 Found 191 hits with Last Name = 'maresca' and Initial = 'p'
Found 191 hits with Last Name = 'maresca' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265453
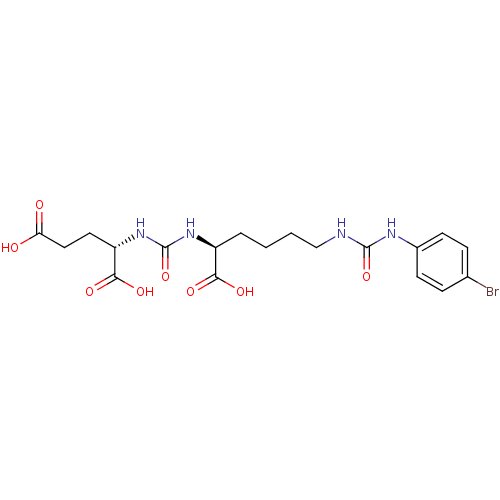
((S)-2-(3-((S)-1-Carboxy-5-(3-(4-bromophenyl)ureido...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccc(Br)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H25BrN4O8/c20-11-4-6-12(7-5-11)22-18(31)21-10-2-1-3-13(16(27)28)23-19(32)24-14(17(29)30)8-9-15(25)26/h4-7,13-14H,1-3,8-10H2,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)(H2,23,24,32)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265381
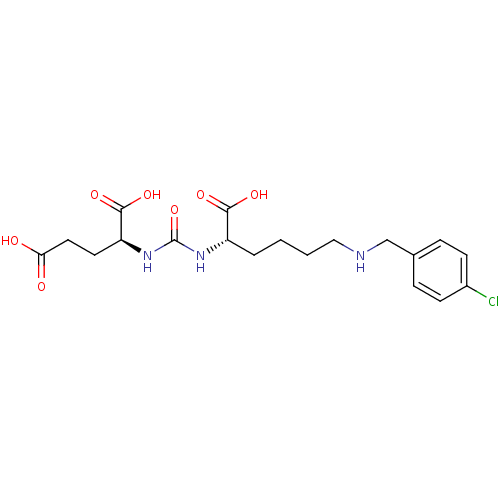
((S)-2-(3-((S)-1-Carboxy-5-(4-chlorobenzylamino)pen...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccc(Cl)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26ClN3O7/c20-13-6-4-12(5-7-13)11-21-10-2-1-3-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h4-7,14-15,21H,1-3,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265471
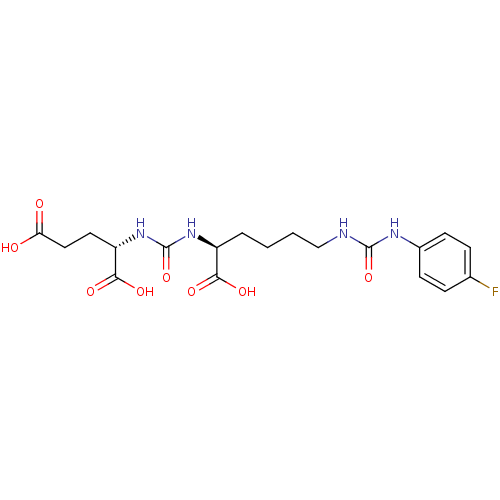
((S)-2-(3-((S)-1-Carboxy-5-(3-(4-fluorophenyl)ureid...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccc(F)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H25FN4O8/c20-11-4-6-12(7-5-11)22-18(31)21-10-2-1-3-13(16(27)28)23-19(32)24-14(17(29)30)8-9-15(25)26/h4-7,13-14H,1-3,8-10H2,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)(H2,23,24,32)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438224

(CHEMBL2407759)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cccc2nnsc12 Show InChI InChI=1S/C23H21N7O2S/c1-13(31)29-7-5-15(6-8-29)30-12-14(10-26-30)18-11-25-23(24)21-17(18)9-20(32-21)16-3-2-4-19-22(16)33-28-27-19/h2-4,9-12,15H,5-8H2,1H3,(H2,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265454
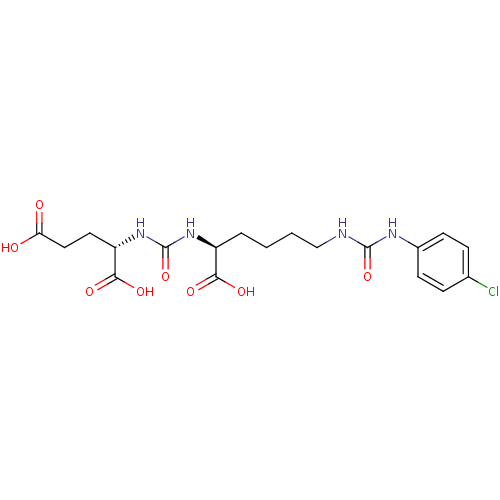
((S)-2-(3-((S)-1-Carboxy-5-(3-(4-chlorophenyl)ureid...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccc(Cl)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H25ClN4O8/c20-11-4-6-12(7-5-11)22-18(31)21-10-2-1-3-13(16(27)28)23-19(32)24-14(17(29)30)8-9-15(25)26/h4-7,13-14H,1-3,8-10H2,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)(H2,23,24,32)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438335
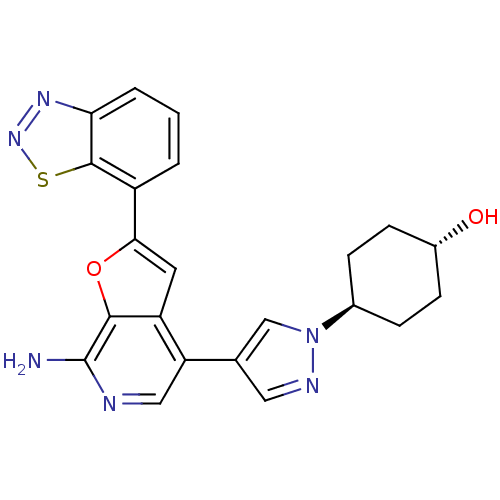
(CHEMBL2408610)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2cc(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(9.91,-9.17,;11.37,-8.7,;12.51,-9.73,;13.97,-9.25,;14.29,-7.76,;15.76,-7.28,;16.23,-5.82,;17.77,-5.82,;18.25,-7.28,;17,-8.19,;19.71,-7.76,;20.03,-9.27,;21.48,-9.75,;22.63,-8.72,;24.1,-9.21,;22.32,-7.22,;20.85,-6.73,;13.16,-6.73,;13.16,-5.19,;11.69,-4.72,;10.79,-5.97,;11.69,-7.2,;11.22,-3.25,;12.26,-2.12,;11.8,-.65,;10.29,-.32,;9.25,-1.47,;7.71,-1.46,;7.23,-2.93,;8.48,-3.83,;9.72,-2.93,)| Show InChI InChI=1S/C22H20N6O2S/c23-22-20-16(8-19(30-20)15-2-1-3-18-21(15)31-27-26-18)17(10-24-22)12-9-25-28(11-12)13-4-6-14(29)7-5-13/h1-3,8-11,13-14,29H,4-7H2,(H2,23,24)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265474

