 Found 419 hits with Last Name = 'marshall' and Initial = 'j'
Found 419 hits with Last Name = 'marshall' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50072996
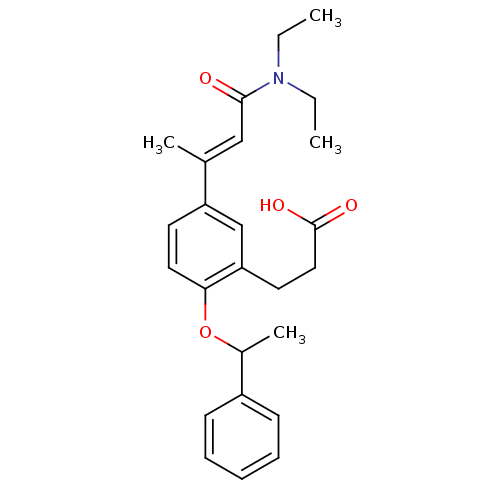
(3-[5-((E)-2-Diethylcarbamoyl-1-methyl-vinyl)-2-(1-...)Show SMILES CCN(CC)C(=O)\C=C(/C)c1ccc(OC(C)c2ccccc2)c(CCC(O)=O)c1 Show InChI InChI=1S/C25H31NO4/c1-5-26(6-2)24(27)16-18(3)21-12-14-23(22(17-21)13-15-25(28)29)30-19(4)20-10-8-7-9-11-20/h7-12,14,16-17,19H,5-6,13,15H2,1-4H3,(H,28,29)/b18-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Leukotriene B4 receptor antagonistic activity was measured by the inhibition of LTB4 induced [Ca2+] release from human PMNs |
J Med Chem 42: 164-72 (1999)
Article DOI: 10.1021/jm980540v
BindingDB Entry DOI: 10.7270/Q26972QG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6/beta-2
(Homo sapiens (Human)) | BDBM50481208

(CHEMBL594330)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C65H104N22O21S4/c1-28(2)11-34(52(94)73-31(7)51(93)75-36(13-32-18-69-26-71-32)55(97)81-40(20-88)57(99)79-38(15-47(67)90)56(98)77-35(12-29(3)4)53(95)85-45(25-112)65(107)108)76-54(96)37(14-33-19-70-27-72-33)78-60(102)44(24-111)84-63(105)50(30(5)6)86-62(104)46-9-8-10-87(46)64(106)39(16-48(68)91)80-58(100)41(21-89)82-61(103)43(23-110)83-59(101)42(22-109)74-49(92)17-66/h18-19,26-31,34-46,50,88-89,109-112H,8-17,20-25,66H2,1-7H3,(H2,67,90)(H2,68,91)(H,69,71)(H,70,72)(H,73,94)(H,74,92)(H,75,93)(H,76,96)(H,77,98)(H,78,102)(H,79,99)(H,80,100)(H,81,97)(H,82,103)(H,83,101)(H,84,105)(H,85,95)(H,86,104)(H,107,108)/t31-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,50-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha6beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6/beta-2
(Homo sapiens (Human)) | BDBM50481207
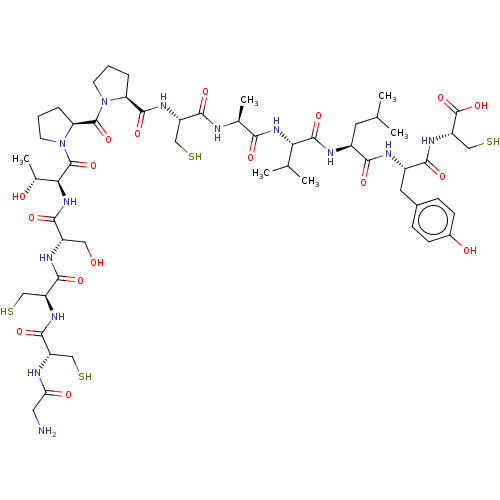
(CHEMBL595286)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C54H85N13O17S4/c1-25(2)17-31(44(73)58-32(18-29-11-13-30(70)14-12-29)45(74)63-37(24-88)54(83)84)59-51(80)41(26(3)4)64-43(72)27(5)56-47(76)35(22-86)62-50(79)38-9-7-15-66(38)52(81)39-10-8-16-67(39)53(82)42(28(6)69)65-46(75)33(20-68)60-49(78)36(23-87)61-48(77)34(21-85)57-40(71)19-55/h11-14,25-28,31-39,41-42,68-70,85-88H,7-10,15-24,55H2,1-6H3,(H,56,76)(H,57,71)(H,58,73)(H,59,80)(H,60,78)(H,61,77)(H,62,79)(H,63,74)(H,64,72)(H,65,75)(H,83,84)/t27-,28+,31-,32-,33-,34-,35-,36-,37-,38-,39-,41-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha6beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6/beta-2
(Homo sapiens (Human)) | BDBM50481206

(CHEMBL595311)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C67H106N22O23S4/c1-29(2)12-35(54(98)76-34(9-10-51(95)96)53(97)79-38(15-33-20-72-28-74-33)57(101)83-41(21-90)59(103)81-39(16-48(69)92)58(102)78-36(13-30(3)4)55(99)87-46(26-116)67(111)112)77-56(100)37(14-32-19-71-27-73-32)80-62(106)45(25-115)86-65(109)52(31(5)6)88-64(108)47-8-7-11-89(47)66(110)40(17-49(70)93)82-60(104)42(22-91)84-63(107)44(24-114)85-61(105)43(23-113)75-50(94)18-68/h19-20,27-31,34-47,52,90-91,113-116H,7-18,21-26,68H2,1-6H3,(H2,69,92)(H2,70,93)(H,71,73)(H,72,74)(H,75,94)(H,76,98)(H,77,100)(H,78,102)(H,79,97)(H,80,106)(H,81,103)(H,82,104)(H,83,101)(H,84,107)(H,85,105)(H,86,109)(H,87,99)(H,88,108)(H,95,96)(H,111,112)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha6beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Homo sapiens (Human)) | BDBM50481206

(CHEMBL595311)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C67H106N22O23S4/c1-29(2)12-35(54(98)76-34(9-10-51(95)96)53(97)79-38(15-33-20-72-28-74-33)57(101)83-41(21-90)59(103)81-39(16-48(69)92)58(102)78-36(13-30(3)4)55(99)87-46(26-116)67(111)112)77-56(100)37(14-32-19-71-27-73-32)80-62(106)45(25-115)86-65(109)52(31(5)6)88-64(108)47-8-7-11-89(47)66(110)40(17-49(70)93)82-60(104)42(22-91)84-63(107)44(24-114)85-61(105)43(23-113)75-50(94)18-68/h19-20,27-31,34-47,52,90-91,113-116H,7-18,21-26,68H2,1-6H3,(H2,69,92)(H2,70,93)(H,71,73)(H,72,74)(H,75,94)(H,76,98)(H,77,100)(H,78,102)(H,79,97)(H,80,106)(H,81,103)(H,82,104)(H,83,101)(H,84,107)(H,85,105)(H,86,109)(H,87,99)(H,88,108)(H,95,96)(H,111,112)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha3beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360024

