

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21125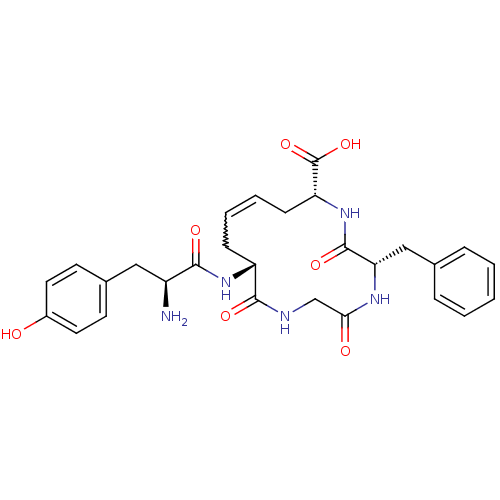 ((5S,8R,10Z,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.430 | -53.5 | n/a | n/a | 0.830 | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21129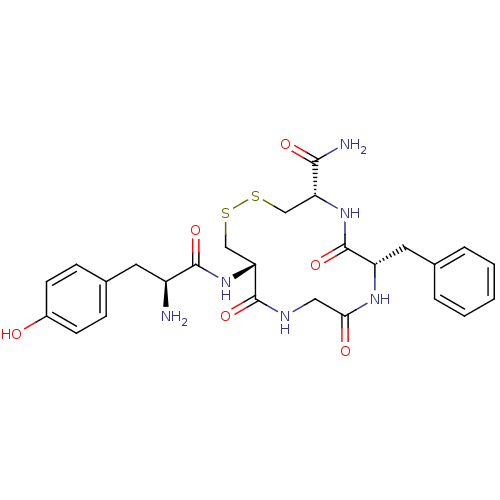 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.550 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21126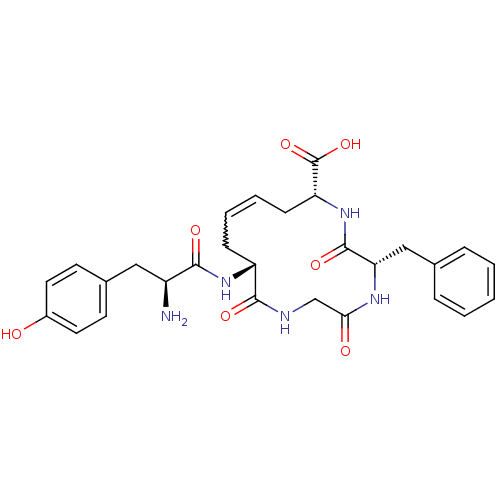 ((5S,8R,10E,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.570 | -52.8 | n/a | n/a | 0.880 | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21129 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.822 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21126 ((5S,8R,10E,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | -50.7 | n/a | n/a | 2.65 | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21125 ((5S,8R,10Z,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.35 | -50.6 | n/a | n/a | 2.35 | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008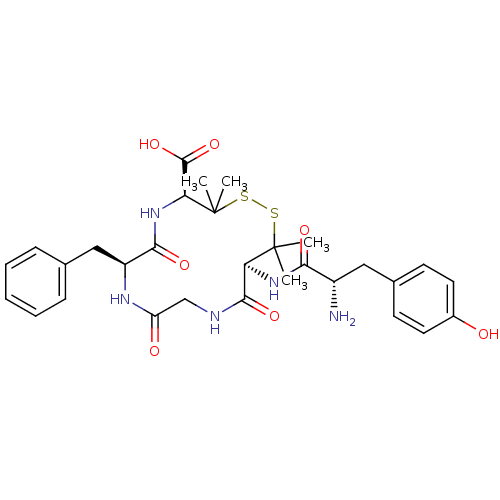 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.60 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21127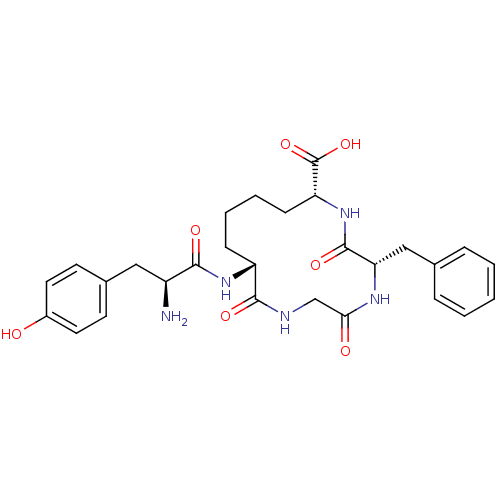 ((5S,8R,13R)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.90 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21128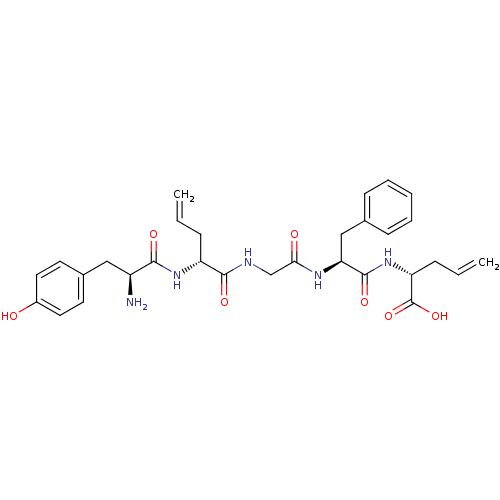 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.10 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50397109 (CHEMBL2171847) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21129 