

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098965 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding to [125I]- fibrinogen by washed platelet fibrinogen receptor | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50414514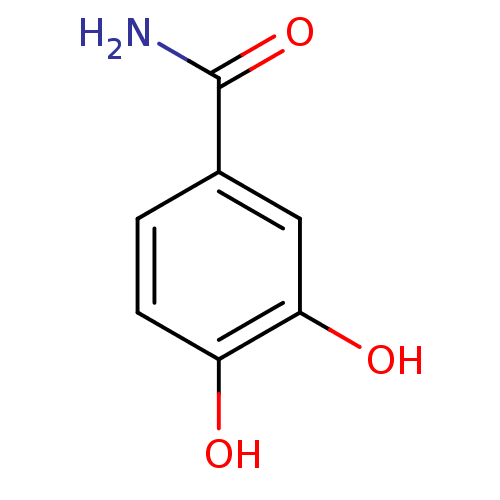 (PROTOCATECHUAMIDE) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology Curated by ChEMBL | Assay Description Apparent noncompetitive inhibition of catecholase activity of tyrosinase in mouse B16 cells by Lineweaver-Burke plot analysis | Bioorg Med Chem Lett 19: 4178-82 (2009) Article DOI: 10.1016/j.bmcl.2009.05.115 BindingDB Entry DOI: 10.7270/Q2BR8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50296245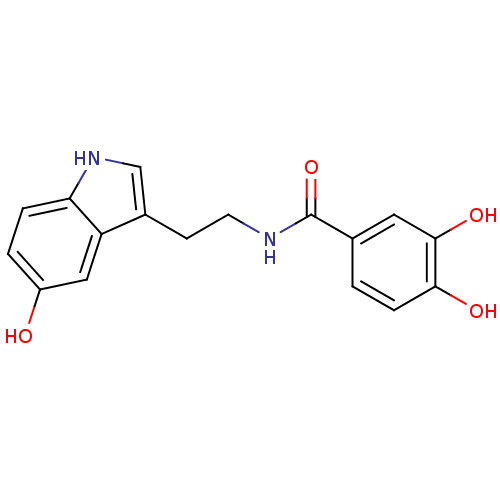 (3,4-Dihydroxy-N-[2-(5-hydroxyindol-3-yl)ethyl]benz...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology Curated by ChEMBL | Assay Description Apparent noncompetitive inhibition of catecholase activity of tyrosinase in mouse B16 cells by Lineweaver-Burke plot analysis | Bioorg Med Chem Lett 19: 4178-82 (2009) Article DOI: 10.1016/j.bmcl.2009.05.115 BindingDB Entry DOI: 10.7270/Q2BR8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM29612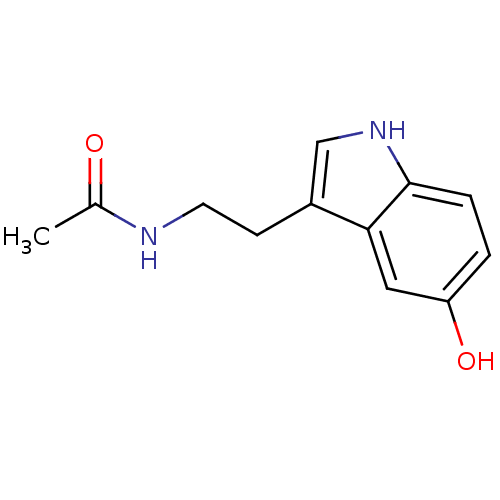 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology Curated by ChEMBL | Assay Description Apparent noncompetitive inhibition of catecholase activity of tyrosinase in mouse B16 cells by Lineweaver-Burke plot analysis | Bioorg Med Chem Lett 19: 4178-82 (2009) Article DOI: 10.1016/j.bmcl.2009.05.115 BindingDB Entry DOI: 10.7270/Q2BR8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098965 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098971 (3-({3-Benzyl-2-[(Z)-4-carbamimidoyl-benzoylimino]-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098967 (3-({2-[(Z)-4-Carbamimidoyl-benzoylimino]-3-ethyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of immobilized HUVEC adhesion on fibronectin | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098964 (3-({3-Butyl-2-[(Z)-4-carbamimidoyl-benzoylimino]-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding to [125I]- fibrinogen by washed platelet fibrinogen receptor | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098966 (3-[3-(4-Carbamimidoyl-benzoylamino)-phenyl]-propio...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of ADP (2 microM) induced platelet aggregation of human platelet rich plasma | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098969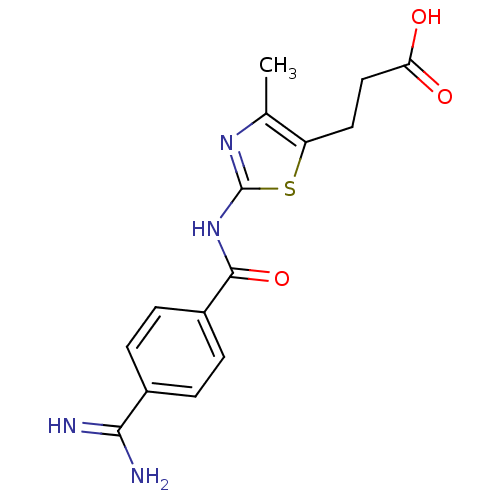 (3-[2-(4-Carbamimidoyl-benzoylamino)-4-methyl-thiaz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of ADP (3 microM)induced platelet aggregation of guinea pig platelet rich plasma | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098968 (3-({2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of immobilized HUVEC adhesion on fibrinogen | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098972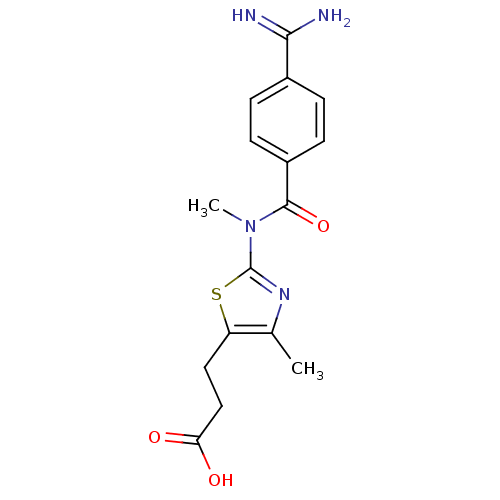 (3-{2-[(4-Carbamimidoyl-benzoyl)-methyl-amino]-4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding to [125I]- fibrinogen by washed platelet fibrinogen receptor | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098970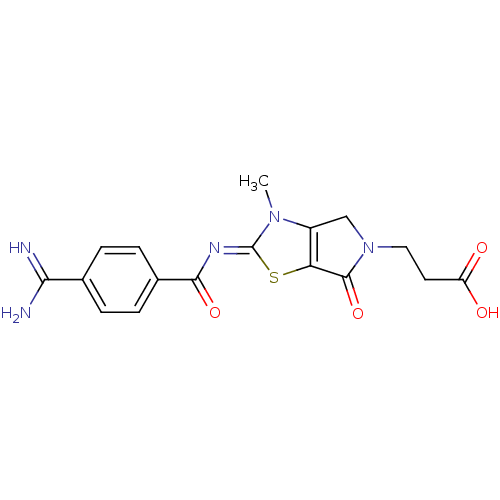 