(CHEMBL1928541)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O4S/c1-12-3-4-15(23(24)25)8-17(12)28(26,27)21(2)19-10-14-11-20-22-6-5-13(9-18)7-16(14)22/h3-8,10-11H,1-2H3/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6/beta-2
(Homo sapiens (Human)) | BDBM50481209

(CHEMBL595047)Show SMILES [H][C@](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCCNC(N)=N)C(C)C)[C@@H](C)O)C(C)C)([C@@H](C)CC)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C79H128N26O26S4/c1-8-36(6)59(73(125)98-49(32-135)78(130)131)101-62(114)40(17-18-53(81)108)89-68(120)50-14-10-20-103(50)75(127)42(24-54(82)109)92-63(115)41(23-38-27-86-33-88-38)90-71(123)58(35(4)5)100-74(126)60(37(7)107)102-67(119)48(31-134)97-72(124)57(34(2)3)99-70(122)52-16-12-22-105(52)76(128)43(25-55(83)110)93-64(116)45(28-106)94-65(117)46(29-132)95-66(118)47(30-133)96-69(121)51-15-11-21-104(51)77(129)44(26-56(111)112)91-61(113)39(80)13-9-19-87-79(84)85/h27,33-37,39-52,57-60,106-107,132-135H,8-26,28-32,80H2,1-7H3,(H2,81,108)(H2,82,109)(H2,83,110)(H,86,88)(H,89,120)(H,90,123)(H,91,113)(H,92,115)(H,93,116)(H,94,117)(H,95,118)(H,96,121)(H,97,124)(H,98,125)(H,99,122)(H,100,126)(H,101,114)(H,102,119)(H,111,112)(H,130,131)(H4,84,85,87)/t36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,57-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha6beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Homo sapiens (Human)) | BDBM50481212
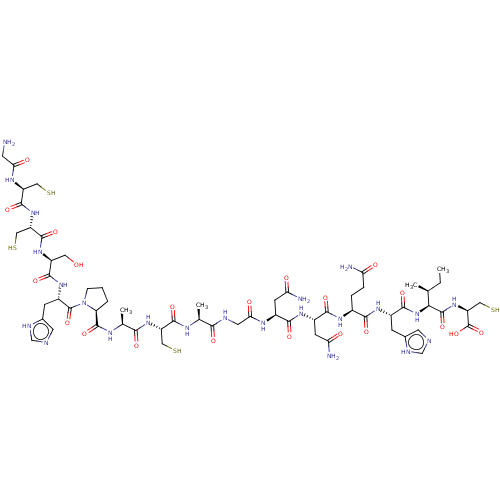
(CHEMBL609593)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C61H95N23O21S4/c1-5-26(2)47(59(102)82-40(23-109)61(104)105)83-53(96)32(11-29-16-66-24-69-29)76-50(93)31(8-9-42(63)86)75-52(95)34(14-44(65)88)77-51(94)33(13-43(64)87)73-46(90)18-68-48(91)27(3)71-55(98)38(21-107)80-49(92)28(4)72-58(101)41-7-6-10-84(41)60(103)35(12-30-17-67-25-70-30)78-54(97)36(19-85)79-57(100)39(22-108)81-56(99)37(20-106)74-45(89)15-62/h16-17,24-28,31-41,47,85,106-109H,5-15,18-23,62H2,1-4H3,(H2,63,86)(H2,64,87)(H2,65,88)(H,66,69)(H,67,70)(H,68,91)(H,71,98)(H,72,101)(H,73,90)(H,74,89)(H,75,95)(H,76,93)(H,77,94)(H,78,97)(H,79,100)(H,80,92)(H,81,99)(H,82,102)(H,83,96)(H,104,105)/t26-,27-,28-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,47-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha3beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360040

(CHEMBL1928536)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#C)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C18H15N5O4S/c1-4-14-7-8-22-17(9-14)15(12-20-22)11-19-21(3)28(26,27)18-10-16(23(24)25)6-5-13(18)2/h1,5-12H,2-3H3/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360037

(CHEMBL1928533)Show SMILES CN(\N=C\c1cnn2ccc(Cl)cc12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14ClN5O4S/c1-11-3-4-14(22(23)24)8-16(11)27(25,26)20(2)18-9-12-10-19-21-6-5-13(17)7-15(12)21/h3-10H,1-2H3/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-6/beta-2
(Homo sapiens (Human)) | BDBM50481212

(CHEMBL609593)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C61H95N23O21S4/c1-5-26(2)47(59(102)82-40(23-109)61(104)105)83-53(96)32(11-29-16-66-24-69-29)76-50(93)31(8-9-42(63)86)75-52(95)34(14-44(65)88)77-51(94)33(13-43(64)87)73-46(90)18-68-48(91)27(3)71-55(98)38(21-107)80-49(92)28(4)72-58(101)41-7-6-10-84(41)60(103)35(12-30-17-67-25-70-30)78-54(97)36(19-85)79-57(100)39(22-108)81-56(99)37(20-106)74-45(89)15-62/h16-17,24-28,31-41,47,85,106-109H,5-15,18-23,62H2,1-4H3,(H2,63,86)(H2,64,87)(H2,65,88)(H,66,69)(H,67,70)(H,68,91)(H,71,98)(H,72,101)(H,73,90)(H,74,89)(H,75,95)(H,76,93)(H,77,94)(H,78,97)(H,79,100)(H,80,92)(H,81,99)(H,82,102)(H,83,96)(H,104,105)/t26-,27-,28-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,47-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha6beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Homo sapiens (Human)) | BDBM50481211

(CHEMBL595312)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C65H107N21O22S4/c1-6-30(4)49(60(103)72-31(5)50(93)75-34(20-45(67)88)52(95)78-36(21-46(68)89)62(105)84-16-8-12-42(84)58(101)77-35(22-48(91)92)53(96)76-33(19-29(2)3)51(94)82-41(28-112)64(107)108)83-57(100)40(27-111)81-59(102)43-13-9-17-85(43)63(106)44-14-10-18-86(44)61(104)32(11-7-15-71-65(69)70)74-54(97)37(24-87)79-56(99)39(26-110)80-55(98)38(25-109)73-47(90)23-66/h29-44,49,87,109-112H,6-28,66H2,1-5H3,(H2,67,88)(H2,68,89)(H,72,103)(H,73,90)(H,74,97)(H,75,93)(H,76,96)(H,77,101)(H,78,95)(H,79,99)(H,80,98)(H,81,102)(H,82,94)(H,83,100)(H,91,92)(H,107,108)(H4,69,70,71)/t30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha3beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360028