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44.9 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21127 ((5S,8R,13R)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56.9 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50397110 (CHEMBL2171846) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21128 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 64.5 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21126 ((5S,8R,10E,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 74.8 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50386560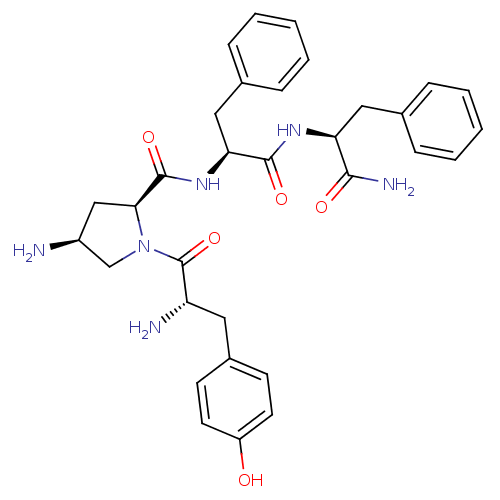 (CHEMBL2048250) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from rat mu opioid receptor expressed in HN9.10 cells after 3 hrs by scintillation counting | J Med Chem 55: 3027-35 (2012) Article DOI: 10.1021/jm201402v BindingDB Entry DOI: 10.7270/Q2571D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Bos taurus) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in calf frontal cortex after 1 hr by gamma counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21125 ((5S,8R,10Z,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 576 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21008 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 609 | -35.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50386559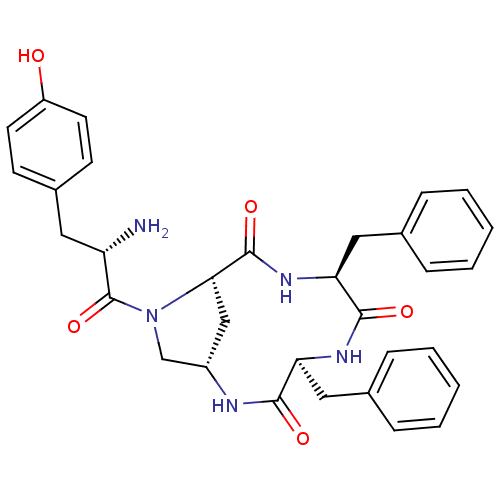 (CHEMBL2048248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from rat mu opioid receptor expressed in HN9.10 cells after 3 hrs by