((S)-2-(3-((S)-1-Carboxy-5-(4-iodophenylsulfonamido...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNS(=O)(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C18H24IN3O9S/c19-11-4-6-12(7-5-11)32(30,31)20-10-2-1-3-13(16(25)26)21-18(29)22-14(17(27)28)8-9-15(23)24/h4-7,13-14,20H,1-3,8-10H2,(H,23,24)(H,25,26)(H,27,28)(H2,21,22,29)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265783

((S)-2-(3-((S)-1-Carboxy-5-(3-(4-iodophenyl)ureido)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H25IN4O8/c20-11-4-6-12(7-5-11)22-18(31)21-10-2-1-3-13(16(27)28)23-19(32)24-14(17(29)30)8-9-15(25)26/h4-7,13-14H,1-3,8-10H2,(H,25,26)(H,27,28)(H,29,30)(H2,21,22,31)(H2,23,24,32)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438223

(CHEMBL2407758)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cccc2ncsc12 Show InChI InChI=1S/C24H22N6O2S/c1-14(31)29-7-5-16(6-8-29)30-12-15(10-28-30)19-11-26-24(25)22-18(19)9-21(32-22)17-3-2-4-20-23(17)33-13-27-20/h2-4,9-13,16H,5-8H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265472

((S)-2-(3-((S)-1-Carboxy-5-(3-phenylureido)pentyl)u...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)Nc1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26N4O8/c24-15(25)10-9-14(17(28)29)23-19(31)22-13(16(26)27)8-4-5-11-20-18(30)21-12-6-2-1-3-7-12/h1-3,6-7,13-14H,4-5,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,20,21,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438224

(CHEMBL2407759)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cccc2nnsc12 Show InChI InChI=1S/C23H21N7O2S/c1-13(31)29-7-5-15(6-8-29)30-12-14(10-26-30)18-11-25-23(24)21-17(18)9-20(32-21)16-3-2-4-19-22(16)33-28-27-19/h2-4,9-12,15H,5-8H2,1H3,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438223

(CHEMBL2407758)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cccc2ncsc12 Show InChI InChI=1S/C24H22N6O2S/c1-14(31)29-7-5-16(6-8-29)30-12-15(10-28-30)19-11-26-24(25)22-18(19)9-21(32-22)17-3-2-4-20-23(17)33-13-27-20/h2-4,9-13,16H,5-8H2,1H3,(H2,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438329

(CHEMBL2408616)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(C(F)F)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(47.16,-24.66,;48.63,-24.19,;49.76,-25.22,;51.23,-24.74,;51.55,-23.24,;53.01,-22.77,;53.49,-21.3,;55.03,-21.3,;55.51,-22.77,;54.26,-23.67,;56.97,-23.24,;57.28,-24.76,;58.74,-25.24,;59.89,-24.21,;61.35,-24.69,;59.57,-22.7,;58.1,-22.22,;50.41,-22.22,;50.41,-20.68,;51.66,-19.77,;53.06,-20.4,;51.5,-18.24,;48.95,-20.2,;48.04,-21.45,;48.95,-22.69,;48.48,-18.74,;49.52,-17.6,;49.05,-16.14,;47.55,-15.81,;46.51,-16.95,;44.97,-16.95,;44.49,-18.41,;45.73,-19.32,;46.98,-18.42,)| Show InChI InChI=1S/C23H20F2N6O2S/c24-22(25)18-17-15(11-8-28-31(10-11)12-4-6-13(32)7-5-12)9-27-23(26)20(17)33-19(18)14-2-1-3-16-21(14)34-30-29-16/h1-3,8-10,12-13,22,32H,4-7H2,(H2,26,27)/t12-,13- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438335

(CHEMBL2408610)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2cc(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(9.91,-9.17,;11.37,-8.7,;12.51,-9.73,;13.97,-9.25,;14.29,-7.76,;15.76,-7.28,;16.23,-5.82,;17.77,-5.82,;18.25,-7.28,;17,-8.19,;19.71,-7.76,;20.03,-9.27,;21.48,-9.75,;22.63,-8.72,;24.1,-9.21,;22.32,-7.22,;20.85,-6.73,;13.16,-6.73,;13.16,-5.19,;11.69,-4.72,;10.79,-5.97,;11.69,-7.2,;11.22,-3.25,;12.26,-2.12,;11.8,-.65,;10.29,-.32,;9.25,-1.47,;7.71,-1.46,;7.23,-2.93,;8.48,-3.83,;9.72,-2.93,)| Show InChI InChI=1S/C22H20N6O2S/c23-22-20-16(8-19(30-20)15-2-1-3-18-21(15)31-27-26-18)17(10-24-22)12-9-25-28(11-12)13-4-6-14(29)7-5-13/h1-3,8-11,13-14,29H,4-7H2,(H2,23,24)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438322
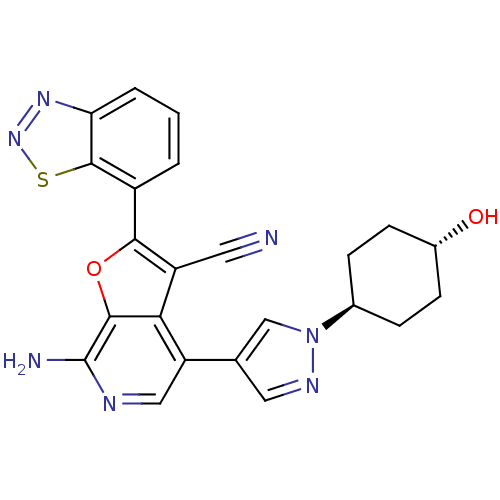
(CHEMBL2408612)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(C#N)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(47.34,-10.13,;48.81,-9.66,;49.94,-10.69,;51.41,-10.21,;51.72,-8.71,;53.19,-8.24,;53.67,-6.77,;55.21,-6.77,;55.68,-8.24,;54.44,-9.14,;57.15,-8.71,;57.46,-10.23,;58.92,-10.71,;60.07,-9.68,;61.53,-10.16,;59.75,-8.17,;58.28,-7.69,;50.59,-7.69,;50.59,-6.15,;51.83,-5.25,;53.07,-4.34,;49.13,-5.68,;48.22,-6.92,;49.13,-8.16,;48.66,-4.21,;49.7,-3.07,;49.23,-1.61,;47.72,-1.28,;46.69,-2.42,;45.15,-2.42,;44.67,-3.88,;45.91,-4.79,;47.16,-3.89,)| Show InChI InChI=1S/C23H19N7O2S/c24-8-16-19-17(12-9-27-30(11-12)13-4-6-14(31)7-5-13)10-26-23(25)21(19)32-20(16)15-2-1-3-18-22(15)33-29-28-18/h1-3,9-11,13-14,31H,4-7H2,(H2,25,26)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438331