(CHEMBL1928525)Show SMILES CN(\N=C\c1cnn2ccc(Br)cc12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-4-14(22(23)24)8-16(11)27(25,26)20(2)18-9-12-10-19-21-6-5-13(17)7-15(12)21/h3-10H,1-2H3/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447094
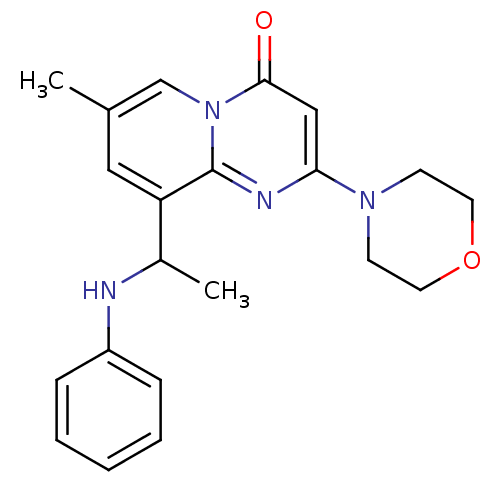
(CHEMBL1972466)Show InChI InChI=1S/C21H24N4O2/c1-15-12-18(16(2)22-17-6-4-3-5-7-17)21-23-19(13-20(26)25(21)14-15)24-8-10-27-11-9-24/h3-7,12-14,16,22H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human p110beta expressed in Sf9 cells co-expressing p85alpha regulatory subunit using phosphatidylinositol substr... |
Bioorg Med Chem 23: 3796-808 (2015)
Article DOI: 10.1016/j.bmc.2015.03.073
BindingDB Entry DOI: 10.7270/Q29K4D0R |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Homo sapiens (Human)) | BDBM50481207

(CHEMBL595286)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C54H85N13O17S4/c1-25(2)17-31(44(73)58-32(18-29-11-13-30(70)14-12-29)45(74)63-37(24-88)54(83)84)59-51(80)41(26(3)4)64-43(72)27(5)56-47(76)35(22-86)62-50(79)38-9-7-15-66(38)52(81)39-10-8-16-67(39)53(82)42(28(6)69)65-46(75)33(20-68)60-49(78)36(23-87)61-48(77)34(21-85)57-40(71)19-55/h11-14,25-28,31-39,41-42,68-70,85-88H,7-10,15-24,55H2,1-6H3,(H,56,76)(H,57,71)(H,58,73)(H,59,80)(H,60,78)(H,61,77)(H,62,79)(H,63,74)(H,64,72)(H,65,75)(H,83,84)/t27-,28+,31-,32-,33-,34-,35-,36-,37-,38-,39-,41-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha3beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360050
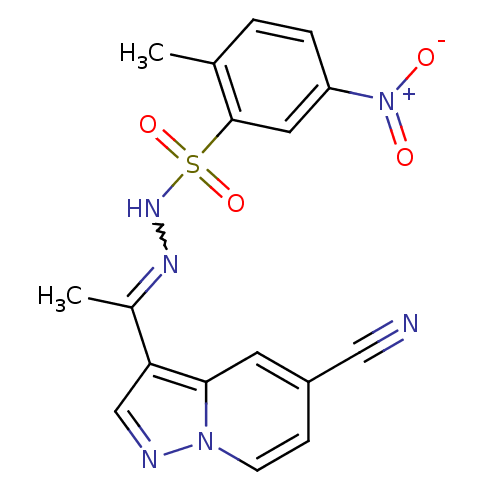
(CHEMBL1928547)Show SMILES CC(=NNS(=O)(=O)c1cc(ccc1C)[N+]([O-])=O)c1cnn2ccc(cc12)C#N |w:2.2| Show InChI InChI=1S/C17H14N6O4S/c1-11-3-4-14(23(24)25)8-17(11)28(26,27)21-20-12(2)15-10-19-22-6-5-13(9-18)7-16(15)22/h3-8,10,21H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Homo sapiens (Human)) | BDBM50481208

(CHEMBL594330)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C65H104N22O21S4/c1-28(2)11-34(52(94)73-31(7)51(93)75-36(13-32-18-69-26-71-32)55(97)81-40(20-88)57(99)79-38(15-47(67)90)56(98)77-35(12-29(3)4)53(95)85-45(25-112)65(107)108)76-54(96)37(14-33-19-70-27-72-33)78-60(102)44(24-111)84-63(105)50(30(5)6)86-62(104)46-9-8-10-87(46)64(106)39(16-48(68)91)80-58(100)41(21-89)82-61(103)43(23-110)83-59(101)42(22-109)74-49(92)17-66/h18-19,26-31,34-46,50,88-89,109-112H,8-17,20-25,66H2,1-7H3,(H2,67,90)(H2,68,91)(H,69,71)(H,70,72)(H,73,94)(H,74,92)(H,75,93)(H,76,96)(H,77,98)(H,78,102)(H,79,99)(H,80,100)(H,81,97)(H,82,103)(H,83,101)(H,84,105)(H,85,95)(H,86,104)(H,107,108)/t31-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,50-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha3beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360051
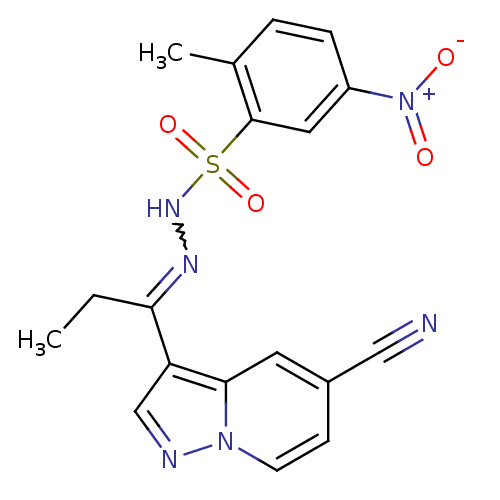
(CHEMBL1928548)Show SMILES CCC(=NNS(=O)(=O)c1cc(ccc1C)[N+]([O-])=O)c1cnn2ccc(cc12)C#N |w:3.3| Show InChI InChI=1S/C18H16N6O4S/c1-3-16(15-11-20-23-7-6-13(10-19)8-17(15)23)21-22-29(27,28)18-9-14(24(25)26)5-4-12(18)2/h4-9,11,22H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Homo sapiens (Human)) | BDBM50481210
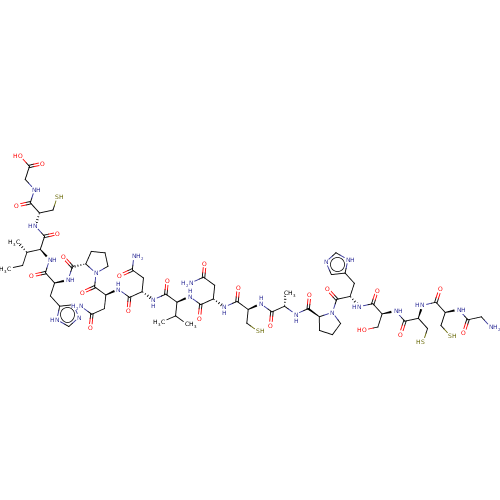
(CHEMBL609594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](CS)C(=O)NCC(O)=O |r| Show InChI InChI=1S/C67H104N24O22S4/c1-6-30(4)52(65(111)87-40(23-114)54(100)74-21-50(97)98)89-56(102)34(13-32-19-72-27-75-32)80-63(109)45-10-8-12-91(45)67(113)38(17-48(71)95)83-55(101)35(15-46(69)93)81-64(110)51(29(2)3)88-57(103)36(16-47(70)94)79-60(106)42(25-116)85-53(99)31(5)77-62(108)44-9-7-11-90(44)66(112)37(14-33-20-73-28-76-33)82-58(104)39(22-92)84-61(107)43(26-117)86-59(105)41(24-115)78-49(96)18-68/h19-20,27-31,34-45,51-52,92,114-117H,6-18,21-26,68H2,1-5H3,(H2,69,93)(H2,70,94)(H2,71,95)(H,72,75)(H,73,76)(H,74,100)(H,77,108)(H,78,96)(H,79,106)(H,80,109)(H,81,110)(H,82,104)(H,83,101)(H,84,107)(H,85,99)(H,86,105)(H,87,111)(H,88,103)(H,89,102)(H,97,98)/t30-,31-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha3beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360049
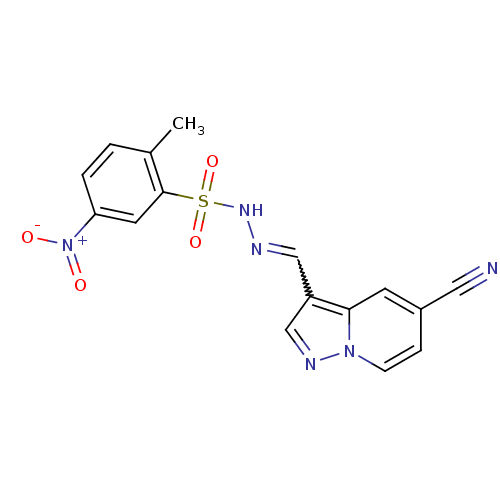
(CHEMBL1928546)Show SMILES Cc1ccc(cc1S(=O)(=O)NN=Cc1cnn2ccc(cc12)C#N)[N+]([O-])=O |w:12.13| Show InChI InChI=1S/C16H12N6O4S/c1-11-2-3-14(22(23)24)7-16(11)27(25,26)20-18-9-13-10-19-21-5-4-12(8-17)6-15(13)21/h2-7,9-10,20H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50360024