scintillation counting | J Med Chem 55: 3027-35 (2012) Article DOI: 10.1021/jm201402v BindingDB Entry DOI: 10.7270/Q2571D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50397115 (CHEMBL2171850) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50397113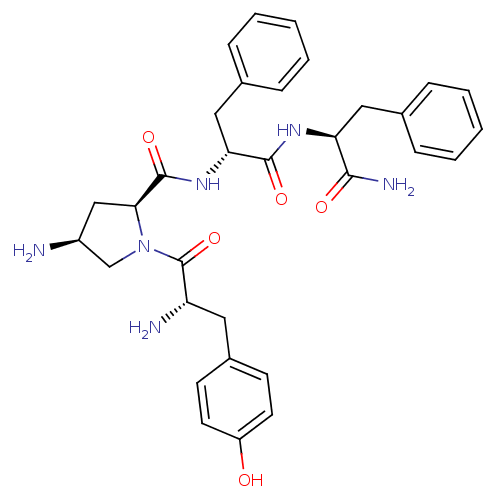 (CHEMBL2171852) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50397109 (CHEMBL2171847) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50397110 (CHEMBL2171846) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50397116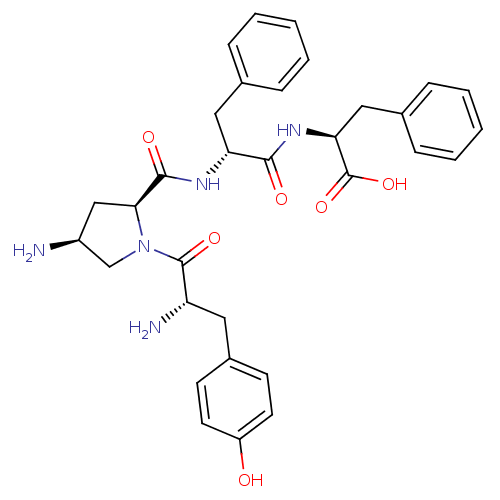 (CHEMBL2171849) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50386561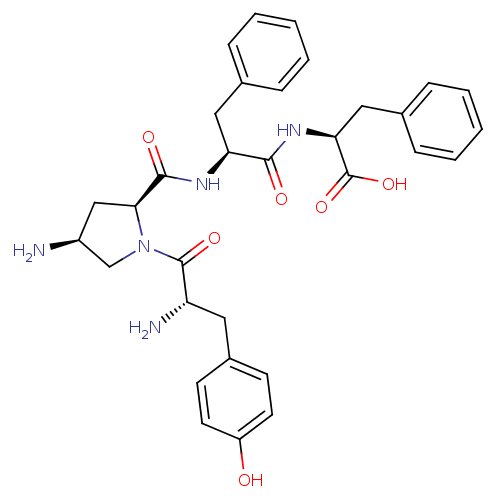 (CHEMBL2048249) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from rat mu opioid receptor expressed in HN9.10 cells after 3 hrs by scintillation counting | J Med Chem 55: 3027-35 (2012) Article DOI: 10.1021/jm201402v BindingDB Entry DOI: 10.7270/Q2571D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50397114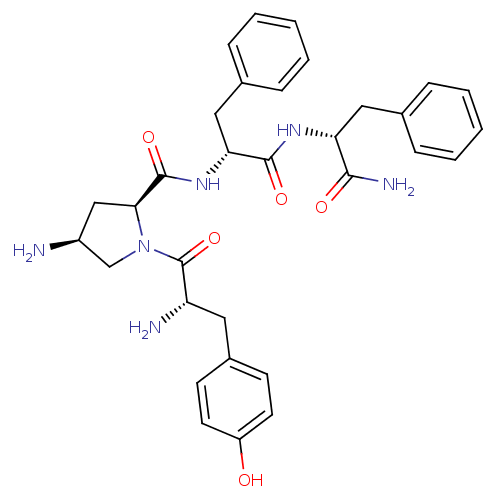 (CHEMBL2171851) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21128 ((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.87E+3 | -30.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21127 ((5S,8R,13R)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.96E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 3138-3142 (2007) Article DOI: 10.1021/jm061048b BindingDB Entry DOI: 10.7270/Q28G8J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50397111 (CHEMBL2171853) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50397112 (CHEMBL2171848) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50397109 (CHEMBL2171847) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50397112 (CHEMBL2171848) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor transfected in human HN9.10 cells after 3 hrs by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50397110 (CHEMBL2171846) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counter | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013754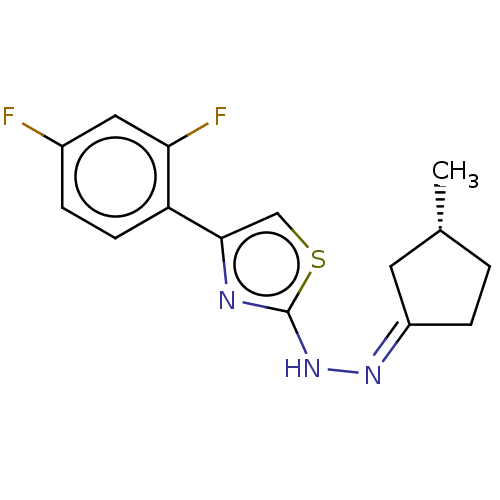 (CHEMBL3264960) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013747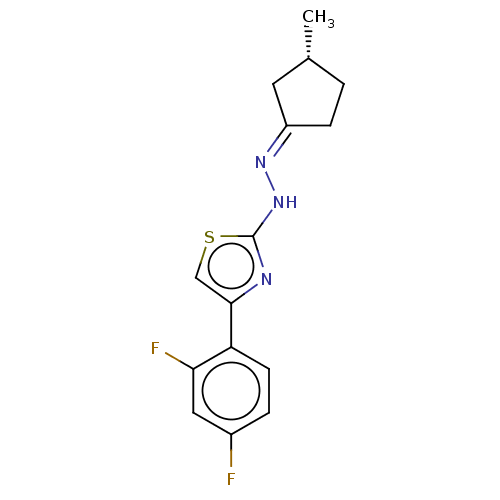 (CHEMBL3264959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013747 (CHEMBL3264959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013756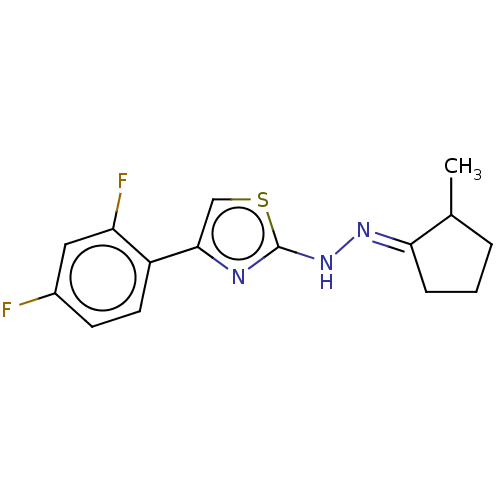 (CHEMBL3264954) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013749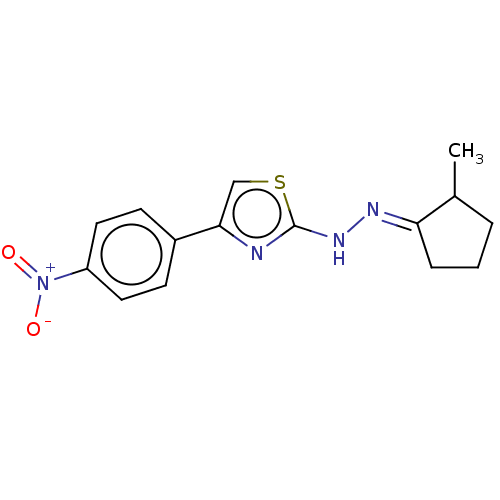 (CHEMBL3264952) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013755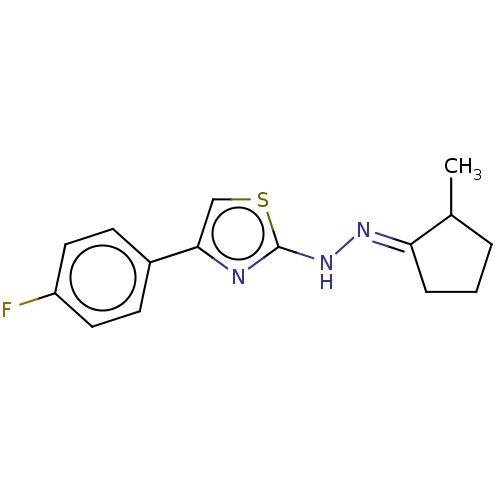 (CHEMBL3264951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013748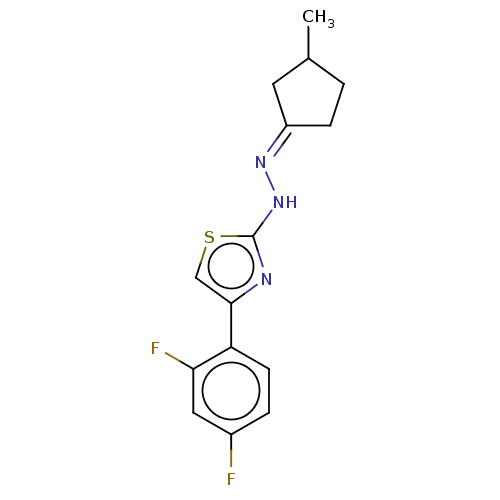 (CHEMBL3264958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013750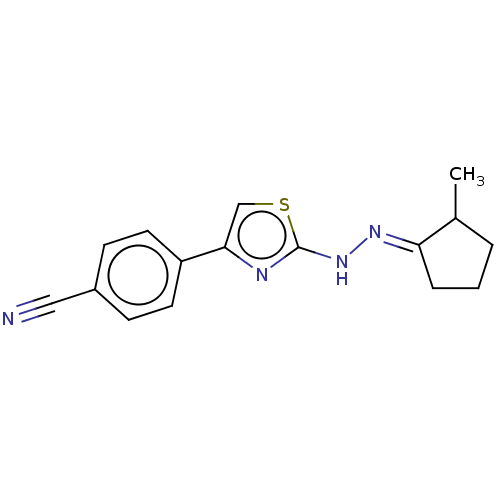 (CHEMBL3264953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013751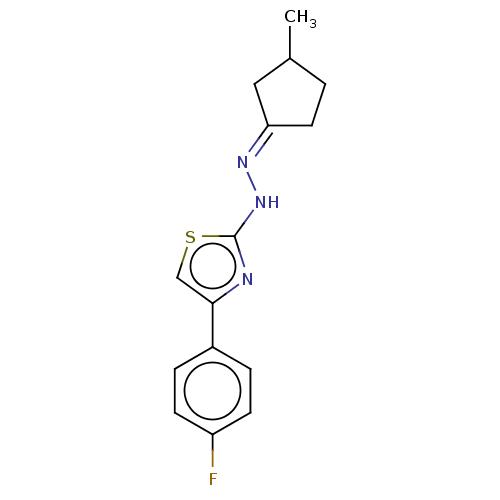 (CHEMBL3264955) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50029816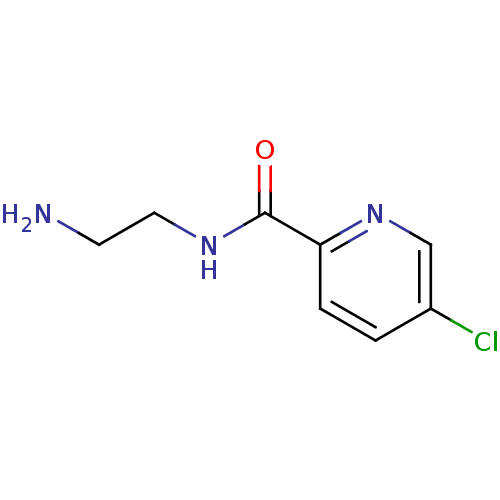 (5-Chloro-pyridine-2-carboxylic