(CHEMBL2408615)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(CF)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(28.58,-24.18,;30.05,-23.7,;31.18,-24.73,;32.65,-24.26,;32.97,-22.76,;34.43,-22.29,;34.91,-20.82,;36.45,-20.82,;36.92,-22.28,;35.68,-23.19,;38.39,-22.76,;38.7,-24.28,;40.16,-24.75,;41.31,-23.73,;42.77,-24.21,;40.99,-22.22,;39.52,-21.74,;31.83,-21.74,;31.83,-20.2,;33.08,-19.29,;32.91,-17.76,;30.37,-19.72,;29.46,-20.97,;30.37,-22.21,;29.9,-18.26,;30.94,-17.12,;30.47,-15.65,;28.96,-15.32,;27.93,-16.47,;26.39,-16.47,;25.91,-17.93,;27.15,-18.84,;28.4,-17.93,)| Show InChI InChI=1S/C23H21FN6O2S/c24-8-16-19-17(12-9-27-30(11-12)13-4-6-14(31)7-5-13)10-26-23(25)21(19)32-20(16)15-2-1-3-18-22(15)33-29-28-18/h1-3,9-11,13-14,31H,4-8H2,(H2,25,26)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438219
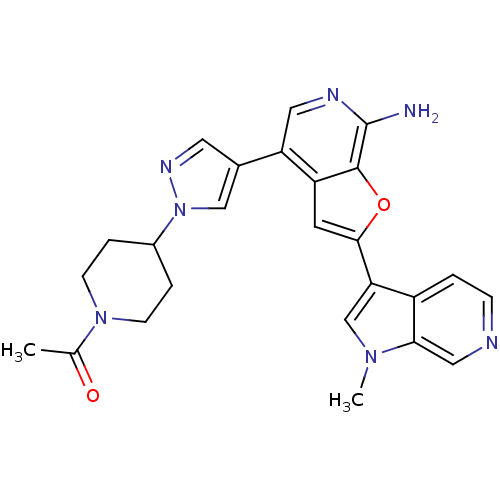
(CHEMBL2407761)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cn(C)c2cnccc12 Show InChI InChI=1S/C25H25N7O2/c1-15(33)31-7-4-17(5-8-31)32-13-16(10-29-32)20-11-28-25(26)24-19(20)9-23(34-24)21-14-30(2)22-12-27-6-3-18(21)22/h3,6,9-14,17H,4-5,7-8H2,1-2H3,(H2,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265473
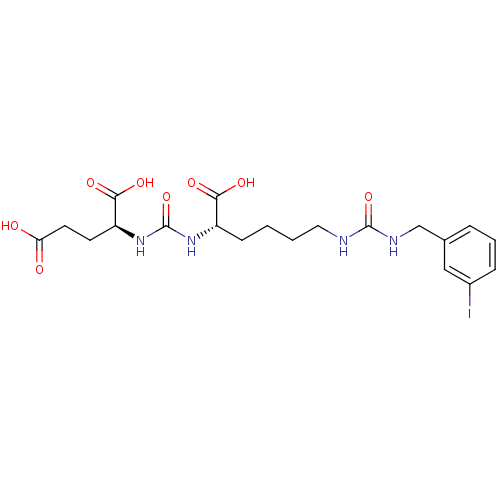
((9S,13S)-1-(3-iodophenyl)-3,11-dioxo-2,4,10,12-tet...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)NCc1cccc(I)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H27IN4O8/c21-13-5-3-4-12(10-13)11-23-19(32)22-9-2-1-6-14(17(28)29)24-20(33)25-15(18(30)31)7-8-16(26)27/h3-5,10,14-15H,1-2,6-9,11H2,(H,26,27)(H,28,29)(H,30,31)(H2,22,23,32)(H2,24,25,33)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438317

(CHEMBL2408611)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(Cl)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(28,-9.67,;29.47,-9.2,;30.61,-10.23,;32.07,-9.75,;32.39,-8.26,;33.86,-7.78,;34.33,-6.31,;35.87,-6.31,;36.35,-7.78,;35.1,-8.68,;37.81,-8.25,;38.13,-9.77,;39.58,-10.25,;40.73,-9.22,;42.2,-9.7,;40.42,-7.71,;38.95,-7.23,;31.26,-7.23,;31.25,-5.69,;32.5,-4.79,;29.79,-5.22,;28.89,-6.46,;29.79,-7.7,;29.32,-3.75,;30.36,-2.61,;29.89,-1.15,;28.39,-.82,;27.35,-1.96,;25.81,-1.96,;25.33,-3.42,;26.58,-4.33,;27.82,-3.43,)| Show InChI InChI=1S/C22H19ClN6O2S/c23-18-17-15(11-8-26-29(10-11)12-4-6-13(30)7-5-12)9-25-22(24)20(17)31-19(18)14-2-1-3-16-21(14)32-28-27-16/h1-3,8-10,12-13,30H,4-7H2,(H2,24,25)/t12-,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438329