(CHEMBL1928541)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O4S/c1-12-3-4-15(23(24)25)8-17(12)28(26,27)21(2)19-10-14-11-20-22-6-5-13(9-18)7-16(14)22/h3-8,10-11H,1-2H3/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110gamma |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360036

(CHEMBL1926704)Show SMILES CN(\N=C\c1cnn2ccc(I)cc12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14IN5O4S/c1-11-3-4-14(22(23)24)8-16(11)27(25,26)20(2)18-9-12-10-19-21-6-5-13(17)7-15(12)21/h3-10H,1-2H3/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50093493
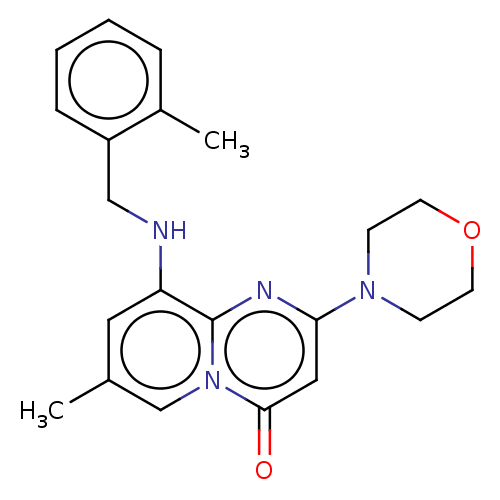
(CHEMBL3218574)Show InChI InChI=1S/C13H20N6O4/c1-2-3-15-10-7-11(18-13(14)17-10)19(5-16-7)12-9(22)8(21)6(4-20)23-12/h5-6,8-9,12,20-22H,2-4H2,1H3,(H3,14,15,17,18)/t6?,8-,9-,12?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human p110beta expressed in Sf9 cells co-expressing p85alpha regulatory subunit using phosphatidylinositol substr... |
Bioorg Med Chem 23: 3796-808 (2015)
Article DOI: 10.1016/j.bmc.2015.03.073
BindingDB Entry DOI: 10.7270/Q29K4D0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50093518
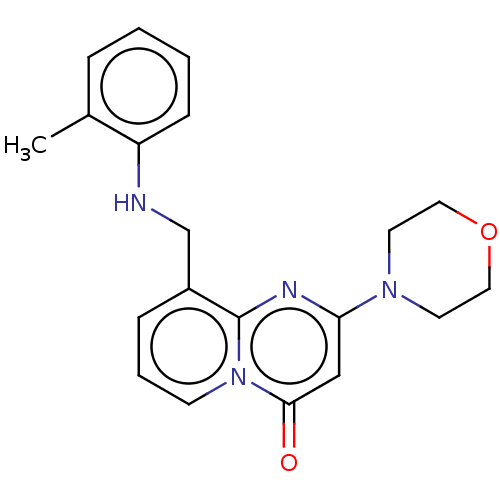
(CHEMBL3585584)Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9?,11-,12-,15?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human p110beta expressed in Sf9 cells co-expressing p85alpha regulatory subunit using phosphatidylinositol substr... |
Bioorg Med Chem 23: 3796-808 (2015)
Article DOI: 10.1016/j.bmc.2015.03.073
BindingDB Entry DOI: 10.7270/Q29K4D0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50093515
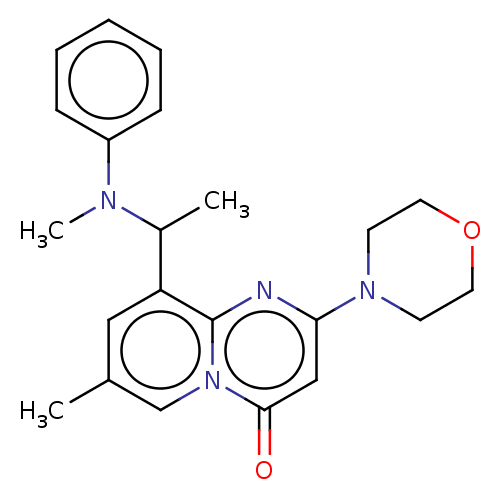
(CHEMBL3585581)Show SMILES CC(N(C)c1ccccc1)c1cc(C)cn2c1nc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C15H22N6O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H3,16,18,19,20)/t8?,10-,11-,14?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human p110beta expressed in Sf9 cells co-expressing p85alpha regulatory subunit using phosphatidylinositol substr... |
Bioorg Med Chem 23: 3796-808 (2015)
Article DOI: 10.1016/j.bmc.2015.03.073
BindingDB Entry DOI: 10.7270/Q29K4D0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50093511
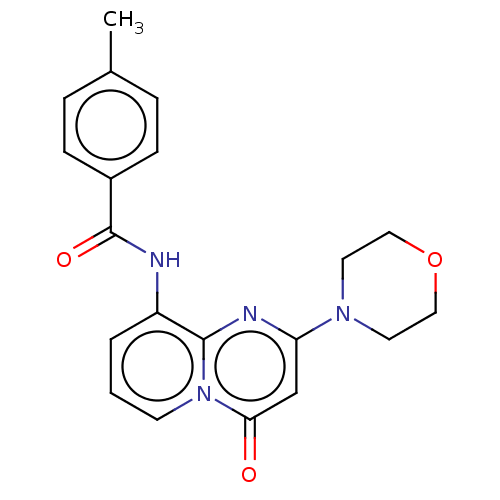
(CHEMBL3585619)Show SMILES Cc1ccc(cc1)C(=O)Nc1cccn2c1nc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C19H24N6O5/c1-29-11-4-2-3-10(7-11)5-6-21-16-13-17(24-19(20)23-16)25(9-22-13)18-15(28)14(27)12(8-26)30-18/h2-4,7,9,12,14-15,18,26-28H,5-6,8H2,1H3,(H3,20,21,23,24)/t12?,14-,15-,18?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human p110delta expressed in Sf9 cells co-expressing p85alpha regulatory subunit using phosphatidylinositol subst... |
Bioorg Med Chem 23: 3796-808 (2015)
Article DOI: 10.1016/j.bmc.2015.03.073
BindingDB Entry DOI: 10.7270/Q29K4D0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50093517

(CHEMBL3585583)Show InChI InChI=1S/C15H18N6O5/c16-12-9-13(20-15(19-12)17-4-7-2-1-3-25-7)21(6-18-9)14-11(24)10(23)8(5-22)26-14/h1-3,6,8,10-11,14,22-24H,4-5H2,(H3,16,17,19,20)/t8?,10-,11-,14?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human p110beta expressed in Sf9 cells co-expressing p85alpha regulatory subunit using phosphatidylinositol substr... |
Bioorg Med Chem 23: 3796-808 (2015)
Article DOI: 10.1016/j.bmc.2015.03.073
BindingDB Entry DOI: 10.7270/Q29K4D0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50093516
(CHEMBL3585582)Show InChI InChI=1S/C26H30N6O4/c33-15-19-21(34)22(35)25(36-19)32-16-29-20-23(27-13-11-17-7-3-1-4-8-17)30-26(31-24(20)32)28-14-12-18-9-5-2-6-10-18/h1-10,16,19,21-22,25,33-35H,11-15H2,(H2,27,28,30,31)/t19?,21-,22-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human p110beta expressed in Sf9 cells co-expressing p85alpha regulatory subunit using phosphatidylinositol substr... |
Bioorg Med Chem 23: 3796-808 (2015)
Article DOI: 10.1016/j.bmc.2015.03.073
BindingDB Entry DOI: 10.7270/Q29K4D0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50360040

(CHEMBL1928536)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#C)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C18H15N5O4S/c1-4-14-7-8-22-17(9-14)15(12-20-22)11-19-21(3)28(26,27)18-10-16(23(24)25)6-5-13(18)2/h1,5-12H,2-3H3/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110beta using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographic... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360053

(CHEMBL1928550)Show SMILES CC(=NNS(=O)(=O)c1cc(ccc1C)[N+]([O-])=O)c1cnn2ccc(Br)cc12 |w:2.2| Show InChI InChI=1S/C16H14BrN5O4S/c1-10-3-4-13(22(23)24)8-16(10)27(25,26)20-19-11(2)14-9-18-21-6-5-12(17)7-15(14)21/h3-9,20H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360052
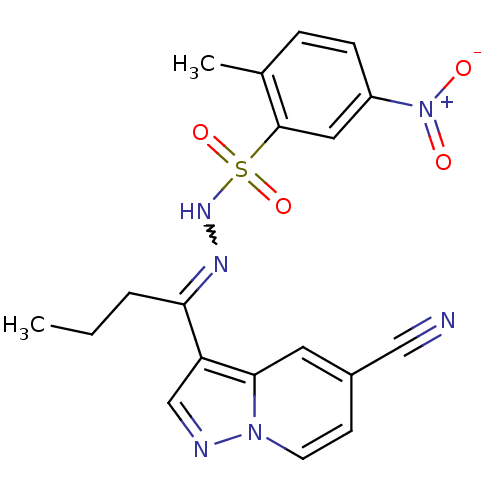
(CHEMBL1928549)Show SMILES CCCC(=NNS(=O)(=O)c1cc(ccc1C)[N+]([O-])=O)c1cnn2ccc(cc12)C#N |w:4.4| Show InChI InChI=1S/C19H18N6O4S/c1-3-4-17(16-12-21-24-8-7-14(11-20)9-18(16)24)22-23-30(28,29)19-10-15(25(26)27)6-5-13(19)2/h5-10,12,23H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50360040

(CHEMBL1928536)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#C)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C18H15N5O4S/c1-4-14-7-8-22-17(9-14)15(12-20-22)11-19-21(3)28(26,27)18-10-16(23(24)25)6-5-13(18)2/h1,5-12H,2-3H3/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110delta using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360045

(CHEMBL1928542)Show SMILES CN(\N=C\c1cnn2cc(F)c(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H13FN6O4S/c1-11-3-4-14(24(25)26)6-17(11)29(27,28)22(2)20-8-13-9-21-23-10-15(18)12(7-19)5-16(13)23/h3-6,8-10H,1-2H3/b20-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50360024

(CHEMBL1928541)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O4S/c1-12-3-4-15(23(24)25)8-17(12)28(26,27)21(2)19-10-14-11-20-22-6-5-13(9-18)7-16(14)22/h3-8,10-11H,1-2H3/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110beta using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographic... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50360024

(CHEMBL1928541)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O4S/c1-12-3-4-15(23(24)25)8-17(12)28(26,27)21(2)19-10-14-11-20-22-6-5-13(9-18)7-16(14)22/h3-8,10-11H,1-2H3/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110delta using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Homo sapiens (Human)) | BDBM50481209