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3-methyl-6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098964 (3-({3-Butyl-2-[(Z)-4-carbamimidoyl-benzoylimino]-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding to [125I]- fibrinogen by washed platelet fibrinogen receptor | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098965 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098965 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098965 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of ADP (5 microM) induced platelet aggregation of mouse platelet rich plasma | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098965 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding to [125I]- fibrinogen by washed platelet fibrinogen receptor | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098965 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding to [125I]- fibrinogen by washed platelet fibrinogen receptor | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098968 (3-({2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098969 (3-[2-(4-Carbamimidoyl-benzoylamino)-4-methyl-thiaz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098970 (3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3-methyl-6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50098966 (3-[3-(4-Carbamimidoyl-benzoylamino)-phenyl]-propio...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Affinity for purified activated GPIIb/IIIa fibrinogen receptor in ELISA | Bioorg Med Chem Lett 11: 1031-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50094938 (5-Isopropoxy-2-(4-methylsulfanyl-pyridin-2-ylmetha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity towards Protein tyrosine phosphatase of CD45 | Bioorg Med Chem Lett 10: 2657-60 (2000) BindingDB Entry DOI: 10.7270/Q2MK6C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50302545 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C19 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50302545 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2D6 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50302545 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C9 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50302544 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C19 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50228518 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C9 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50302543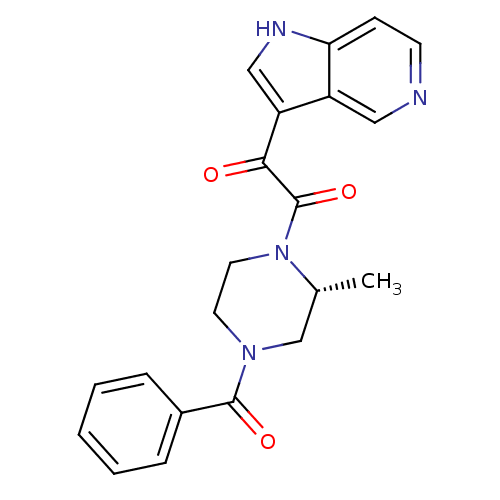 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 using 7-benzyloxyresorufin as substrate | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50133282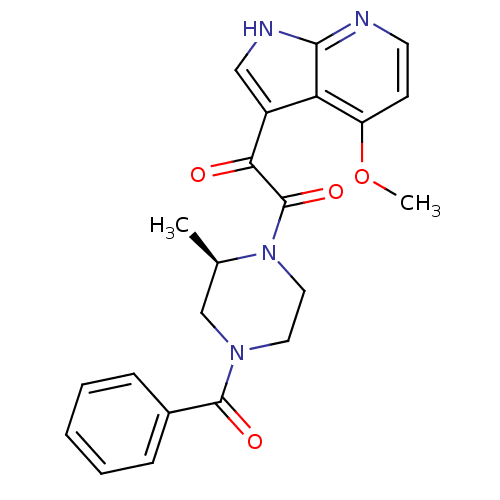 ((R)-1-(4-benzoyl-2-methylpiperazin-1-yl)-2-(4-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2D6 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50228518 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C19 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50228518 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2D6 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50302544 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C9 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50302543 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C19 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50302543 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 using benzyloxy-4-(trifluoromethyl)coumarin as substrate | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50094938 (5-Isopropoxy-2-(4-methylsulfanyl-pyridin-2-ylmetha...