(CHEMBL2408616)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(C(F)F)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(47.16,-24.66,;48.63,-24.19,;49.76,-25.22,;51.23,-24.74,;51.55,-23.24,;53.01,-22.77,;53.49,-21.3,;55.03,-21.3,;55.51,-22.77,;54.26,-23.67,;56.97,-23.24,;57.28,-24.76,;58.74,-25.24,;59.89,-24.21,;61.35,-24.69,;59.57,-22.7,;58.1,-22.22,;50.41,-22.22,;50.41,-20.68,;51.66,-19.77,;53.06,-20.4,;51.5,-18.24,;48.95,-20.2,;48.04,-21.45,;48.95,-22.69,;48.48,-18.74,;49.52,-17.6,;49.05,-16.14,;47.55,-15.81,;46.51,-16.95,;44.97,-16.95,;44.49,-18.41,;45.73,-19.32,;46.98,-18.42,)| Show InChI InChI=1S/C23H20F2N6O2S/c24-22(25)18-17-15(11-8-28-31(10-11)12-4-6-13(32)7-5-12)9-27-23(26)20(17)33-19(18)14-2-1-3-16-21(14)34-30-29-16/h1-3,8-10,12-13,22,32H,4-7H2,(H2,26,27)/t12-,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM103917
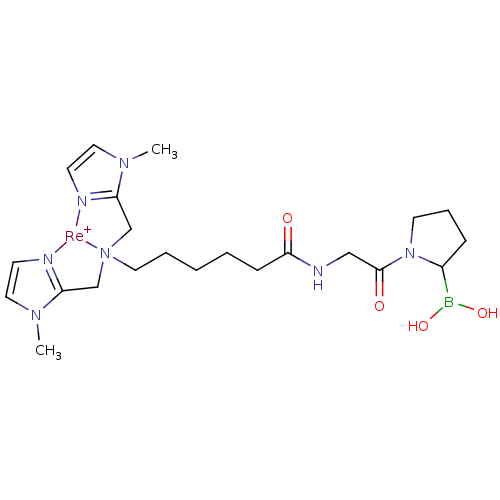
(US8562945, 253)Show SMILES CN1C=C[N]2=C1C[N]1(CCCCCC(=O)NCC(=O)N3CCCC3B(O)O)CC3=[N](C=CN3C)[Re+]21 |c:2,4,32,t:30| Show InChI InChI=1S/C22H36BN7O4.Re/c1-27-13-9-24-19(27)16-29(17-20-25-10-14-28(20)2)11-5-3-4-8-21(31)26-15-22(32)30-12-6-7-18(30)23(33)34;/h9-10,13-14,18,33-34H,3-8,11-12,15-17H2,1-2H3,(H,26,31);/q;+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit separase enzyme. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM103918
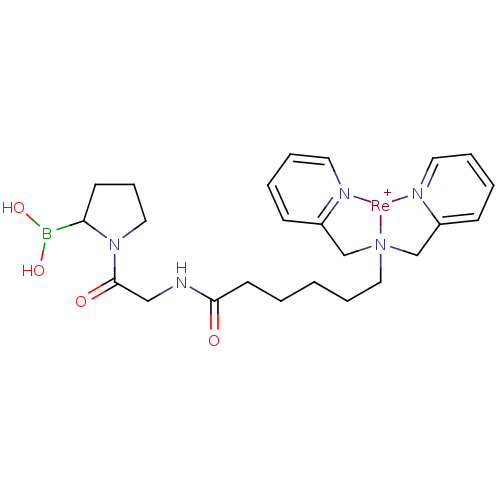
(US8562945, 254)Show SMILES OB(O)C1CCCN1C(=O)CNC(=O)CCCCC[N]12CC3=CC=CC=[N]3[Re+]1[N]1=C(C2)C=CC=C1 |c:24,26,31,35,37,t:22| Show InChI InChI=1S/C24H34BN5O4.Re/c31-23(28-17-24(32)30-16-8-11-22(30)25(33)34)12-2-1-7-15-29(18-20-9-3-5-13-26-20)19-21-10-4-6-14-27-21;/h3-6,9-10,13-14,22,33-34H,1-2,7-8,11-12,15-19H2,(H,28,31);/q;+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit separase enzyme. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265377

((S)-2-(3-((S)-1-Carboxy-5-(4-iodobenzylamino)penty...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26IN3O7/c20-13-6-4-12(5-7-13)11-21-10-2-1-3-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h4-7,14-15,21H,1-3,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM103909
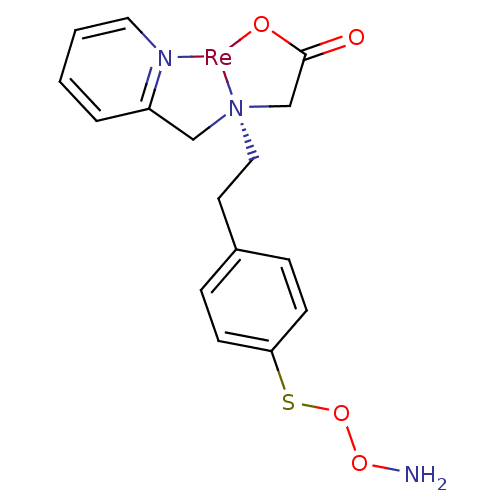
(JNK3 inhibitor 6 | US8562945, 242)Show SMILES NOOSc1ccc(CC[N@@]23CC(=O)O[Re]2[N]2=C(C3)C=CC=C2)cc1 |r,c:17,21,23| Show InChI InChI=1S/C16H19N3O4S.Re/c17-22-23-24-15-6-4-13(5-7-15)8-10-19(12-16(20)21)11-14-3-1-2-9-18-14;/h1-7,9H,8,10-12,17H2,(H,20,21);/q;+1/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438222
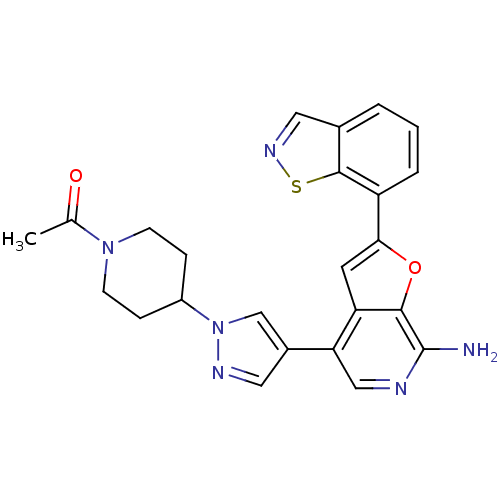
(CHEMBL2407757)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cccc2cnsc12 Show InChI InChI=1S/C24H22N6O2S/c1-14(31)29-7-5-17(6-8-29)30-13-16(10-27-30)20-12-26-24(25)22-19(20)9-21(32-22)18-4-2-3-15-11-28-33-23(15)18/h2-4,9-13,17H,5-8H2,1H3,(H2,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438317

(CHEMBL2408611)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(Cl)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(28,-9.67,;29.47,-9.2,;30.61,-10.23,;32.07,-9.75,;32.39,-8.26,;33.86,-7.78,;34.33,-6.31,;35.87,-6.31,;36.35,-7.78,;35.1,-8.68,;37.81,-8.25,;38.13,-9.77,;39.58,-10.25,;40.73,-9.22,;42.2,-9.7,;40.42,-7.71,;38.95,-7.23,;31.26,-7.23,;31.25,-5.69,;32.5,-4.79,;29.79,-5.22,;28.89,-6.46,;29.79,-7.7,;29.32,-3.75,;30.36,-2.61,;29.89,-1.15,;28.39,-.82,;27.35,-1.96,;25.81,-1.96,;25.33,-3.42,;26.58,-4.33,;27.82,-3.43,)| Show InChI InChI=1S/C22H19ClN6O2S/c23-18-17-15(11-8-26-29(10-11)12-4-6-13(30)7-5-12)9-25-22(24)20(17)31-19(18)14-2-1-3-16-21(14)32-28-27-16/h1-3,8-10,12-13,30H,4-7H2,(H2,24,25)/t12-,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human TOV21G cells assessed as inhibition of JNK phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438317

