

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50246766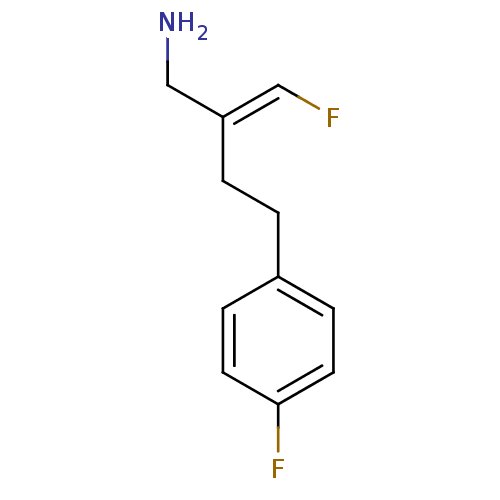 (CHEMBL489079 | Mofegiline | US9302986, Mofegiline) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in Pichia pastoris by kinetic assay | J Med Chem 51: 8019-26 (2008) Article DOI: 10.1021/jm8011867 BindingDB Entry DOI: 10.7270/Q2542NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p-hydroxybenzoate hydroxylase (Pseudomonas fluorescens) | BDBM50038194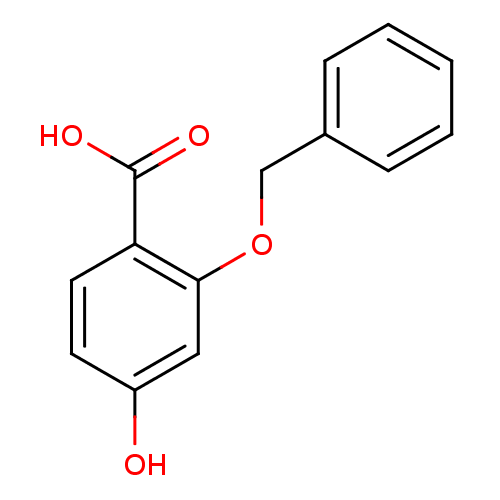 (2-Benzyloxy-4-hydroxy-benzoic acid | CHEMBL130259) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound against p-hydroxybenzoate hydroxylase (PHBH) from Pseudomonas fluorescence competing with p-hydroxy benzoic acid | J Med Chem 37: 4076-8 (1995) BindingDB Entry DOI: 10.7270/Q2959GKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p-hydroxybenzoate hydroxylase (Pseudomonas fluorescens) | BDBM50038194 (2-Benzyloxy-4-hydroxy-benzoic acid | CHEMBL130259) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound against p-hydroxybenzoate hydroxylase (PHBH) from Pseudomonas fluorescence competing with NADPH | J Med Chem 37: 4076-8 (1995) BindingDB Entry DOI: 10.7270/Q2959GKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50246766 (CHEMBL489079 | Mofegiline | US9302986, Mofegiline) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA expressed in Pichia pastoris by kinetic assay | J Med Chem 51: 8019-26 (2008) Article DOI: 10.1021/jm8011867 BindingDB Entry DOI: 10.7270/Q2542NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50246766 (CHEMBL489079 | Mofegiline | US9302986, Mofegiline) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human SSAO/VAP1 measuring H2O2 production by Kitz and Wilson plot analysis | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384084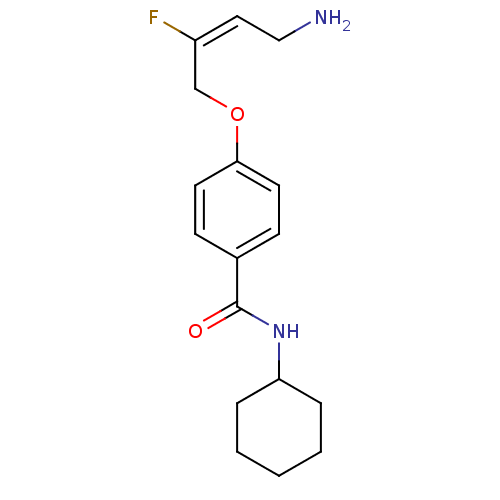 (CHEMBL2029546) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human