(CHEMBL595047)Show SMILES [H][C@](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCCNC(N)=N)C(C)C)[C@@H](C)O)C(C)C)([C@@H](C)CC)C(=O)N[C@@H](CS)C(O)=O |r| Show InChI InChI=1S/C79H128N26O26S4/c1-8-36(6)59(73(125)98-49(32-135)78(130)131)101-62(114)40(17-18-53(81)108)89-68(120)50-14-10-20-103(50)75(127)42(24-54(82)109)92-63(115)41(23-38-27-86-33-88-38)90-71(123)58(35(4)5)100-74(126)60(37(7)107)102-67(119)48(31-134)97-72(124)57(34(2)3)99-70(122)52-16-12-22-105(52)76(128)43(25-55(83)110)93-64(116)45(28-106)94-65(117)46(29-132)95-66(118)47(30-133)96-69(121)51-15-11-21-104(51)77(129)44(26-56(111)112)91-61(113)39(80)13-9-19-87-79(84)85/h27,33-37,39-52,57-60,106-107,132-135H,8-26,28-32,80H2,1-7H3,(H2,81,108)(H2,82,109)(H2,83,110)(H,86,88)(H,89,120)(H,90,123)(H,91,113)(H,92,115)(H,93,116)(H,94,117)(H,95,118)(H,96,121)(H,97,124)(H,98,125)(H,99,122)(H,100,126)(H,101,114)(H,102,119)(H,111,112)(H,130,131)(H4,84,85,87)/t36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,57-,58-,59-,60-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Boise State University
Curated by ChEMBL
| Assay Description
Inhibition of alpha3beta2 nAChR |
Bioorg Med Chem 17: 5894-9 (2009)
Article DOI: 10.1016/j.bmc.2009.07.005
BindingDB Entry DOI: 10.7270/Q2474DN8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360054
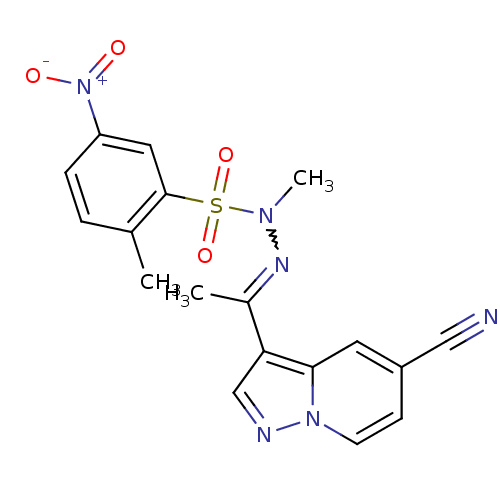
(CHEMBL1928551)Show SMILES CN(N=C(C)c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O |w:2.1| Show InChI InChI=1S/C18H16N6O4S/c1-12-4-5-15(24(25)26)9-18(12)29(27,28)22(3)21-13(2)16-11-20-23-7-6-14(10-19)8-17(16)23/h4-9,11H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50360037

(CHEMBL1928533)Show SMILES CN(\N=C\c1cnn2ccc(Cl)cc12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14ClN5O4S/c1-11-3-4-14(22(23)24)8-16(11)27(25,26)20(2)18-9-12-10-19-21-6-5-13(17)7-15(12)21/h3-10H,1-2H3/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110delta using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
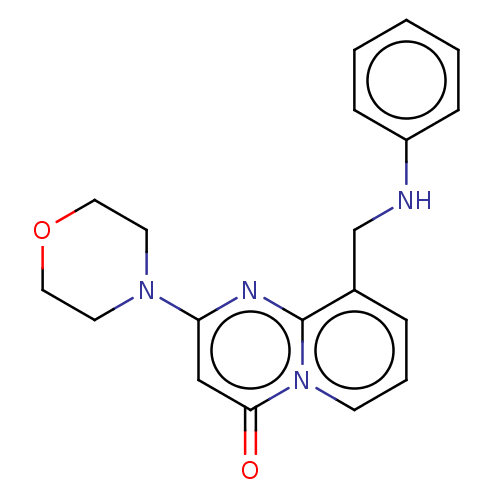
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501736
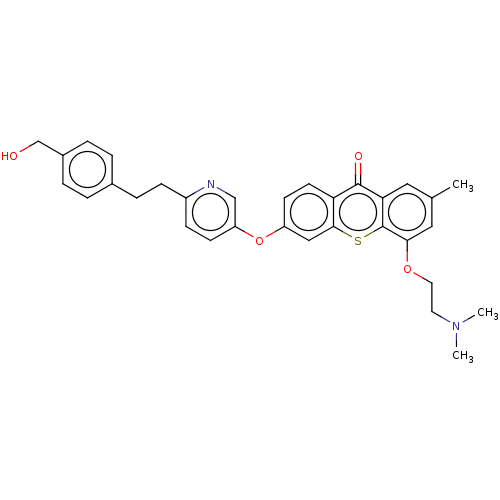
(4-(2-(Dimethylamino)ethoxy)-6-((6-(4-methoxyphenet...)Show SMILES CN(C)CCOc1cc(C)cc2c1sc1cc(Oc3ccc(CCc4ccc(CO)cc4)nc3)ccc1c2=O Show InChI InChI=1S/C32H32N2O4S/c1-21-16-28-31(36)27-13-12-25(18-30(27)39-32(28)29(17-21)37-15-14-34(2)3)38-26-11-10-24(33-19-26)9-8-22-4-6-23(20-35)7-5-22/h4-7,10-13,16-19,35H,8-9,14-15,20H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501737
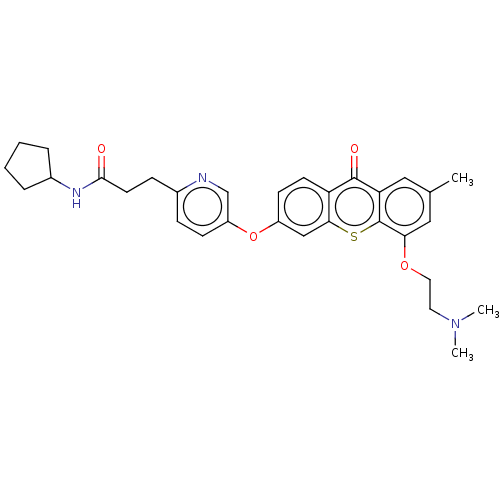
(N-Cyclopentyl-3-(5-((5-(2-(dimethylamino)ethoxy)-7...)Show SMILES CN(C)CCOc1cc(C)cc2c1sc1cc(Oc3ccc(CCC(=O)NC4CCCC4)nc3)ccc1c2=O Show InChI InChI=1S/C31H35N3O4S/c1-20-16-26-30(36)25-12-11-23(18-28(25)39-31(26)27(17-20)37-15-14-34(2)3)38-24-10-8-21(32-19-24)9-13-29(35)33-22-6-4-5-7-22/h8,10-12,16-19,22H,4-7,9,13-15H2,1-3H3,(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501740
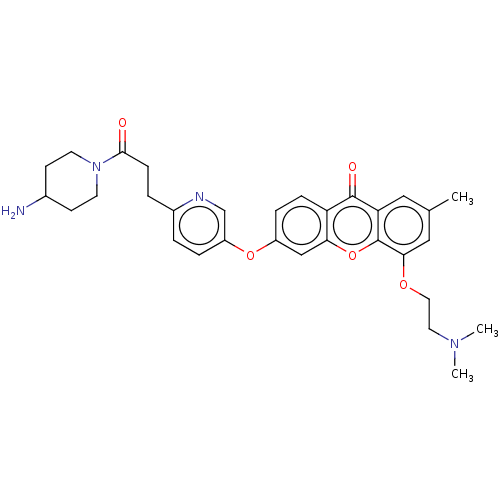
(4-(2-(Dimethylamino)ethoxy)-2-methyl-6-((6-(3-(4-m...)Show SMILES CN(C)CCOc1cc(C)cc2c1oc1cc(Oc3ccc(CCC(=O)N4CCC(N)CC4)nc3)ccc1c2=O Show InChI InChI=1S/C31H36N4O5/c1-20-16-26-30(37)25-8-7-23(18-27(25)40-31(26)28(17-20)38-15-14-34(2)3)39-24-6-4-22(33-19-24)5-9-29(36)35-12-10-21(32)11-13-35/h4,6-8,16-19,21H,5,9-15,32H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501741
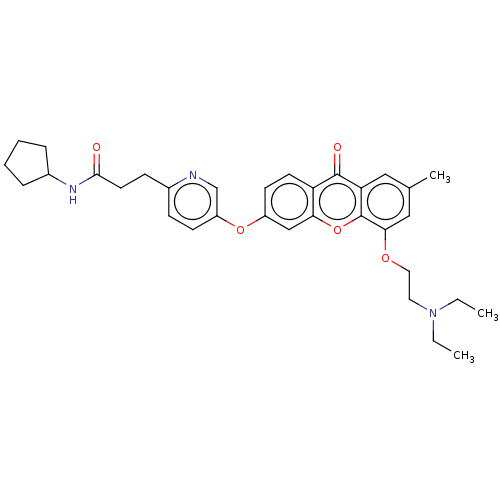
(N-Cyclopentyl-3-(5-((5-(2-(diethylamino)ethoxy)-7-...)Show SMILES CCN(CC)CCOc1cc(C)cc2c1oc1cc(Oc3ccc(CCC(=O)NC4CCCC4)nc3)ccc1c2=O Show InChI InChI=1S/C33H39N3O5/c1-4-36(5-2)16-17-39-30-19-22(3)18-28-32(38)27-14-13-25(20-29(27)41-33(28)30)40-26-12-10-23(34-21-26)11-15-31(37)35-24-8-6-7-9-24/h10,12-14,18-21,24H,4-9,11,15-17H2,1-3H3,(H,35,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501742
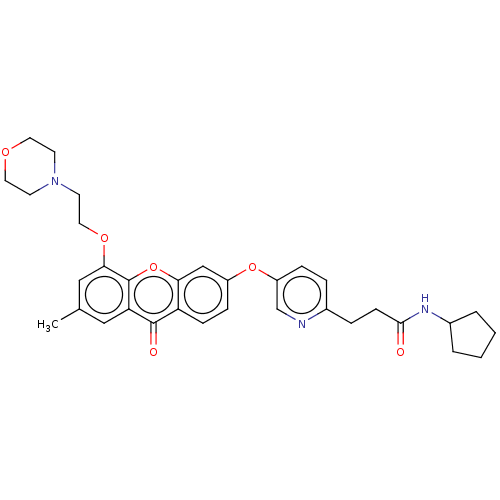
(N-Cyclopentyl-3-[5-({7-methyl-5-[2-(4-morpholinyl)...)Show SMILES Cc1cc(OCCN2CCOCC2)c2oc3cc(Oc4ccc(CCC(=O)NC5CCCC5)nc4)ccc3c(=O)c2c1 Show InChI InChI=1S/C33H37N3O6/c1-22-18-28-32(38)27-10-9-25(20-29(27)42-33(28)30(19-22)40-17-14-36-12-15-39-16-13-36)41-26-8-6-23(34-21-26)7-11-31(37)35-24-4-2-3-5-24/h6,8-10,18-21,24H,2-5,7,11-17H2,1H3,(H,35,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501743
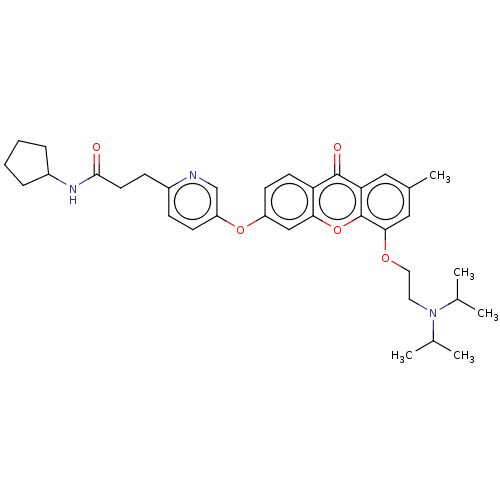
(N-Cyclopentyl-3-[5-({5-[2-(diisopropylamino)ethoxy...)Show SMILES CC(C)N(CCOc1cc(C)cc2c1oc1cc(Oc3ccc(CCC(=O)NC4CCCC4)nc3)ccc1c2=O)C(C)C Show InChI InChI=1S/C35H43N3O5/c1-22(2)38(23(3)4)16-17-41-32-19-24(5)18-30-34(40)29-14-13-27(20-31(29)43-35(30)32)42-28-12-10-25(36-21-28)11-15-33(39)37-26-8-6-7-9-26/h10,12-14,18-23,26H,6-9,11,15-17H2,1-5H3,(H,37,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501744
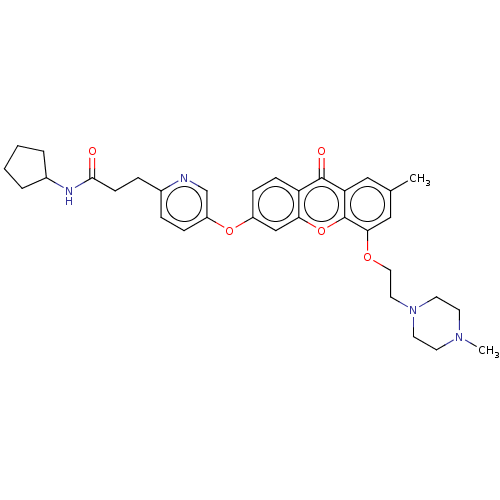