(CHEMBL2408611)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(Cl)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(28,-9.67,;29.47,-9.2,;30.61,-10.23,;32.07,-9.75,;32.39,-8.26,;33.86,-7.78,;34.33,-6.31,;35.87,-6.31,;36.35,-7.78,;35.1,-8.68,;37.81,-8.25,;38.13,-9.77,;39.58,-10.25,;40.73,-9.22,;42.2,-9.7,;40.42,-7.71,;38.95,-7.23,;31.26,-7.23,;31.25,-5.69,;32.5,-4.79,;29.79,-5.22,;28.89,-6.46,;29.79,-7.7,;29.32,-3.75,;30.36,-2.61,;29.89,-1.15,;28.39,-.82,;27.35,-1.96,;25.81,-1.96,;25.33,-3.42,;26.58,-4.33,;27.82,-3.43,)| Show InChI InChI=1S/C22H19ClN6O2S/c23-18-17-15(11-8-26-29(10-11)12-4-6-13(30)7-5-12)9-25-22(24)20(17)31-19(18)14-2-1-3-16-21(14)32-28-27-16/h1-3,8-10,12-13,30H,4-7H2,(H2,24,25)/t12-,13- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438221
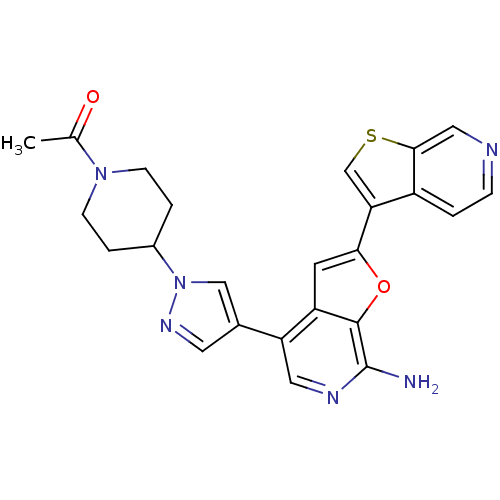
(CHEMBL2407760)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1csc2cnccc12 Show InChI InChI=1S/C24H22N6O2S/c1-14(31)29-6-3-16(4-7-29)30-12-15(9-28-30)19-10-27-24(25)23-18(19)8-21(32-23)20-13-33-22-11-26-5-2-17(20)22/h2,5,8-13,16H,3-4,6-7H2,1H3,(H2,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438330

(CHEMBL2408614)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(-c3cncs3)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(9.62,-24.57,;11.08,-24.1,;12.22,-25.13,;13.69,-24.65,;14,-23.16,;15.47,-22.68,;15.95,-21.22,;17.49,-21.22,;17.96,-22.68,;16.72,-23.59,;19.43,-23.16,;19.74,-24.67,;21.2,-25.15,;22.35,-24.12,;23.81,-24.61,;22.03,-22.62,;20.56,-22.13,;12.87,-22.13,;12.87,-20.59,;14.12,-19.69,;14.12,-18.15,;15.58,-17.67,;16.49,-18.92,;15.58,-20.16,;11.4,-20.12,;10.5,-21.37,;11.41,-22.6,;10.94,-18.65,;11.98,-17.52,;11.51,-16.05,;10,-15.72,;8.96,-16.86,;7.42,-16.86,;6.95,-18.33,;8.19,-19.23,;9.44,-18.33,)| Show InChI InChI=1S/C25H21N7O2S2/c26-25-23-20(17(9-28-25)13-8-29-32(11-13)14-4-6-15(33)7-5-14)21(19-10-27-12-35-19)22(34-23)16-2-1-3-18-24(16)36-31-30-18/h1-3,8-12,14-15,33H,4-7H2,(H2,26,28)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265378
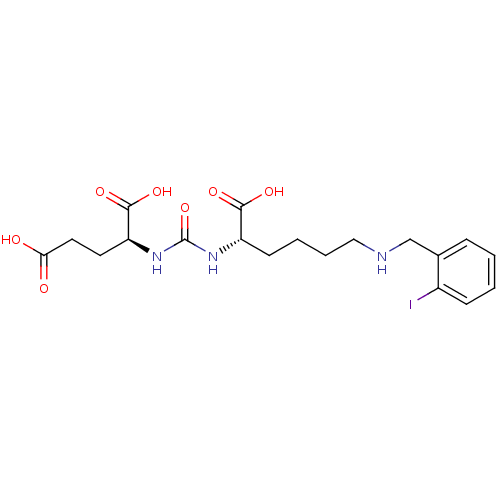
((S)-2-(3-((S)-1-Carboxy-5-(2-iodobenzylamino)penty...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccccc1I)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26IN3O7/c20-13-6-2-1-5-12(13)11-21-10-4-3-7-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h1-2,5-6,14-15,21H,3-4,7-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM103908
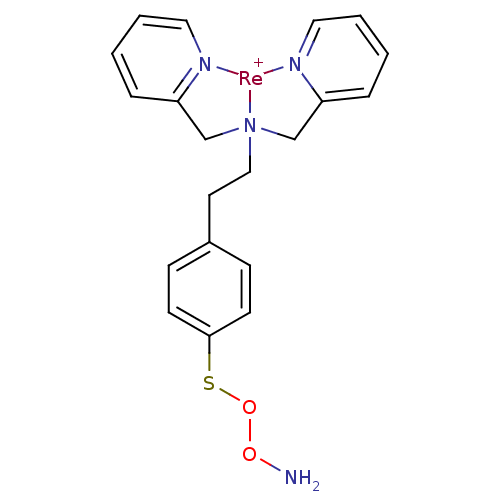
(JNK3 inhibitor 5 | US8562945, 241)Show SMILES NOOSc1ccc(CC[N]23CC4=CC=CC=[N]4[Re+]2[N]2=C(C3)C=CC=C2)cc1 |c:14,16,21,25,27,t:12| Show InChI InChI=1S/C20H22N4O2S.Re/c21-25-26-27-20-9-7-17(8-10-20)11-14-24(15-18-5-1-3-12-22-18)16-19-6-2-4-13-23-19;/h1-10,12-13H,11,14-16,21H2;/q;+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM103911
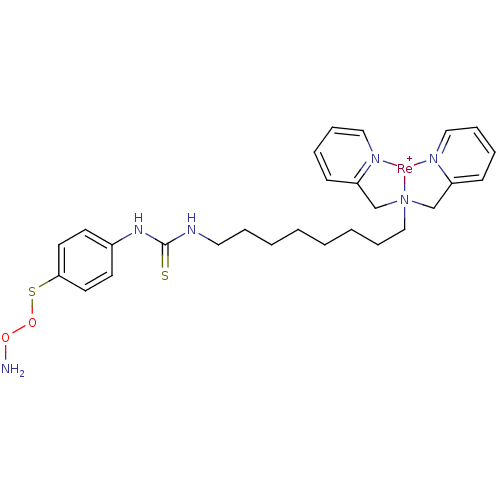
(JNK3 inhibitor 8 | US8562945, 246)Show SMILES NOOSc1ccc(NC(=S)NCCCCCCCC[N]23CC4=CC=CC=[N]4[Re+]2[N]2=C(C3)C=CC=C2)cc1 |c:24,26,31,35,37,t:22| Show InChI InChI=1S/C27H36N6O2S2.Re/c28-34-35-37-26-15-13-23(14-16-26)32-27(36)31-19-7-3-1-2-4-10-20-33(21-24-11-5-8-17-29-24)22-25-12-6-9-18-30-25;/h5-6,8-9,11-18H,1-4,7,10,19-22,28H2,(H2,31,32,36);/q;+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438330