SSAO/VAP1 measuring H2O2 production by Kitz and Wilson plot analysis | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50113840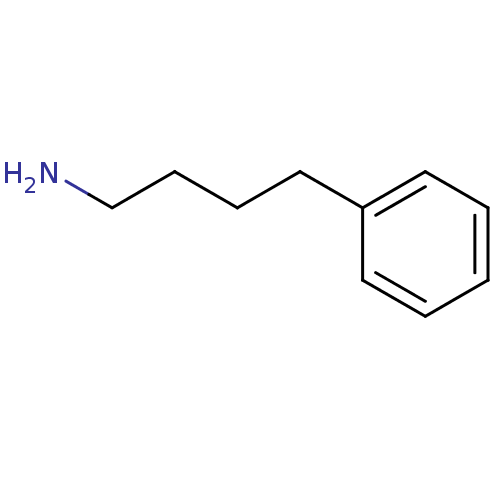 (4-Phenyl-butylamine | 4-phenylbutylamine | CHEMBL7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of human MAOA | J Med Chem 51: 8019-26 (2008) Article DOI: 10.1021/jm8011867 BindingDB Entry DOI: 10.7270/Q2542NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50004897 ((APV)2-Amino-5-phosphono-pentanoic acid | (R)-2-Am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50010893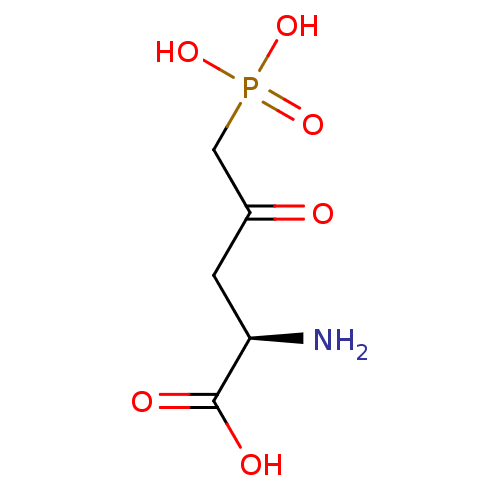 ((R)-2-Amino-4-oxo-5-phosphono-pentanoic acid | 2-A...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50004897 ((APV)2-Amino-5-phosphono-pentanoic acid | (R)-2-Am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50010893 ((R)-2-Amino-4-oxo-5-phosphono-pentanoic acid | 2-A...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50002363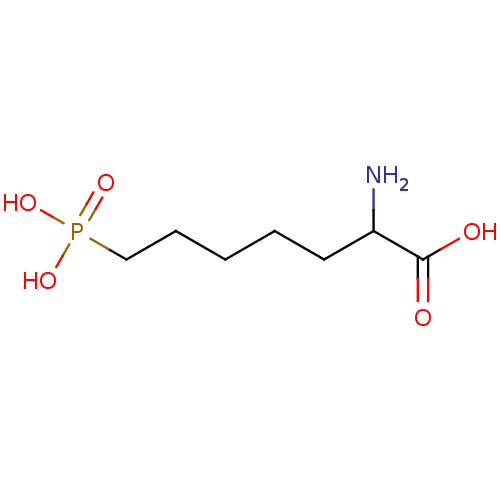 ((APH)2-Amino-7-phosphono-heptanoic acid | 2-Amino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50004927 (4-Phosphonomethyl-piperidine-2-carboxylic acid | 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50004927 (4-Phosphonomethyl-piperidine-2-carboxylic acid | 4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50002363 ((APH)2-Amino-7-phosphono-heptanoic acid | 2-Amino-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50002360 ((CPP)4-(3-Phosphono-propyl)-piperazine-2-carboxyli...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50002360 ((CPP)4-(3-Phosphono-propyl)-piperazine-2-carboxyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; Inactive | J Med Chem 33: 2961-3 (1990) BindingDB Entry DOI: 10.7270/Q2BK1CXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-9 (RAT) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences Inc. Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor by the displacement of [3H]-nicotine from rat cortical membranes | J Med Chem 42: 1684-6 (1999) Article DOI: 10.1021/jm990035d BindingDB Entry DOI: 10.7270/Q2QC02PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50246766 (CHEMBL489079 | Mofegiline | US9302986, Mofegiline) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of rat membrane MAOB | J Med Chem 51: 8019-26 (2008) Article DOI: 10.1021/jm8011867 BindingDB Entry DOI: 10.7270/Q2542NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A/B (Rattus norvegicus (rat)) | BDBM50225462 (CHEMBL554775) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against rat brain mitochondrial Monoamine oxidase | J Med Chem 28: 186-93 (1985) BindingDB Entry DOI: 10.7270/Q26M3910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50246766 (CHEMBL489079 | Mofegiline | US9302986, Mofegiline) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
BOEHRINGER INGELHEIM INTERNATIONAL GMBH US Patent | Assay Description Recombinant human MAO-B (0.06 mg/mL; Sigma Aldrich) was used as source of MAO-B enzyme activities. The assay was performed in a similar way as for hu... | US Patent US9302986 (2016) BindingDB Entry DOI: 10.7270/Q2ZC81QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A/B (Rattus norvegicus (rat)) | BDBM50225466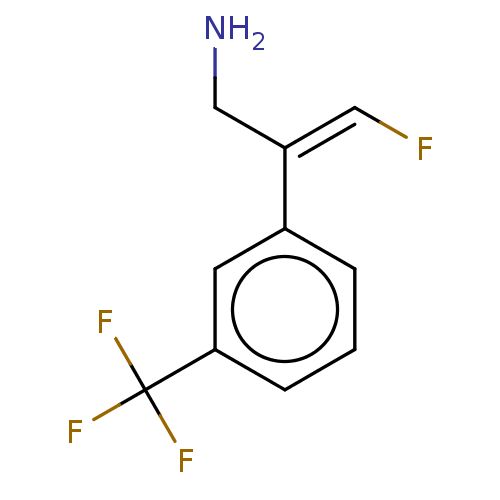 (CHEMBL544509) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against rat brain mitochondrial Monoamine oxidase | J Med Chem 28: 186-93 (1985) BindingDB Entry DOI: 10.7270/Q26M3910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50213061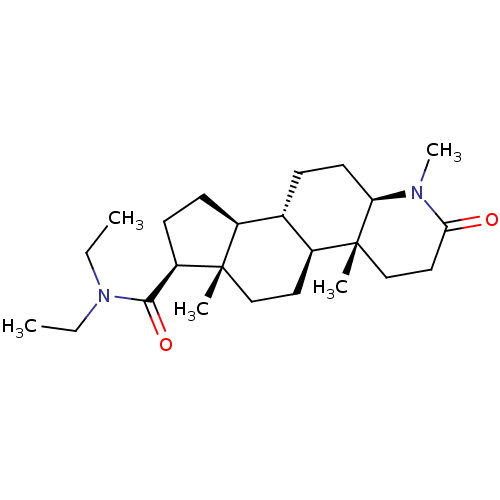 (CHEMBL2298601) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductase | Bioorg Med Chem Lett 4: 847-851 (1994) Article DOI: 10.1016/S0960-894X(01)80861-1 BindingDB Entry DOI: 10.7270/Q21C1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A/B (Rattus norvegicus (rat)) | BDBM50225455 (CHEMBL555332) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against rat brain mitochondrial Monoamine oxidase | J Med Chem 28: 186-93 (1985) BindingDB Entry DOI: 10.7270/Q26M3910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Mus musculus) | BDBM50246766 (CHEMBL489079 | Mofegiline | US9302986, Mofegiline) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of SSAO/VAP1 in mouse adipocytes measuring H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50246766 (CHEMBL489079 | Mofegiline | US9302986, Mofegiline) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50384098 (CHEMBL2029537) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384084 (CHEMBL2029546) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A/B (Rattus norvegicus (rat)) | BDBM50225456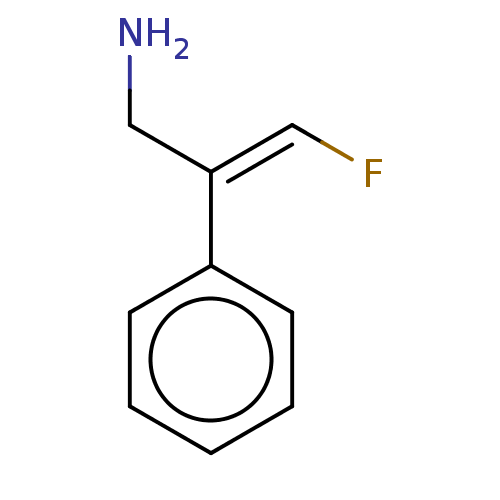 (CHEMBL545207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against rat brain mitochondrial Monoamine oxidase | J Med Chem 28: 186-93 (1985) BindingDB Entry DOI: 10.7270/Q26M3910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50246766 (CHEMBL489079 | Mofegiline | US9302986, Mofegiline) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384083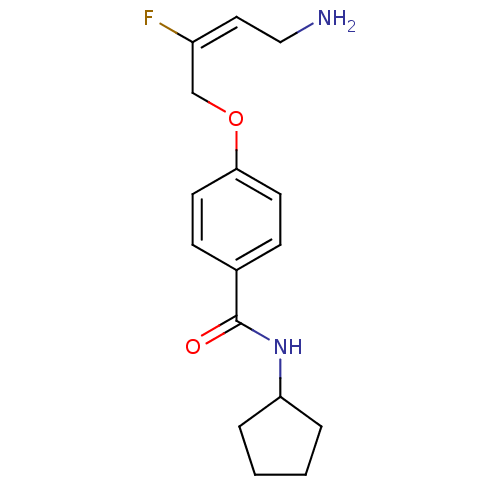 (CHEMBL2029545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A/B (Rattus norvegicus (rat)) | BDBM50225453 (CHEMBL541803) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against rat brain mitochondrial Monoamine oxidase | J Med Chem 28: 186-93 (1985) BindingDB Entry DOI: 10.7270/Q26M3910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384095 (CHEMBL2029534) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A/B (Rattus norvegicus (rat)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against rat brain mitochondrial Monoamine oxidase | J Med Chem 28: 186-93 (1985) BindingDB Entry DOI: 10.7270/Q26M3910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50384101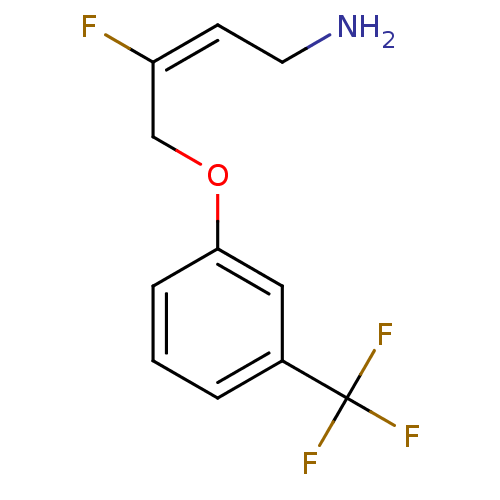 (CHEMBL2029540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50384094 (CHEMBL2029533) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384087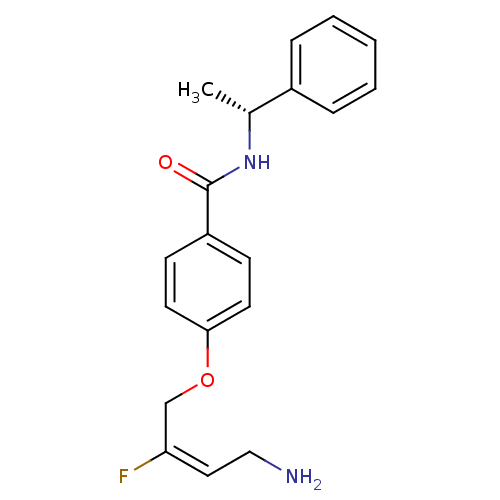 (CHEMBL2029549) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384100 (CHEMBL2029539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384086 (CHEMBL2029548) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384088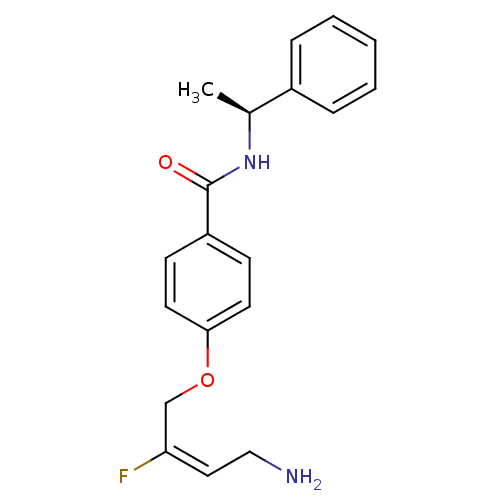 (CHEMBL2029550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A/B (Rattus norvegicus (rat)) | BDBM50225463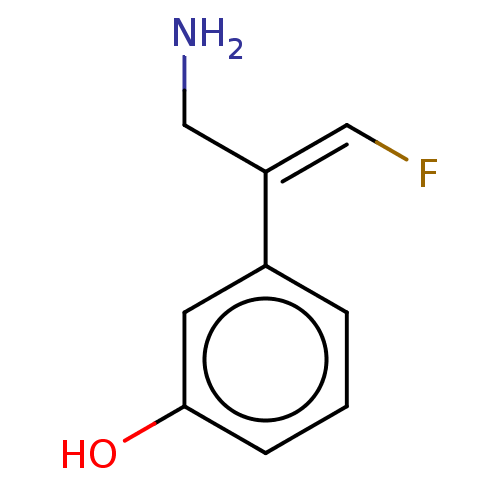 (CHEMBL538992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against rat brain mitochondrial Monoamine oxidase | J Med Chem 28: 186-93 (1985) BindingDB Entry DOI: 10.7270/Q26M3910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A/B (Rattus norvegicus (rat)) | BDBM50225449 (CHEMBL543328) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against rat brain mitochondrial Monoamine oxidase | J Med Chem 28: 186-93 (1985) BindingDB Entry DOI: 10.7270/Q26M3910 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50384097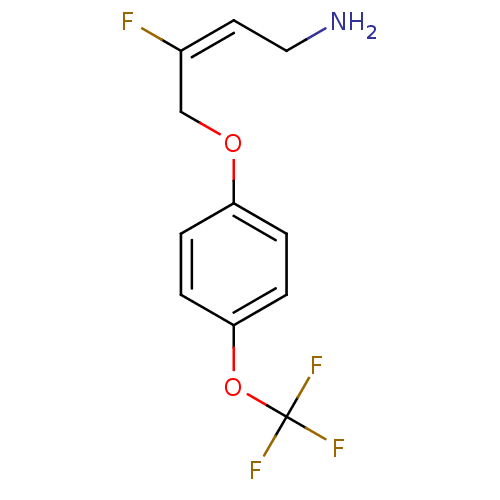 (CHEMBL2029536) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384098 (CHEMBL2029537) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50384085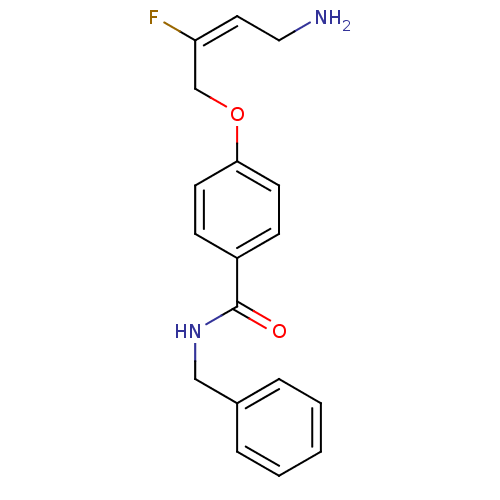 (CHEMBL2029547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant SSAO/VAP1 assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50384099 (CHEMBL2029538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B assessed as H2O2 production by Resorufin/Amplex Red assay | Bioorg Med Chem Lett 22: 3935-40 (2012) Article DOI: 10.1016/j.bmcl.2012.04.111 BindingDB Entry DOI: 10.7270/Q2SQ91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM220871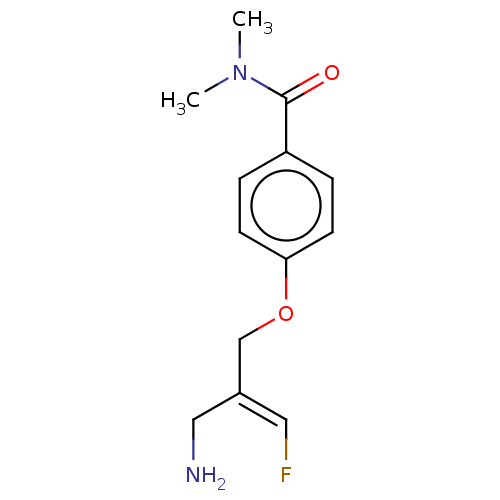 (US9302986, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | 7.4 | 37 |