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity towards Protein tyrosine phosphatase of PTP1B | Bioorg Med Chem Lett 10: 2657-60 (2000) BindingDB Entry DOI: 10.7270/Q2MK6C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50296247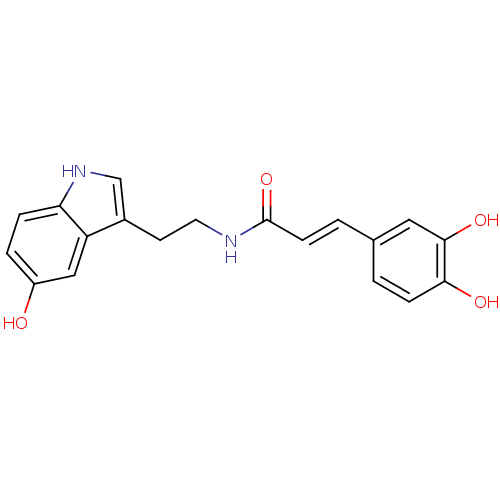 (3-(3,4-Dihydroxyphenyl)-N-[2-(5-hydroxyindol-3-yl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology Curated by ChEMBL | Assay Description Inhibition of catecholase activity of tyrosinase in mouse B16 cells assessed as dopachrome formation | Bioorg Med Chem Lett 19: 4178-82 (2009) Article DOI: 10.1016/j.bmcl.2009.05.115 BindingDB Entry DOI: 10.7270/Q2BR8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50228465 (1-(4-benzoylpiperazin-1-yl)-2-(4,7-dimethoxy-1H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C9 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50094936 (CHEMBL86473 | Vanadate) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity towards Protein tyrosine phosphatase of PP2A | Bioorg Med Chem Lett 10: 2657-60 (2000) BindingDB Entry DOI: 10.7270/Q2MK6C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50094936 (CHEMBL86473 | Vanadate) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity towards Protein tyrosine phosphatase of PP1 | Bioorg Med Chem Lett 10: 2657-60 (2000) BindingDB Entry DOI: 10.7270/Q2MK6C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50094936 (CHEMBL86473 | Vanadate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity towards Protein tyrosine phosphatase of CD45 | Bioorg Med Chem Lett 10: 2657-60 (2000) BindingDB Entry DOI: 10.7270/Q2MK6C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50302542 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C19 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50302544 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2D6 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50094936 (CHEMBL86473 | Vanadate) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity towards Protein tyrosine phosphatase of PTP-S2 | Bioorg Med Chem Lett 10: 2657-60 (2000) BindingDB Entry DOI: 10.7270/Q2MK6C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50302542 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP1A2 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50302542 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C9 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50302542 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2D6 | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50302542 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 using benzyloxy-4-(trifluoromethyl)coumarin as substrate | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50302542 ((R)-1-(4-Benzoyl-2-methylpiperazin-1-yl)-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 using 7-benzyloxyresorufin as substrate | J Med Chem 52: 7778-87 (2009) Article DOI: 10.1021/jm900843g BindingDB Entry DOI: 10.7270/Q2Q81D5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |