

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50454212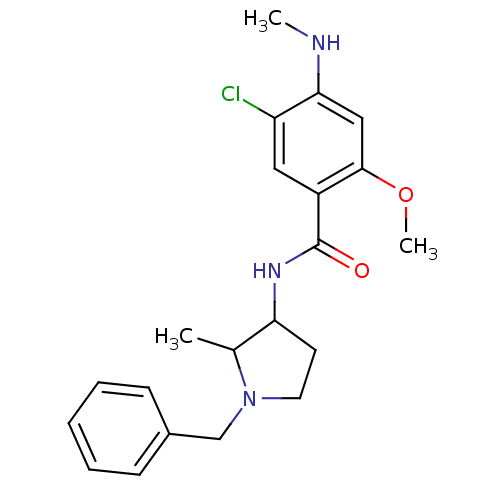 (CHEBI:64217 | Emonapride | Nemonapride) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50454212 (CHEBI:64217 | Emonapride | Nemonapride) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052196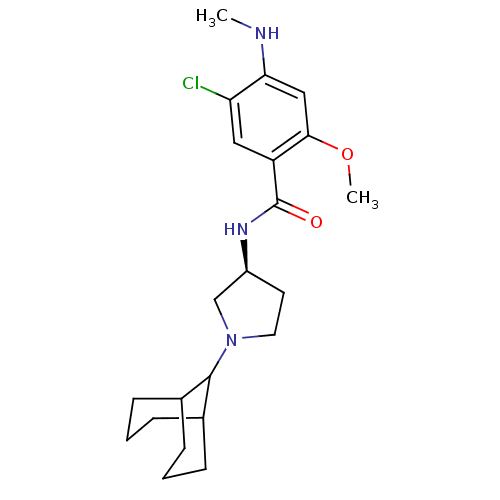 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052194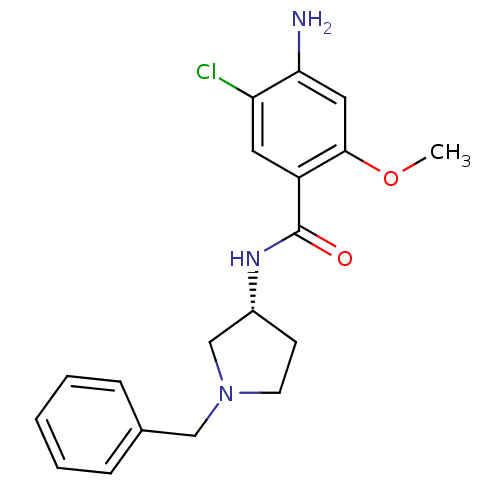 (4-Amino-N-((R)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052196 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052196 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052194 (4-Amino-N-((R)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052186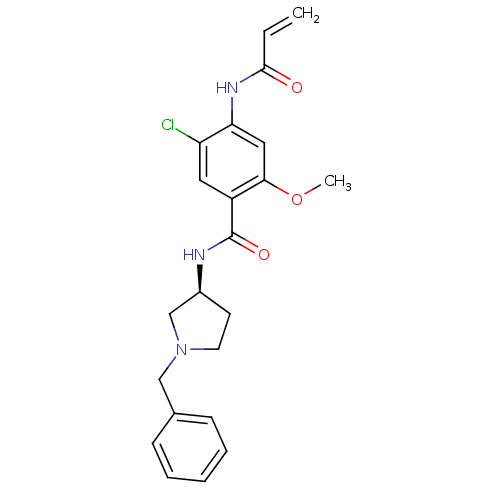 (4-Acryloylamino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052195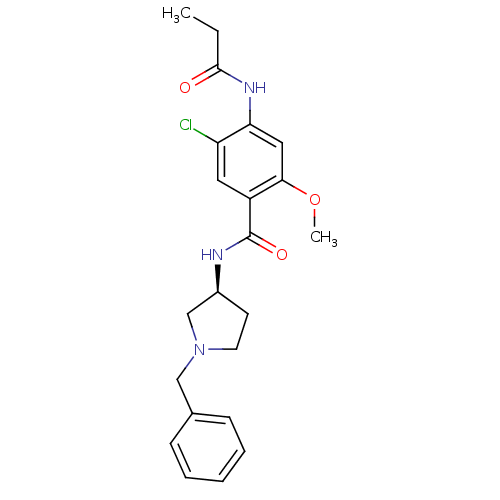 (CHEMBL93198 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052184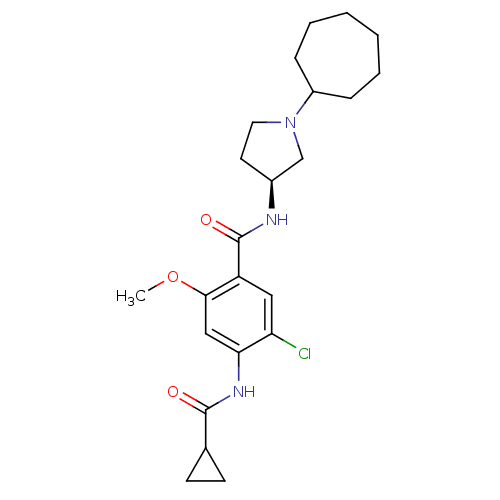 (5-Chloro-N-((S)-1-cycloheptyl-pyrrolidin-3-yl)-4-(...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052181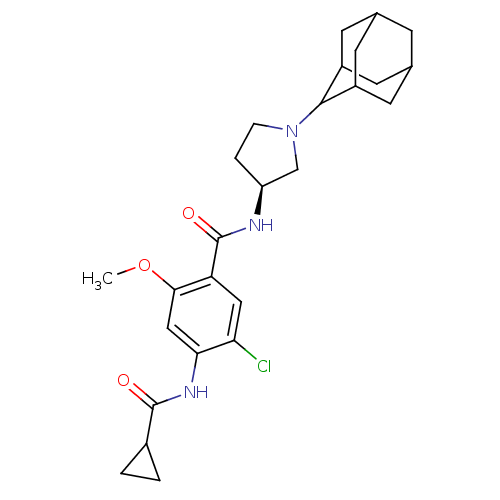 (CHEMBL92924 | N-((S)-1-Adamantan-2-yl-pyrrolidin-3...