BOEHRINGER INGELHEIM INTERNATIONAL GMBH US Patent | Assay Description Recombinant human MAO-B (0.06 mg/mL; Sigma Aldrich) was used as source of MAO-B enzyme activities. The assay was performed in a similar way as for hu... | US Patent US9302986 (2016) BindingDB Entry DOI: 10.7270/Q2ZC81QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM220861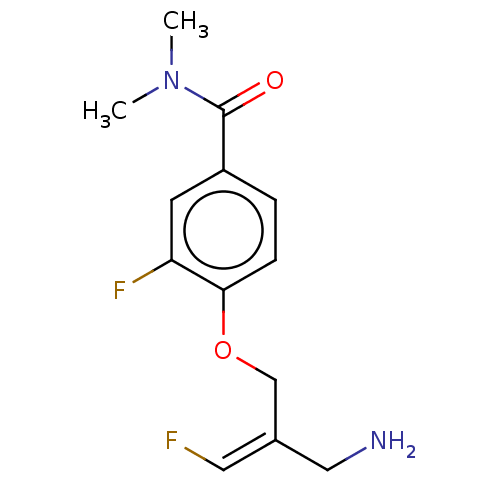 (US9302986, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | 7.4 | 37 |
BOEHRINGER INGELHEIM INTERNATIONAL GMBH US Patent | Assay Description Recombinant human MAO-B (0.06 mg/mL; Sigma Aldrich) was used as source of MAO-B enzyme activities. The assay was performed in a similar way as for hu... | US Patent US9302986 (2016) BindingDB Entry DOI: 10.7270/Q2ZC81QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM220884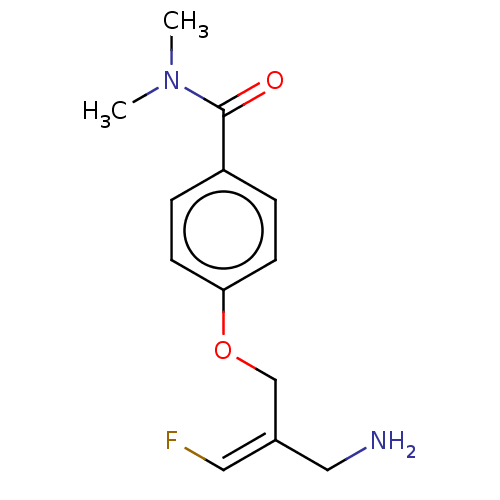 (US9302986, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | 7.4 | 37 |
BOEHRINGER INGELHEIM INTERNATIONAL GMBH US Patent | Assay Description Recombinant human MAO-B (0.06 mg/mL; Sigma Aldrich) was used as source of MAO-B enzyme activities. The assay was performed in a similar way as for hu... | US Patent US9302986 (2016) BindingDB Entry DOI: 10.7270/Q2ZC81QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amiloride-sensitive amine oxidase [copper-containing] (Homo sapiens (Human)) | BDBM220869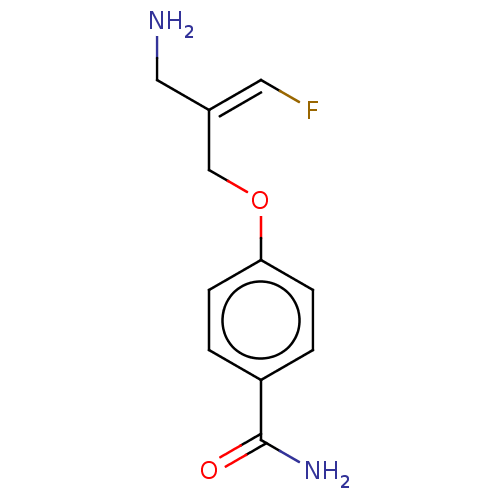 (US9302986, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 7.4 | 37 |
BOEHRINGER INGELHEIM INTERNATIONAL GMBH US Patent | Assay Description Recombinant human DAO (2.4 μg/mL) was used as source of DAO enzyme activities. The assay was performed as described in the method for human SSAO/V... | US Patent US9302986 (2016) BindingDB Entry DOI: 10.7270/Q2ZC81QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 259 total ) | Next | Last >> |