(N-Cyclopentyl-3-(5-((7-methyl-5-(2-(4-methylpipera...)Show SMILES CN1CCN(CCOc2cc(C)cc3c2oc2cc(Oc4ccc(CCC(=O)NC5CCCC5)nc4)ccc2c3=O)CC1 Show InChI InChI=1S/C34H40N4O5/c1-23-19-29-33(40)28-11-10-26(42-27-9-7-24(35-22-27)8-12-32(39)36-25-5-3-4-6-25)21-30(28)43-34(29)31(20-23)41-18-17-38-15-13-37(2)14-16-38/h7,9-11,19-22,25H,3-6,8,12-18H2,1-2H3,(H,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501745
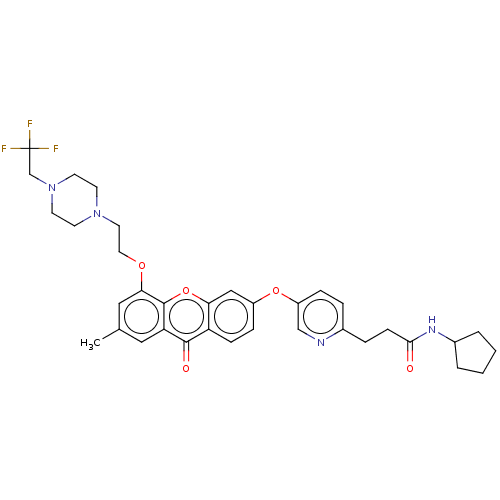
(N-Cyclopentyl-3-(5-((7-methyl-9-oxo-5-(2-(4-(2,2,2...)Show SMILES Cc1cc(OCCN2CCN(CC(F)(F)F)CC2)c2oc3cc(Oc4ccc(CCC(=O)NC5CCCC5)nc4)ccc3c(=O)c2c1 Show InChI InChI=1S/C35H39F3N4O5/c1-23-18-29-33(44)28-10-9-26(46-27-8-6-24(39-21-27)7-11-32(43)40-25-4-2-3-5-25)20-30(28)47-34(29)31(19-23)45-17-16-41-12-14-42(15-13-41)22-35(36,37)38/h6,8-10,18-21,25H,2-5,7,11-17,22H2,1H3,(H,40,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501746
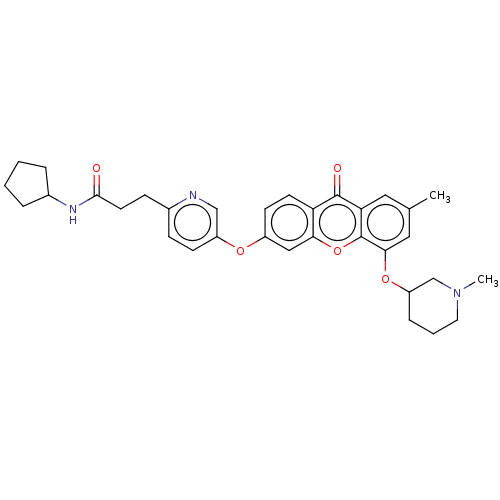
(N-Cyclopentyl-3-(5-((7-methyl-5-((1-methylpiperidi...)Show SMILES CN1CCCC(C1)Oc1cc(C)cc2c1oc1cc(Oc3ccc(CCC(=O)NC4CCCC4)nc3)ccc1c2=O Show InChI InChI=1S/C33H37N3O5/c1-21-16-28-32(38)27-13-12-24(18-29(27)41-33(28)30(17-21)40-26-8-5-15-36(2)20-26)39-25-11-9-22(34-19-25)10-14-31(37)35-23-6-3-4-7-23/h9,11-13,16-19,23,26H,3-8,10,14-15,20H2,1-2H3,(H,35,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501747
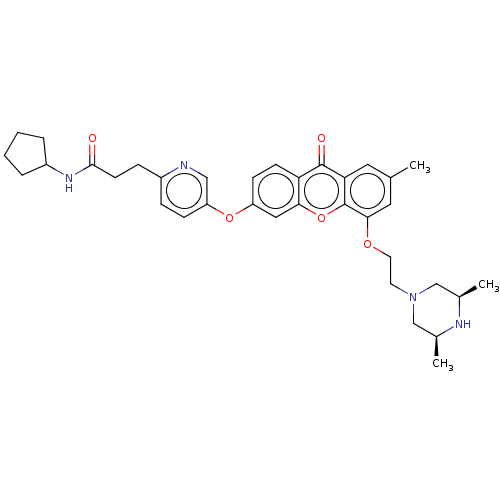
(N-Cyclopentyl-3-(5-((5-(2-((3S,5R)-3,5-dimethylpip...)Show SMILES C[C@H]1CN(CCOc2cc(C)cc3c2oc2cc(Oc4ccc(CCC(=O)NC5CCCC5)nc4)ccc2c3=O)C[C@@H](C)N1 |r| Show InChI InChI=1S/C35H42N4O5/c1-22-16-30-34(41)29-12-11-27(43-28-10-8-25(36-19-28)9-13-33(40)38-26-6-4-5-7-26)18-31(29)44-35(30)32(17-22)42-15-14-39-20-23(2)37-24(3)21-39/h8,10-12,16-19,23-24,26,37H,4-7,9,13-15,20-21H2,1-3H3,(H,38,40)/t23-,24+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501748
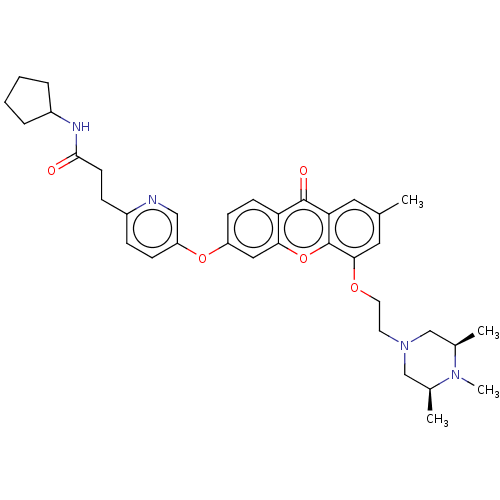
(N-Cyclopentyl-3-(5-((7-methyl-9-oxo-5-(2-((3S,5R)-...)Show SMILES C[C@H]1CN(CCOc2cc(C)cc3c2oc2cc(Oc4ccc(CCC(=O)NC5CCCC5)nc4)ccc2c3=O)C[C@@H](C)N1C |r| Show InChI InChI=1S/C36H44N4O5/c1-23-17-31-35(42)30-13-12-28(44-29-11-9-26(37-20-29)10-14-34(41)38-27-7-5-6-8-27)19-32(30)45-36(31)33(18-23)43-16-15-40-21-24(2)39(4)25(3)22-40/h9,11-13,17-20,24-25,27H,5-8,10,14-16,21-22H2,1-4H3,(H,38,41)/t24-,25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM501749
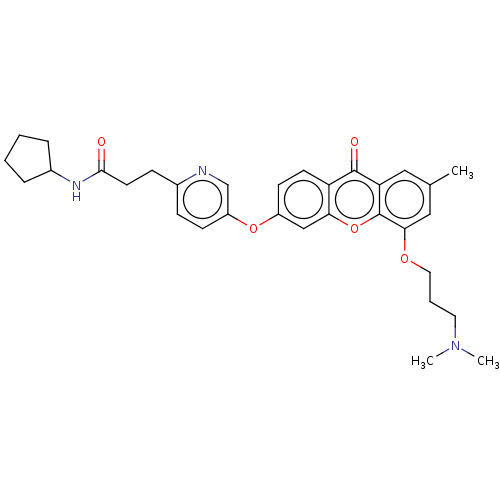
(N-Cyclopentyl-3-(5-((5-(3-(dimethylamino)propoxy)-...)Show SMILES CN(C)CCCOc1cc(C)cc2c1oc1cc(Oc3ccc(CCC(=O)NC4CCCC4)nc3)ccc1c2=O Show InChI InChI=1S/C32H37N3O5/c1-21-17-27-31(37)26-13-12-24(19-28(26)40-32(27)29(18-21)38-16-6-15-35(2)3)39-25-11-9-22(33-20-25)10-14-30(36)34-23-7-4-5-8-23/h9,11-13,17-20,23H,4-8,10,14-16H2,1-3H3,(H,34,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
AUCKLAND UNISERVICES LIMITED
US Patent
| Assay Description
IC50 values were determined using the Z′-LYTEŽ activity assay, with ATP concentration used at Km app for the specific assay (500 μM ATP fo... |
US Patent US11028064 (2021)
BindingDB Entry DOI: 10.7270/Q2ZS30PB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data