(CHEMBL2408614)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(-c3cncs3)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(9.62,-24.57,;11.08,-24.1,;12.22,-25.13,;13.69,-24.65,;14,-23.16,;15.47,-22.68,;15.95,-21.22,;17.49,-21.22,;17.96,-22.68,;16.72,-23.59,;19.43,-23.16,;19.74,-24.67,;21.2,-25.15,;22.35,-24.12,;23.81,-24.61,;22.03,-22.62,;20.56,-22.13,;12.87,-22.13,;12.87,-20.59,;14.12,-19.69,;14.12,-18.15,;15.58,-17.67,;16.49,-18.92,;15.58,-20.16,;11.4,-20.12,;10.5,-21.37,;11.41,-22.6,;10.94,-18.65,;11.98,-17.52,;11.51,-16.05,;10,-15.72,;8.96,-16.86,;7.42,-16.86,;6.95,-18.33,;8.19,-19.23,;9.44,-18.33,)| Show InChI InChI=1S/C25H21N7O2S2/c26-25-23-20(17(9-28-25)13-8-29-32(11-13)14-4-6-15(33)7-5-14)21(19-10-27-12-35-19)22(34-23)16-2-1-3-18-24(16)36-31-30-18/h1-3,8-12,14-15,33H,4-7H2,(H2,26,28)/t14-,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265380
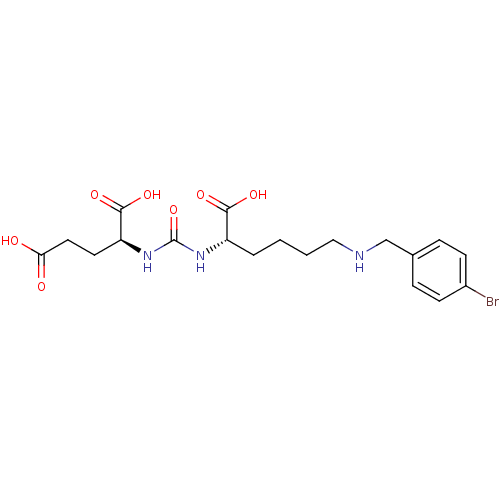
((S)-2-(3-((S)-1-Carboxy-5-(4-bromobenzylamino)pent...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1ccc(Br)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26BrN3O7/c20-13-6-4-12(5-7-13)11-21-10-2-1-3-14(17(26)27)22-19(30)23-15(18(28)29)8-9-16(24)25/h4-7,14-15,21H,1-3,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,22,23,30)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438222

(CHEMBL2407757)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cccc2cnsc12 Show InChI InChI=1S/C24H22N6O2S/c1-14(31)29-7-5-17(6-8-29)30-13-16(10-27-30)20-12-26-24(25)22-19(20)9-21(32-22)18-4-2-3-15-11-28-33-23(15)18/h2-4,9-13,17H,5-8H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438331

(CHEMBL2408615)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(CF)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(28.58,-24.18,;30.05,-23.7,;31.18,-24.73,;32.65,-24.26,;32.97,-22.76,;34.43,-22.29,;34.91,-20.82,;36.45,-20.82,;36.92,-22.28,;35.68,-23.19,;38.39,-22.76,;38.7,-24.28,;40.16,-24.75,;41.31,-23.73,;42.77,-24.21,;40.99,-22.22,;39.52,-21.74,;31.83,-21.74,;31.83,-20.2,;33.08,-19.29,;32.91,-17.76,;30.37,-19.72,;29.46,-20.97,;30.37,-22.21,;29.9,-18.26,;30.94,-17.12,;30.47,-15.65,;28.96,-15.32,;27.93,-16.47,;26.39,-16.47,;25.91,-17.93,;27.15,-18.84,;28.4,-17.93,)| Show InChI InChI=1S/C23H21FN6O2S/c24-8-16-19-17(12-9-27-30(11-12)13-4-6-14(31)7-5-13)10-26-23(25)21(19)32-20(16)15-2-1-3-18-22(15)33-29-28-18/h1-3,9-11,13-14,31H,4-8H2,(H2,25,26)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438211
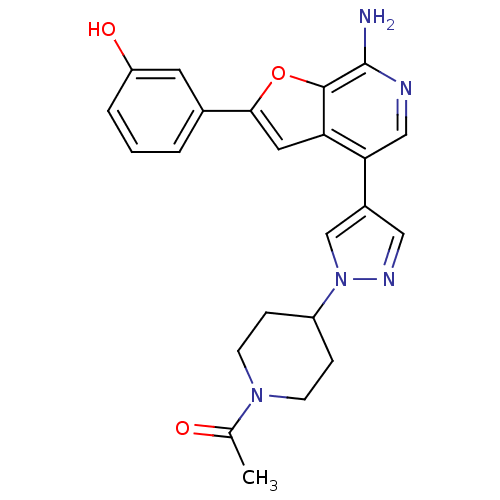
(CHEMBL2407795)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H23N5O3/c1-14(29)27-7-5-17(6-8-27)28-13-16(11-26-28)20-12-25-23(24)22-19(20)10-21(31-22)15-3-2-4-18(30)9-15/h2-4,9-13,17,30H,5-8H2,1H3,(H2,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438220
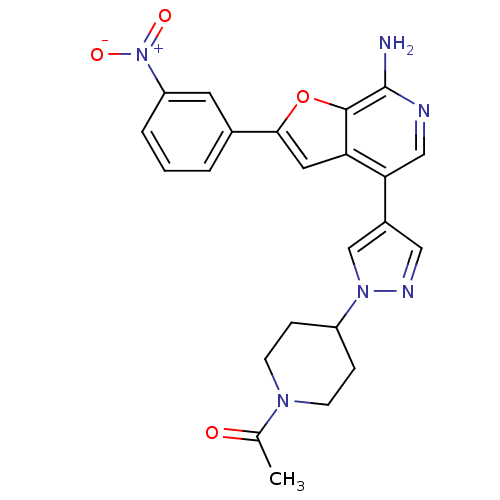
(CHEMBL2407792)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H22N6O4/c1-14(30)27-7-5-17(6-8-27)28-13-16(11-26-28)20-12-25-23(24)22-19(20)10-21(33-22)15-3-2-4-18(9-15)29(31)32/h2-4,9-13,17H,5-8H2,1H3,(H2,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438316
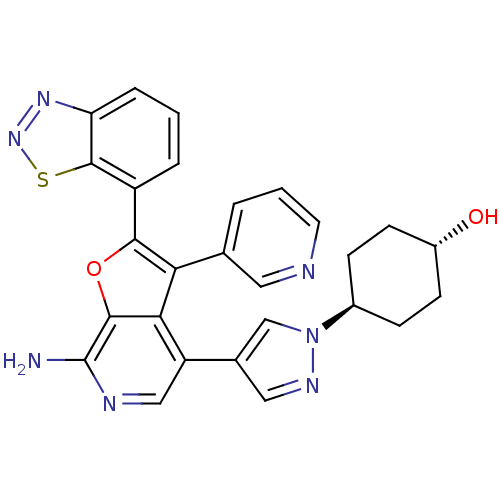
(CHEMBL2408613)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(c(oc12)-c1cccc2nnsc12)-c1cccnc1 |r,wU:13.14,wD:10.10,(-9.96,-23.67,;-8.48,-23.2,;-7.35,-24.23,;-5.89,-23.75,;-5.57,-22.25,;-4.09,-21.78,;-3.62,-20.31,;-2.08,-20.31,;-1.61,-21.78,;-2.85,-22.68,;-.15,-22.25,;.18,-23.77,;1.64,-24.25,;2.79,-23.22,;4.25,-23.7,;2.47,-21.71,;1,-21.23,;-6.71,-21.23,;-6.7,-19.69,;-8.16,-19.21,;-9.08,-20.46,;-8.17,-21.7,;-8.65,-17.74,;-7.6,-16.61,;-8.07,-15.14,;-9.56,-14.81,;-10.61,-15.96,;-12.15,-15.95,;-12.64,-17.42,;-11.39,-18.33,;-10.14,-17.42,;-5.47,-18.79,;-4.06,-19.42,;-2.82,-18.51,;-2.97,-16.98,;-4.38,-16.35,;-5.63,-17.26,)| Show InChI InChI=1S/C27H23N7O2S/c28-27-25-23(20(13-30-27)16-12-31-34(14-16)17-6-8-18(35)9-7-17)22(15-3-2-10-29-11-15)24(36-25)19-4-1-5-21-26(19)37-33-32-21/h1-5,10-14,17-18,35H,6-9H2,(H2,28,30)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438221

(CHEMBL2407760)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1csc2cnccc12 Show InChI InChI=1S/C24H22N6O2S/c1-14(31)29-6-3-16(4-7-29)30-12-15(9-28-30)19-10-27-24(25)23-18(19)8-21(32-23)20-13-33-22-11-26-5-2-17(20)22/h2,5,8-13,16H,3-4,6-7H2,1H3,(H2,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50438316

(CHEMBL2408613)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(c(oc12)-c1cccc2nnsc12)-c1cccnc1 |r,wU:13.14,wD:10.10,(-9.96,-23.67,;-8.48,-23.2,;-7.35,-24.23,;-5.89,-23.75,;-5.57,-22.25,;-4.09,-21.78,;-3.62,-20.31,;-2.08,-20.31,;-1.61,-21.78,;-2.85,-22.68,;-.15,-22.25,;.18,-23.77,;1.64,-24.25,;2.79,-23.22,;4.25,-23.7,;2.47,-21.71,;1,-21.23,;-6.71,-21.23,;-6.7,-19.69,;-8.16,-19.21,;-9.08,-20.46,;-8.17,-21.7,;-8.65,-17.74,;-7.6,-16.61,;-8.07,-15.14,;-9.56,-14.81,;-10.61,-15.96,;-12.15,-15.95,;-12.64,-17.42,;-11.39,-18.33,;-10.14,-17.42,;-5.47,-18.79,;-4.06,-19.42,;-2.82,-18.51,;-2.97,-16.98,;-4.38,-16.35,;-5.63,-17.26,)| Show InChI InChI=1S/C27H23N7O2S/c28-27-25-23(20(13-30-27)16-12-31-34(14-16)17-6-8-18(35)9-7-17)22(15-3-2-10-29-11-15)24(36-25)19-4-1-5-21-26(19)37-33-32-21/h1-5,10-14,17-18,35H,6-9H2,(H2,28,30)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438173
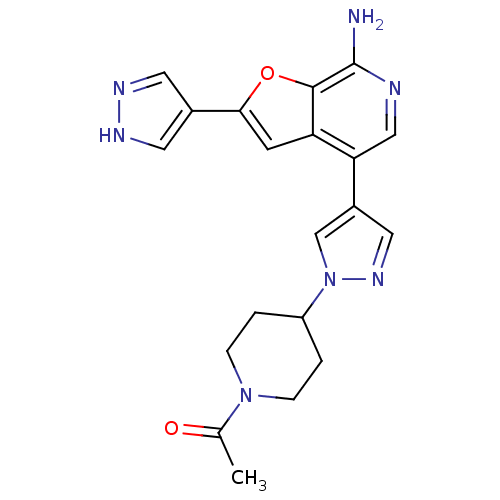
(CHEMBL2407966)Show SMILES CC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc(N)c2oc(cc12)-c1cn[nH]c1 Show InChI InChI=1S/C20H21N7O2/c1-12(28)26-4-2-15(3-5-26)27-11-14(9-25-27)17-10-22-20(21)19-16(17)6-18(29-19)13-7-23-24-8-13/h6-11,15H,2-5H2,1H3,(H2,21,22)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4517-22 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.053
BindingDB Entry DOI: 10.7270/Q2ZK5J2K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM103915
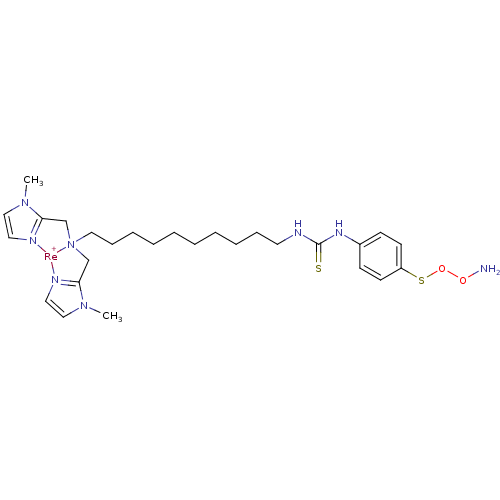
(US8562945, 250)Show SMILES CN1C=C[N]2=C1C[N]1(CCCCCCCCCCNC(=S)Nc3ccc(SOON)cc3)CC3=[N](C=CN3C)[Re+]21 |c:2,4,37,t:35| Show InChI InChI=1S/C27H42N8O2S2.Re/c1-33-19-16-29-25(33)21-35(22-26-30-17-20-34(26)2)18-10-8-6-4-3-5-7-9-15-31-27(38)32-23-11-13-24(14-12-23)39-37-36-28;/h11-14,16-17,19-20H,3-10,15,18,21-22,28H2,1-2H3,(H2,31,32,38);/q;+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM103911

(JNK3 inhibitor 8 | US8562945, 246)Show SMILES NOOSc1ccc(NC(=S)NCCCCCCCC[N]23CC4=CC=CC=[N]4[Re+]2[N]2=C(C3)C=CC=C2)cc1 |c:24,26,31,35,37,t:22| Show InChI InChI=1S/C27H36N6O2S2.Re/c28-34-35-37-26-15-13-23(14-16-26)32-27(36)31-19-7-3-1-2-4-10-20-33(21-24-11-5-8-17-29-24)22-25-12-6-9-18-30-25;/h5-6,8-9,11-18H,1-4,7,10,19-22,28H2,(H2,31,32,36);/q;+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438325
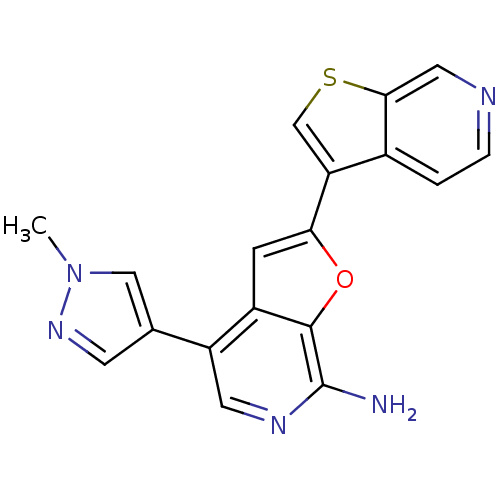
(CHEMBL2408617)Show SMILES Cn1cc(cn1)-c1cnc(N)c2oc(cc12)-c1csc2cnccc12 Show InChI InChI=1S/C18H13N5OS/c1-23-8-10(5-22-23)13-6-21-18(19)17-12(13)4-15(24-17)14-9-25-16-7-20-3-2-11(14)16/h2-9H,1H3,(H2,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50265489
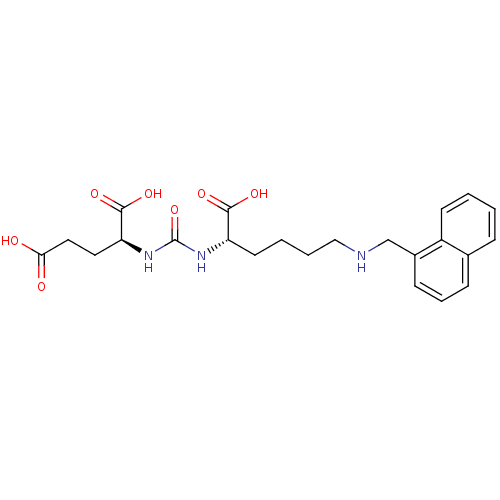
((S)-2-(3-((S)-1-Carboxy-5-(naphthalen-1-ylmethylam...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNCc1cccc2ccccc12)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H29N3O7/c27-20(28)12-11-19(22(31)32)26-23(33)25-18(21(29)30)10-3-4-13-24-14-16-8-5-7-15-6-1-2-9-17(15)16/h1-2,5-9,18-19,24H,3-4,10-14H2,(H,27,28)(H,29,30)(H,31,32)(H2,25,26,33)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [131I]DCIT from PSMA in human LNCAP cells |
J Med Chem 52: 347-57 (2009)
Article DOI: 10.1021/jm800994j
BindingDB Entry DOI: 10.7270/Q2V987ZZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438322

(CHEMBL2408612)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(C#N)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(47.34,-10.13,;48.81,-9.66,;49.94,-10.69,;51.41,-10.21,;51.72,-8.71,;53.19,-8.24,;53.67,-6.77,;55.21,-6.77,;55.68,-8.24,;54.44,-9.14,;57.15,-8.71,;57.46,-10.23,;58.92,-10.71,;60.07,-9.68,;61.53,-10.16,;59.75,-8.17,;58.28,-7.69,;50.59,-7.69,;50.59,-6.15,;51.83,-5.25,;53.07,-4.34,;49.13,-5.68,;48.22,-6.92,;49.13,-8.16,;48.66,-4.21,;49.7,-3.07,;49.23,-1.61,;47.72,-1.28,;46.69,-2.42,;45.15,-2.42,;44.67,-3.88,;45.91,-4.79,;47.16,-3.89,)| Show InChI InChI=1S/C23H19N7O2S/c24-8-16-19-17(12-9-27-30(11-12)13-4-6-14(31)7-5-13)10-26-23(25)21(19)32-20(16)15-2-1-3-18-22(15)33-29-28-18/h1-3,9-11,13-14,31H,4-7H2,(H2,25,26)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50438335

(CHEMBL2408610)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2cc(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(9.91,-9.17,;11.37,-8.7,;12.51,-9.73,;13.97,-9.25,;14.29,-7.76,;15.76,-7.28,;16.23,-5.82,;17.77,-5.82,;18.25,-7.28,;17,-8.19,;19.71,-7.76,;20.03,-9.27,;21.48,-9.75,;22.63,-8.72,;24.1,-9.21,;22.32,-7.22,;20.85,-6.73,;13.16,-6.73,;13.16,-5.19,;11.69,-4.72,;10.79,-5.97,;11.69,-7.2,;11.22,-3.25,;12.26,-2.12,;11.8,-.65,;10.29,-.32,;9.25,-1.47,;7.71,-1.46,;7.23,-2.93,;8.48,-3.83,;9.72,-2.93,)| Show InChI InChI=1S/C22H20N6O2S/c23-22-20-16(8-19(30-20)15-2-1-3-18-21(15)31-27-26-18)17(10-24-22)12-9-25-28(11-12)13-4-6-14(29)7-5-13/h1-3,8-11,13-14,29H,4-7H2,(H2,23,24)/t13-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of aurora B kinase in human HT-29 cells assessed as inhibition of histone H3 S10 phosphorylation |
Bioorg Med Chem Lett 23: 4511-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.054
BindingDB Entry DOI: 10.7270/Q2FN17MJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM103909

(JNK3 inhibitor 6 | US8562945, 242)Show SMILES NOOSc1ccc(CC[N@@]23CC(=O)O[Re]2[N]2=C(C3)C=CC=C2)cc1 |r,c:17,21,23| Show InChI InChI=1S/C16H19N3O4S.Re/c17-22-23-24-15-6-4-13(5-7-15)8-10-19(12-16(20)21)11-14-3-1-2-9-18-14;/h1-7,9H,8,10-12,17H2,(H,20,21);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM103914
(US8562945, 249)Show SMILES NOOSc1ccc(NC(=S)NCCCCCCCC[N@@]23CC(=O)O[Re]2[N]2=C(C3)C=CC=C2)cc1 |r,c:27,31,33| Show InChI InChI=1S/C23H33N5O4S2.Re/c24-31-32-34-21-12-10-19(11-13-21)27-23(33)26-15-6-3-1-2-4-8-16-28(18-22(29)30)17-20-9-5-7-14-25-20;/h5,7,9-14H,1-4,6,8,15-18,24H2,(H,29,30)(H2,26,27,33);/q;+1/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data