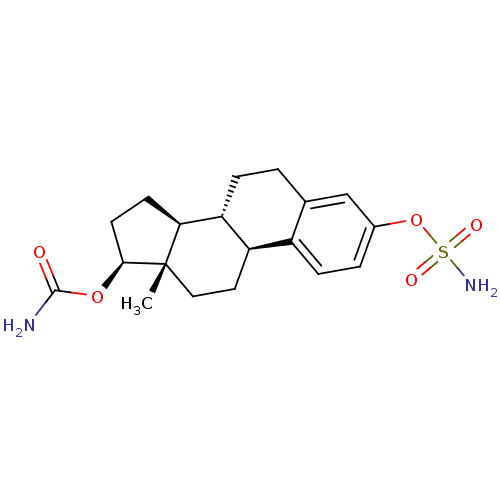 (17beta-carbamoyloxy-3-sulfamoyloxyestra-1,3,5(10)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase in placental microsomes | J Med Chem 50: 4431-43 (2007) Article DOI: 10.1021/jm070405v BindingDB Entry DOI: 10.7270/Q2765F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24321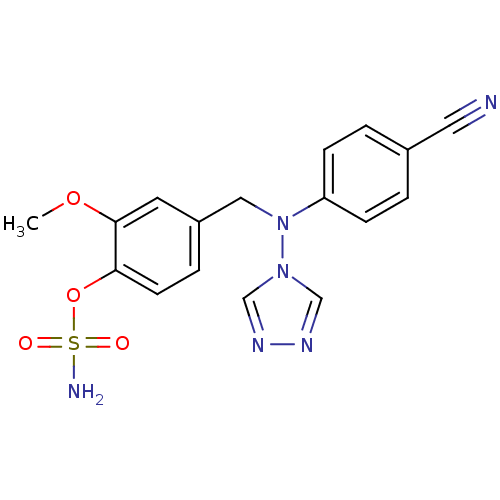 ((4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50171450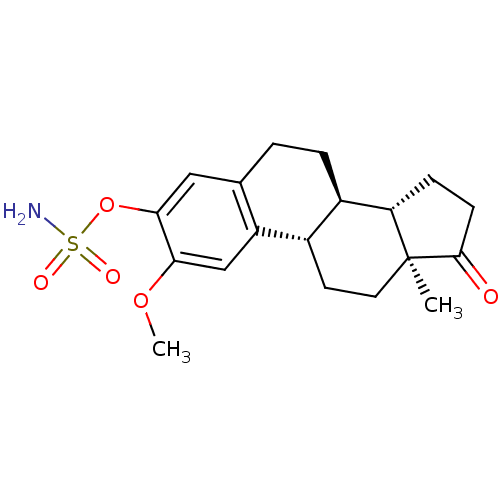 ((8R,9S,13S,14S)-2-Methoxy-13-methyl-17-oxo-7,8,9,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibitory concentration against steroid sulfatase in placental microsomes | J Med Chem 48: 5243-56 (2005) Article DOI: 10.1021/jm050066a BindingDB Entry DOI: 10.7270/Q2NZ875B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50281620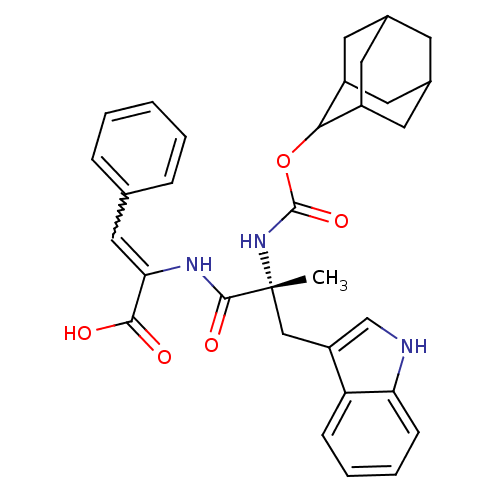 ((Z)-2-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type B receptor in mouse cerebral cortex | Bioorg Med Chem Lett 3: 667-670 (1993) Article DOI: 10.1016/S0960-894X(01)81250-6 BindingDB Entry DOI: 10.7270/Q2K937G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281620 ((Z)-2-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | Bioorg Med Chem Lett 3: 667-670 (1993) Article DOI: 10.1016/S0960-894X(01)81250-6 BindingDB Entry DOI: 10.7270/Q2K937G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 174 total ) | Next | Last >> |