acid (2-amino-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in insect cells assessed as inhibition of oxidation of kynuramine to 4-hydroxyquinoline after 20 mins... | Eur J Med Chem 82: 164-71 (2014) Article DOI: 10.1016/j.ejmech.2014.05.048 BindingDB Entry DOI: 10.7270/Q2M90B7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50022662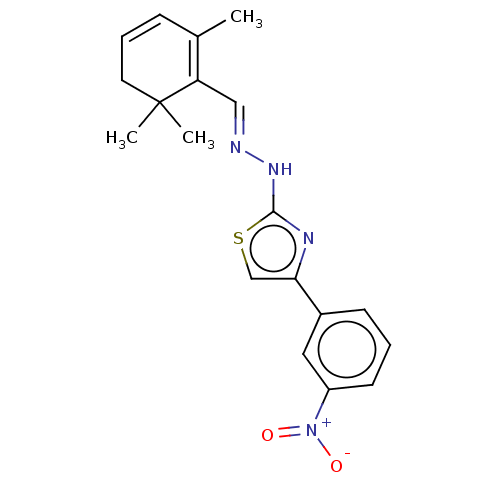 (CHEMBL3299140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in insect cells assessed as inhibition of oxidation of kynuramine to 4-hydroxyquinoline after 20 mins... | Eur J Med Chem 82: 164-71 (2014) Article DOI: 10.1016/j.ejmech.2014.05.048 BindingDB Entry DOI: 10.7270/Q2M90B7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50013752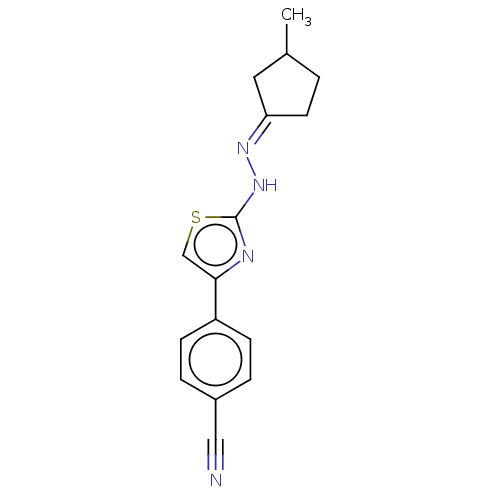 (CHEMBL3264957) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in baculovirus-infected BTI-TN-5B1-4 cell microsomes assessed as decrease in H2O2 production using p-... | Bioorg Med Chem 22: 2887-95 (2014) Article DOI: 10.1016/j.bmc.2014.03.042 BindingDB Entry DOI: 10.7270/Q2C53NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50397109 (CHEMBL2171847) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara G. d'Annunzio Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in Hartley guinea pig ileum longitudinal muscle myenteric plexus assessed as inhibition of electrically-induce... | J Med Chem 55: 8477-82 (2012) Article DOI: 10.1021/jm300947s BindingDB Entry DOI: 10.7270/Q2NP25JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 91 total ) | Next | Last >> |