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052194 (4-Amino-N-((R)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052182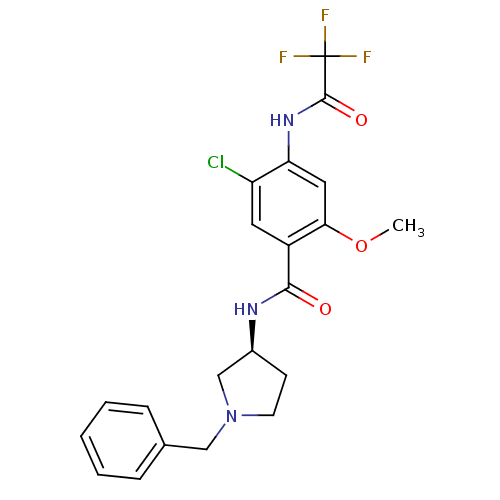 (CHEMBL330318 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052199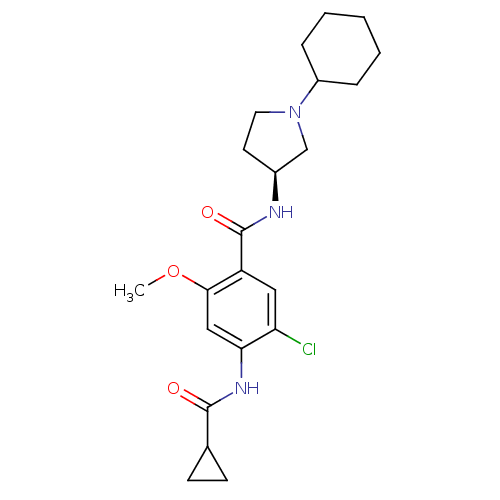 (5-Chloro-N-((S)-1-cyclohexyl-pyrrolidin-3-yl)-4-(c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against rat cortical Muscarinic acetylcholine receptor M1 by radioligand [3H]-pirenzepine binding assay. | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052180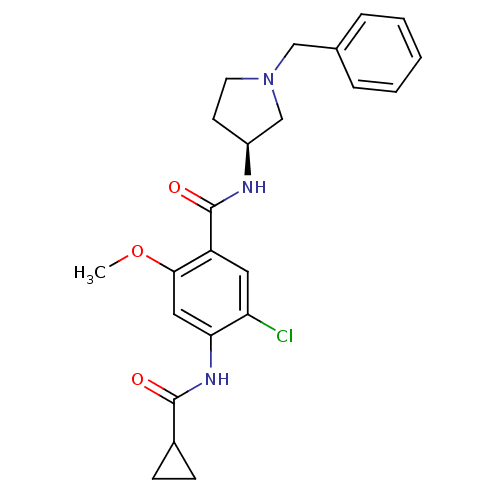 (CHEMBL328866 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052183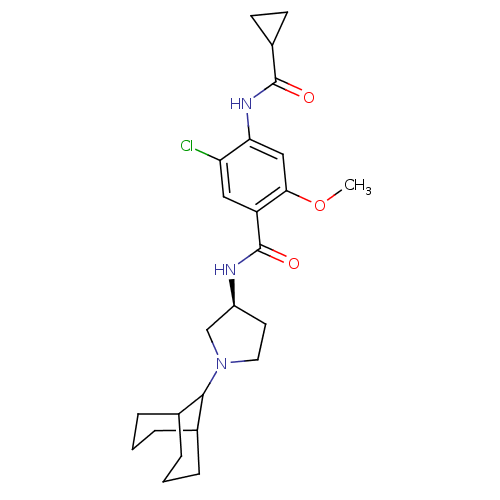 (CHEMBL94050 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyrr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (RABBIT) | BDBM82279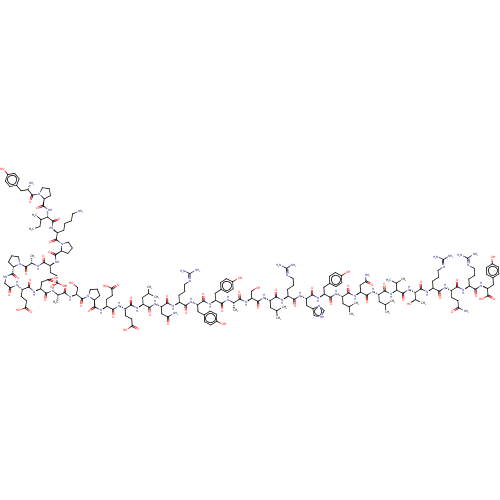 (CAS_118997-30-1 | PYY, human) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Biol Chem 271: 27217-20 (1996) Article DOI: 10.1074/jbc.271.44.27217 BindingDB Entry DOI: 10.7270/Q2K64GKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052184 (5-Chloro-N-((S)-1-cycloheptyl-pyrrolidin-3-yl)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052179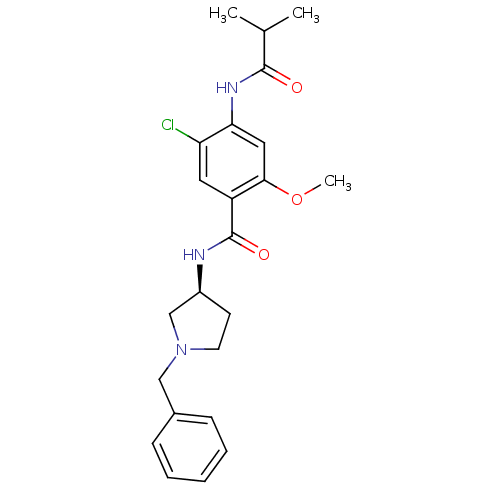 (CHEMBL93021 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052197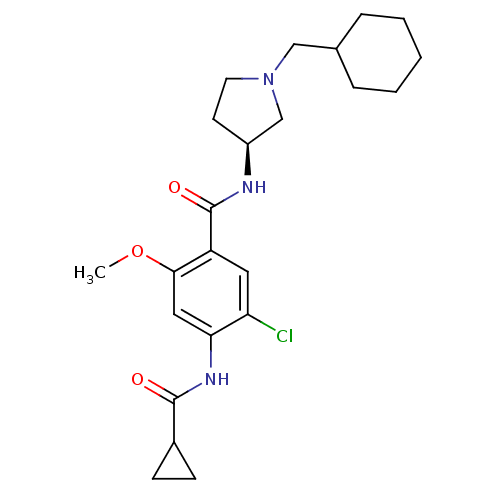 (5-Chloro-N-((S)-1-cyclohexylmethyl-pyrrolidin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052200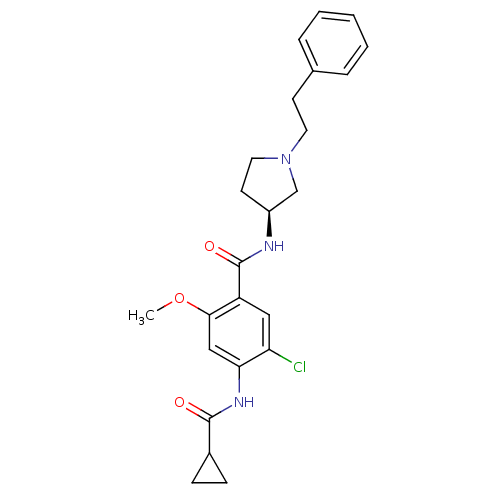 (5-Chloro-4-(cyclopropanecarbonyl-amino)-2-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052197 (5-Chloro-N-((S)-1-cyclohexylmethyl-pyrrolidin-3-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052183 (CHEMBL94050 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (RABBIT) | BDBM82287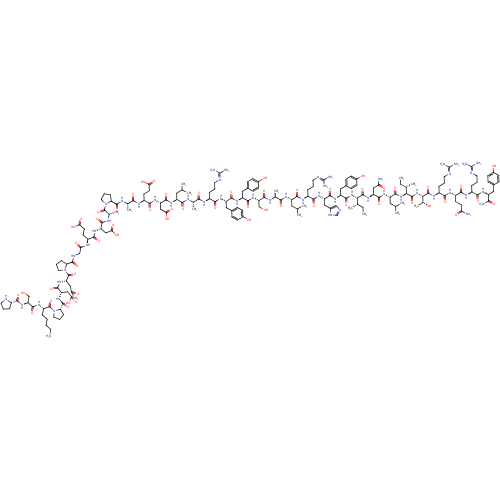 (NPY2-36 | NPY2-36, human | NPY2-36, porcine) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Biol Chem 271: 27217-20 (1996) Article DOI: 10.1074/jbc.271.44.27217 BindingDB Entry DOI: 10.7270/Q2K64GKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052199 (5-Chloro-N-((S)-1-cyclohexyl-pyrrolidin-3-yl)-4-(c...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052181 (CHEMBL92924 | N-((S)-1-Adamantan-2-yl-pyrrolidin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052186 (4-Acryloylamino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (RABBIT) | BDBM82282 (NPY13-36 | NPY13-36, porcine) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Biol Chem 271: 27217-20 (1996) Article DOI: 10.1074/jbc.271.44.27217 BindingDB Entry DOI: 10.7270/Q2K64GKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against rat cortical H1 receptor by radioligand [3H]-pyrilamine binding assay. | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052182 (CHEMBL330318 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052192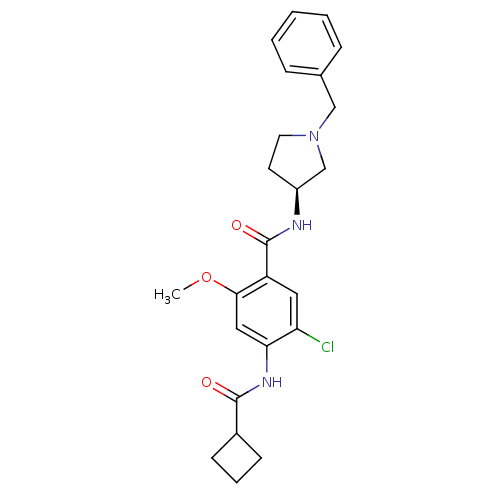 (CHEMBL315446 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052193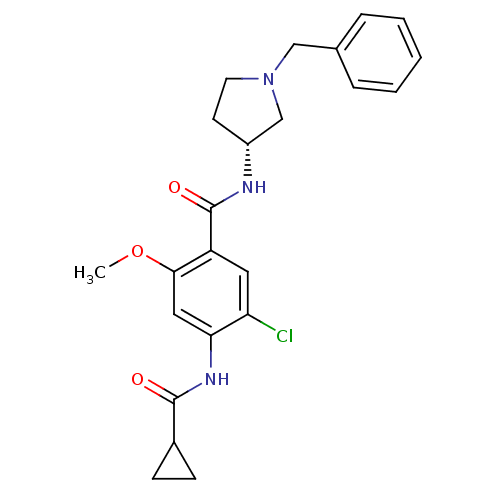 (CHEMBL319079 | N-((R)-1-Benzyl-pyrrolidin-3-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052195 (CHEMBL93198 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-c...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052190 (CHEMBL316317 | N-((R)-1-Benzyl-pyrrolidin-3-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052182 (CHEMBL330318 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052179 (CHEMBL93021 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-c...