

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109062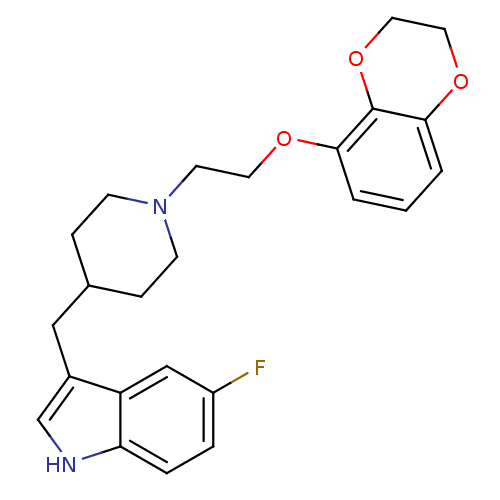 (3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109058 (1H-4-indolyl 2-[4-(1H-3-indolyl)-1,2,3,6-tetrahydr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102006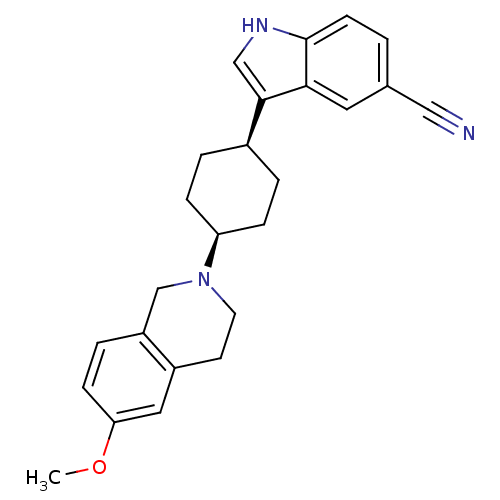 (3-[4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109057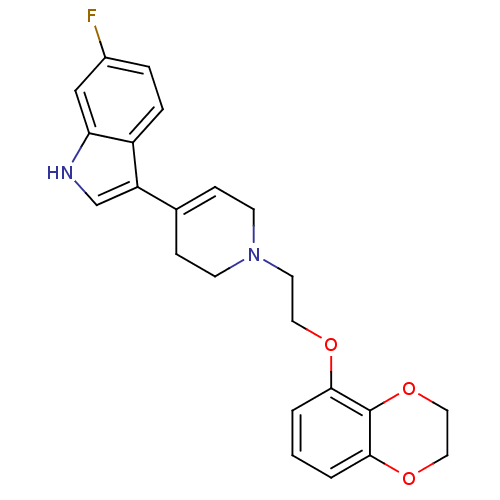 (3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109055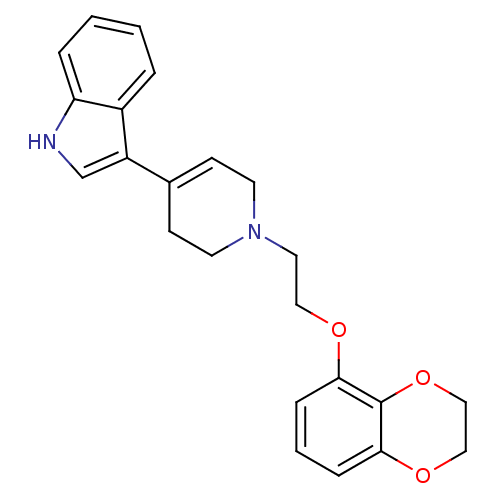 (3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50109058 (1H-4-indolyl 2-[4-(1H-3-indolyl)-1,2,3,6-tetrahydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards Alpha-1 adrenergic receptor was determined by the displacement of [3H]prazosin | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109060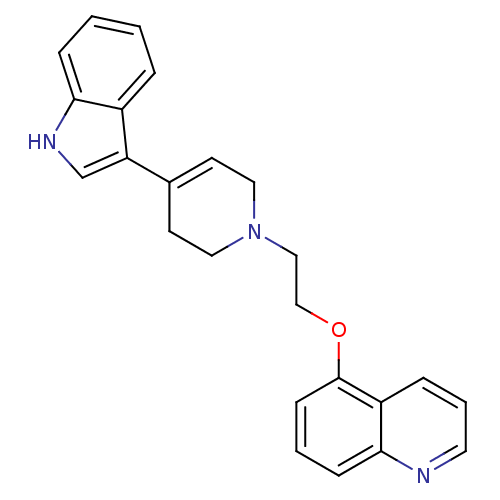 (5-{2-[4-(1H-Indol-3-yl)-3,6-dihydro-2H-pyridin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102023 (3-[4-(5-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102009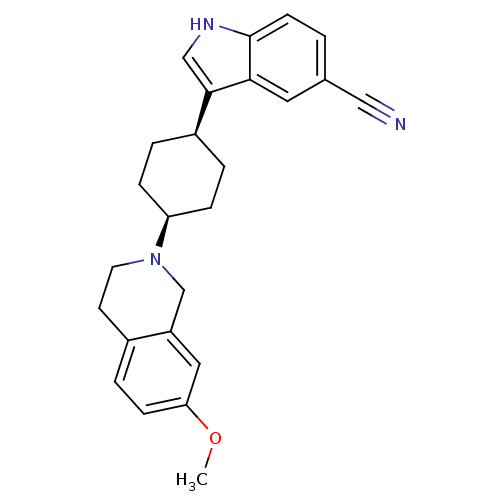 (3-[4-(7-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50109061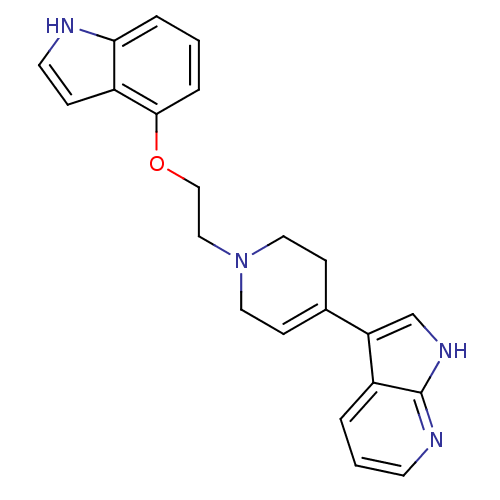 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards Alpha-1 adrenergic receptor was determined by the displacement of [3H]prazosin | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102003 (3-[4-(8-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50252278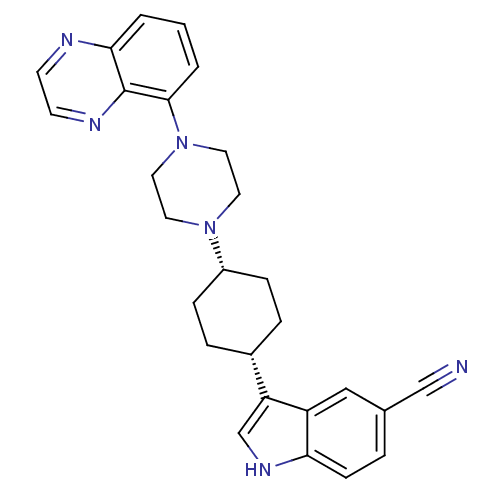 (3-[(1,4-cis)-4-(4-Quinoxalin-5-yl-piperazin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102010 (3-[4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109054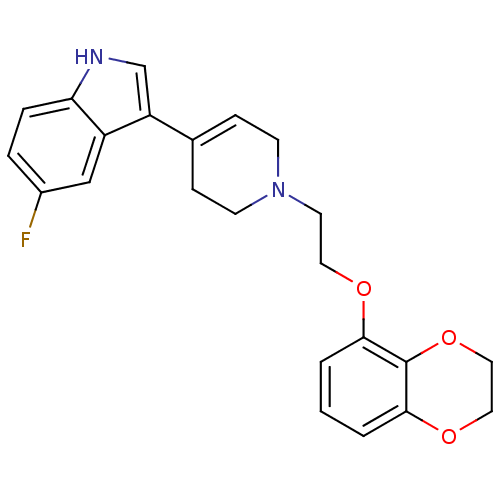 (3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50252221 (3-[(1,4-cis)-4-(4-Quinolin-8-yl-piperazin-1-yl)-cy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102005 (2-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-6-methoxy-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes by [3H]-Citalopram displacement. | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50101999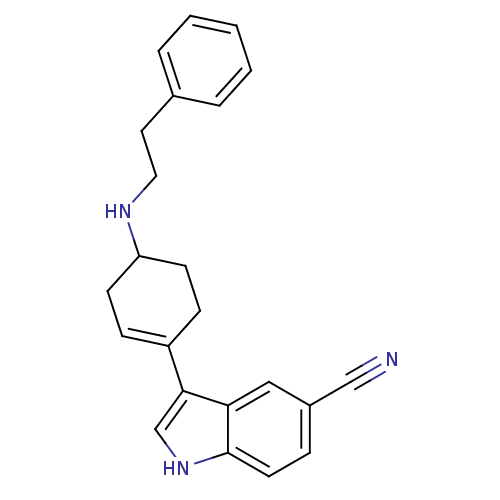 (3-(4-Phenethylamino-cyclohex-1-enyl)-1H-indole-5-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50252591 (3-{4-[(1,4-cis)-4-(1H-Indol-4-yl)-pipera-zinyl-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252084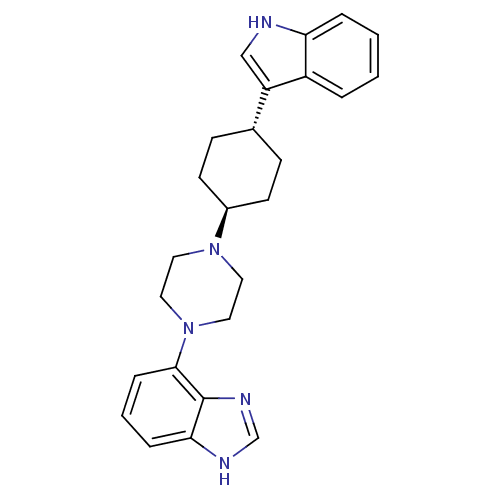 (4-{trans-4-[4-(1H-Indol-3-yl)cyclohexyl]-piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102012 (2-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-5-methoxy-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102016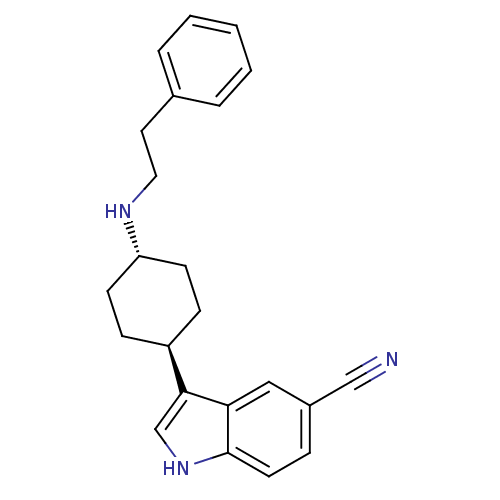 (3-(4-Phenethylamino-cyclohexyl)-1H-indole-5-carbon...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50109059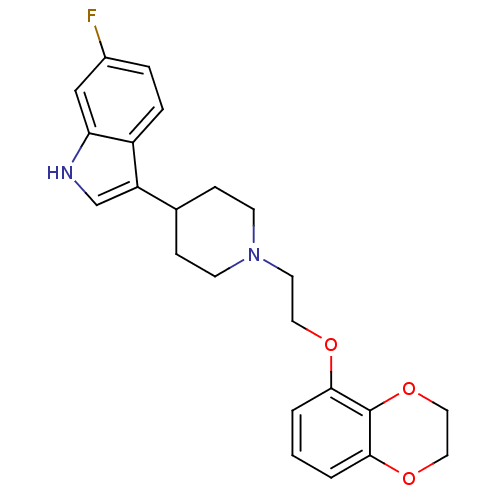 (3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Antagonism of 5-hydroxytryptamine 