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was tested for the Binding affinity against rat frontal cortex 5-hydroxytryptamine 2A receptor by Radio ligand [3H]-ketanserin binding assay... | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052188 (CHEMBL94200 | N-((R)-1-Benzyl-pyrrolidin-3-yl)-5-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052184 (5-Chloro-N-((S)-1-cycloheptyl-pyrrolidin-3-yl)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052185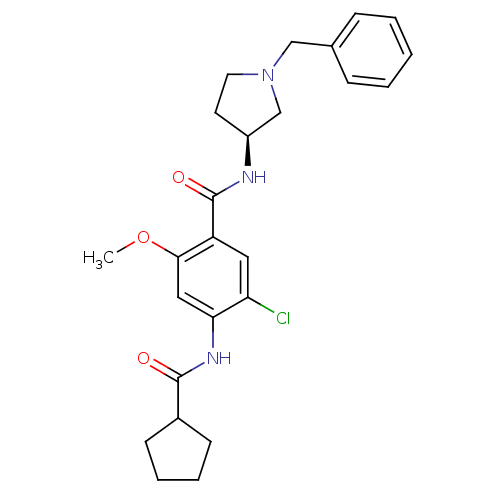 (CHEMBL92030 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (RABBIT) | BDBM82276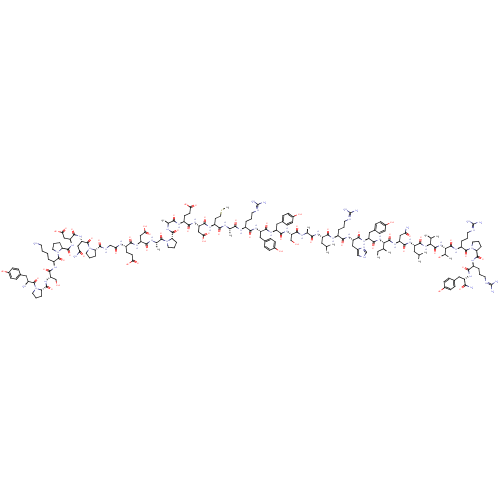 (L31,P34-NPY,human | NPY Leu31, Pro34, human, rat |...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Biol Chem 271: 27217-20 (1996) Article DOI: 10.1074/jbc.271.44.27217 BindingDB Entry DOI: 10.7270/Q2K64GKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052180 (CHEMBL328866 | N-((S)-1-Benzyl-pyrrolidin-3-yl)-5-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052186 (4-Acryloylamino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 472 total ) | Next | Last >> |