1A receptor was determined in vitro using a [35S]-GTP-gammaS, | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252222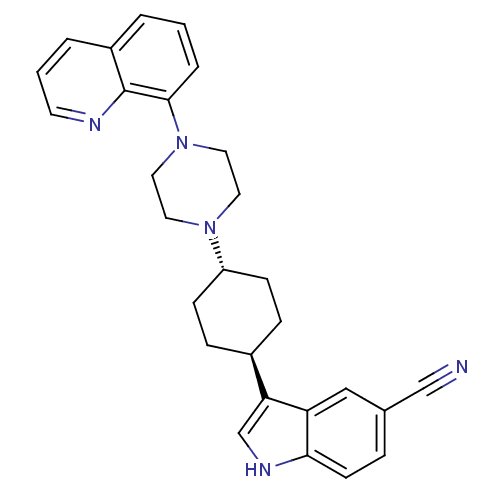 (3-[(1,4-trans)-4-(4-Quinolin-8-yl-piperazin-1-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252026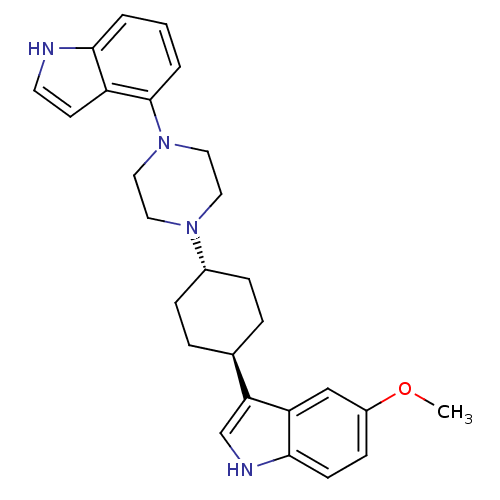 (5-Methoxy-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252129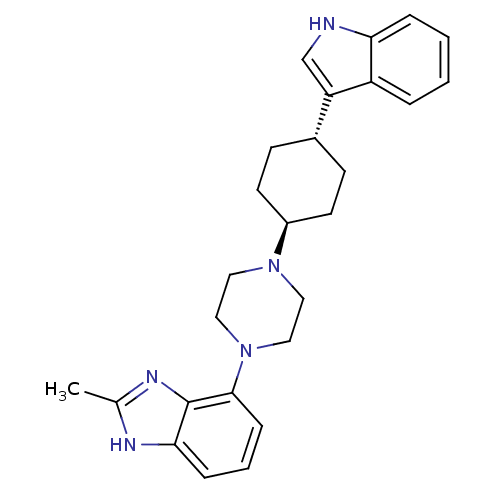 (4-{trans-4-[4-(1H-Indol-3-yl)cyclohexyl]piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102017 (3-(4-Benzylamino-cyclohex-1-enyl)-1H-indole-5-carb...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50252645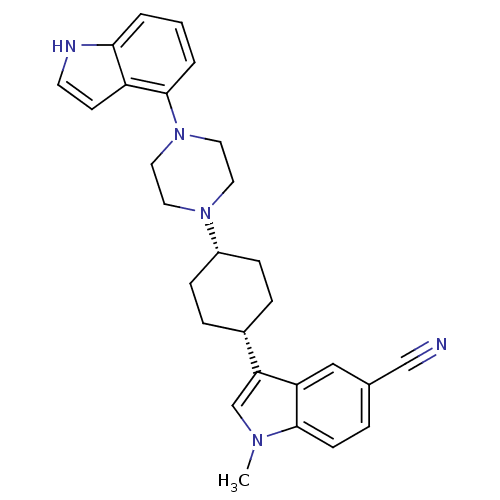 (3-{(1,4-cis)-4-[4-(1H-Indol-4-yl)-piperazin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50252180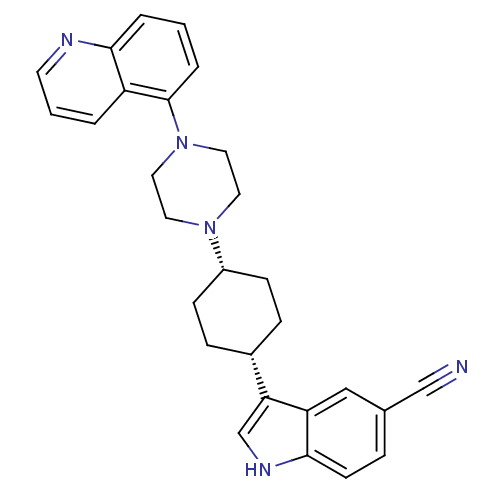 (3-[(1,4-cis)-4-(4-Quinolin-5-yl-piperazin-1-yl)-cy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252644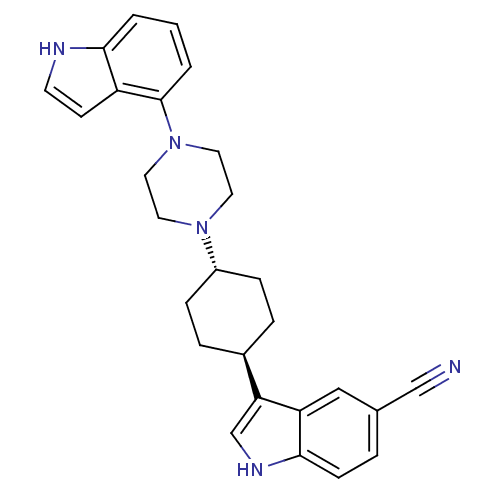 (3-{4-[(1,4-trans)-4-(1H-indol-4-yl)-pipera-zinyl-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50109060 (5-{2-[4-(1H-Indol-3-yl)-3,6-dihydro-2H-pyridin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards Alpha-1 adrenergic receptor was determined by the displacement of [3H]prazosin | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252131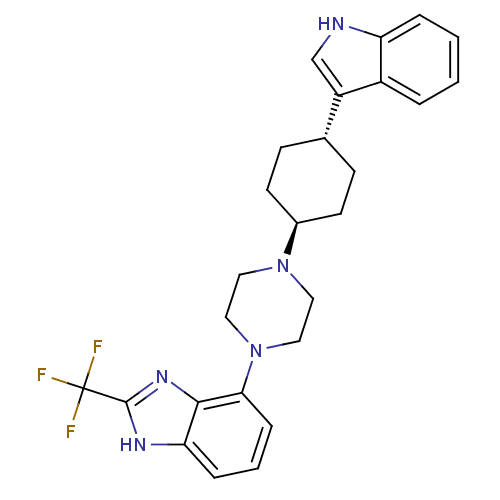 (4-{trans-4-[4-(1H-Indol-3-yl)cyclohexyl]-piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50109057 (3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards Alpha-1 adrenergic receptor was determined by the displacement of [3H]prazosin | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50252219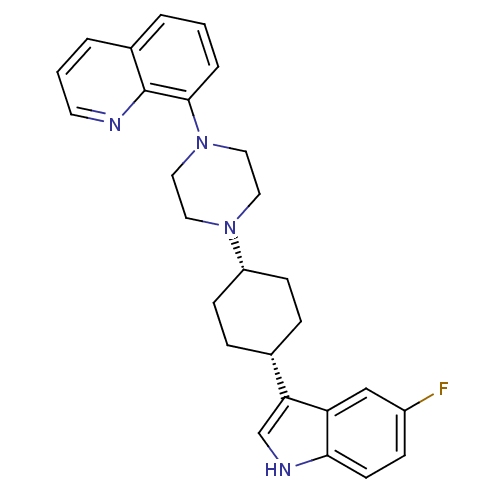 (8-{4-[(1,4-cis)-4-(5-Fluoro-1H-indol-3-yl)-cyclohe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50160613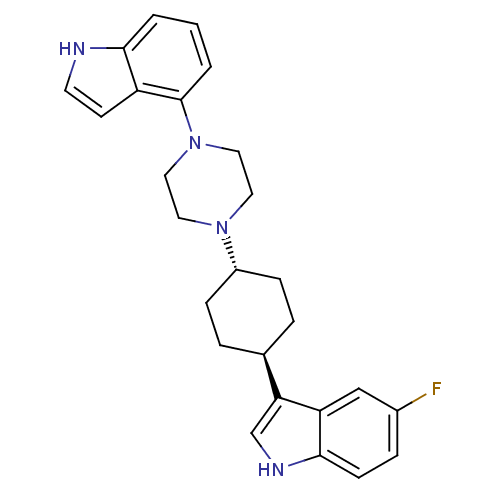 (5-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50109055 (3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards Alpha-1 adrenergic receptor was determined by the displacement of [3H]prazosin | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109059 (3-{1-[2-(2,3-Dihydro-benzo[1,4]dioxin-5-yloxy)-eth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102015 (2-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-1,2,3,4-tetr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50252181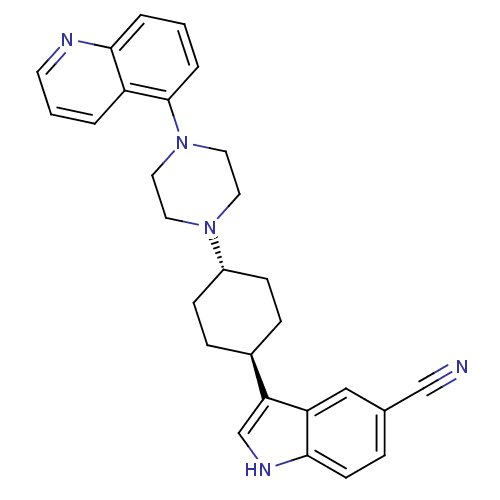 (3-[(1,4-trans)-4-(4-Quinolin-5-yl-piperazin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160603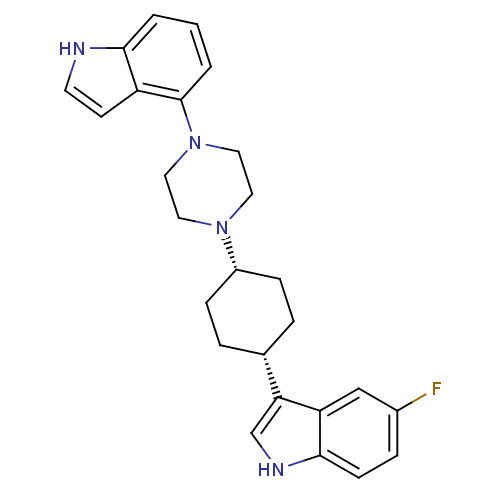 (5-Fluoro-3-[cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252534 (3-[trans-4-[4-(1H-Indol-4-yl)-1-pipera-zinyl]-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252588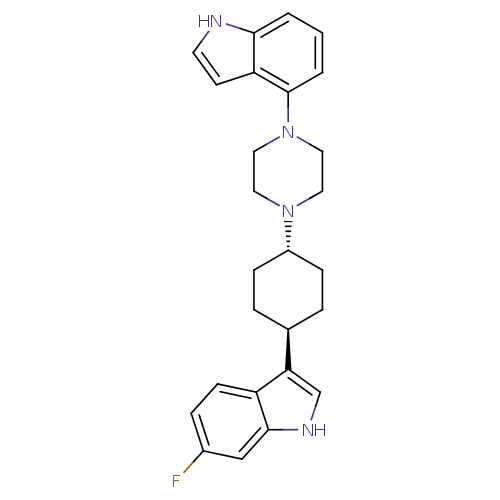 (6-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-pipera-zi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252181 (3-[(1,4-trans)-4-(4-Quinolin-5-yl-piperazin-1-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50252220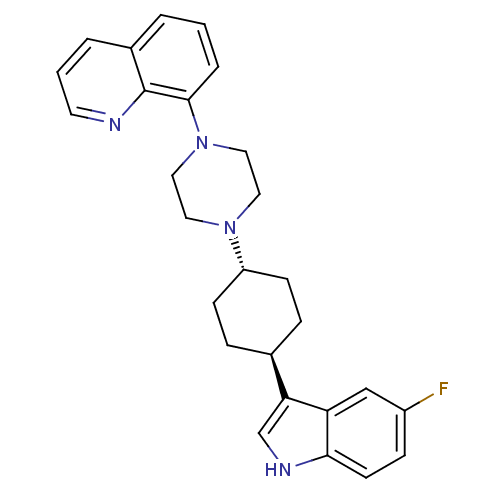 (8-{4-[(1,4-trans)-4-(5-Fluoro-1H-indol-3-yl)-cyclo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109056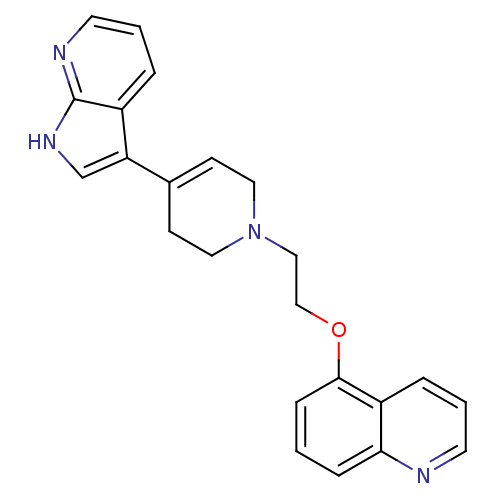 (5-{2-[4-(1H-Pyrrolo[2,3-b]pyridin-3-yl)-3,6-dihydr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102024 (3-(4-Phenethylamino-cyclohexyl)-1H-indole-5-carbon...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102000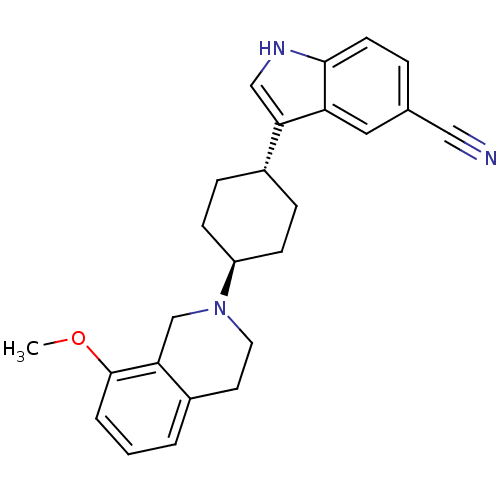 (3-[4-(8-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50102014 (3-(4-Benzylamino-cyclohexyl)-1H-indole-5-carbonitr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro for its binding affinity for serotonin transporter RB5-HT-T in rat cortical membranes | Bioorg Med Chem Lett 11: 1885-8 (2001) BindingDB Entry DOI: 10.7270/Q2GM86K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 334 total ) | Next | Last >> |