 Found 14 hits with Last Name = 'meng' and Initial = 'fw'
Found 14 hits with Last Name = 'meng' and Initial = 'fw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50069691
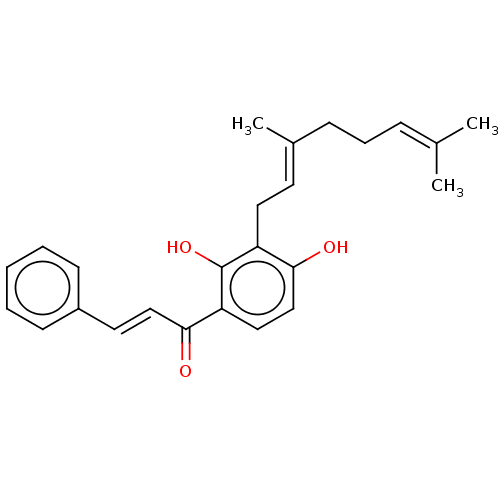
(Xanthoangelol | Xanthoangerol)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-[#6](=O)\[#6]=[#6]\c2ccccc2)c1-[#8] Show InChI InChI=1S/C25H28O3/c1-18(2)8-7-9-19(3)12-14-21-24(27)17-15-22(25(21)28)23(26)16-13-20-10-5-4-6-11-20/h4-6,8,10-13,15-17,27-28H,7,9,14H2,1-3H3/b16-13+,19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50069688
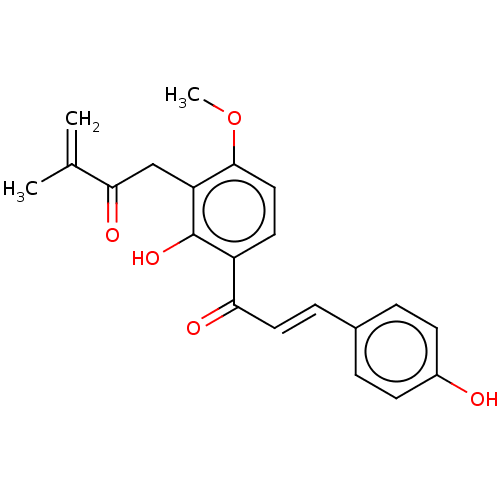
(CHEMBL3402548)Show SMILES COc1ccc(C(=O)\C=C\c2ccc(O)cc2)c(O)c1CC(=O)C(C)=C Show InChI InChI=1S/C21H20O5/c1-13(2)19(24)12-17-20(26-3)11-9-16(21(17)25)18(23)10-6-14-4-7-15(22)8-5-14/h4-11,22,25H,1,12H2,2-3H3/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601
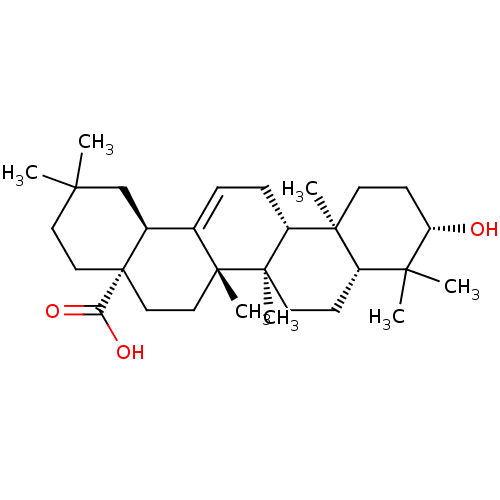
(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50069683
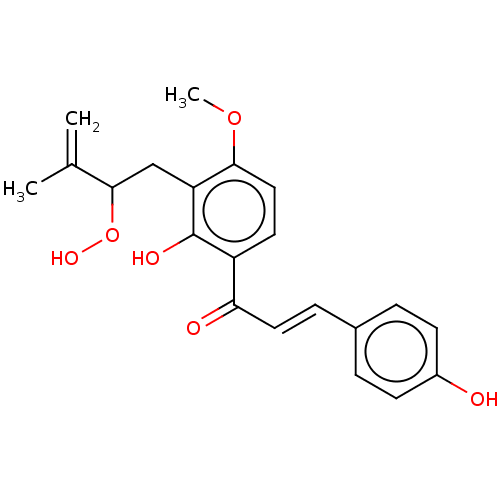
(CHEMBL1718454)Show SMILES COc1ccc(C(=O)\C=C\c2ccc(O)cc2)c(O)c1CC(OO)C(C)=C Show InChI InChI=1S/C21H22O6/c1-13(2)20(27-25)12-17-19(26-3)11-9-16(21(17)24)18(23)10-6-14-4-7-15(22)8-5-14/h4-11,20,22,24-25H,1,12H2,2-3H3/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50352811
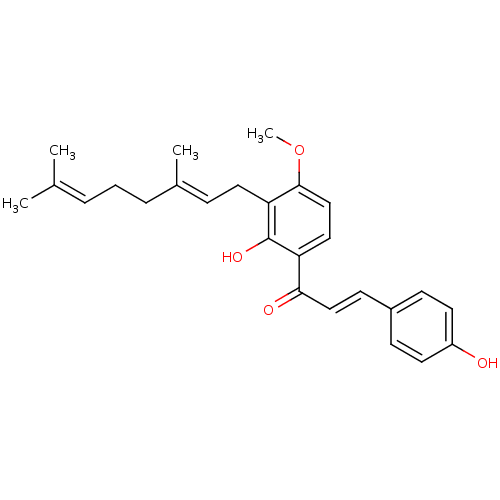
(CHEMBL1722838)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8])cc2)c(-[#8])c1-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C26H30O4/c1-18(2)6-5-7-19(3)8-14-23-25(30-4)17-15-22(26(23)29)24(28)16-11-20-9-12-21(27)13-10-20/h6,8-13,15-17,27,29H,5,7,14H2,1-4H3/b16-11+,19-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50352809
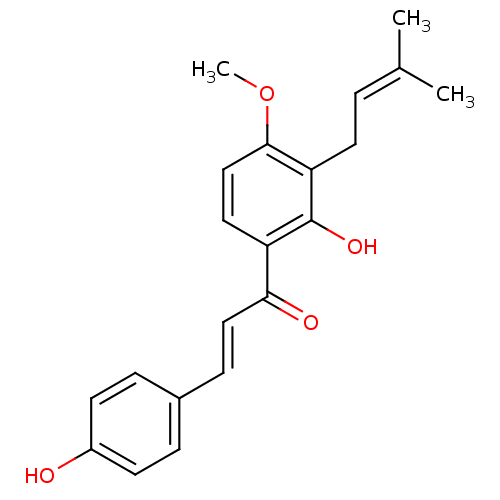
(CHEMBL458094)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8])cc2)c(-[#8])c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C21H22O4/c1-14(2)4-10-18-20(25-3)13-11-17(21(18)24)19(23)12-7-15-5-8-16(22)9-6-15/h4-9,11-13,22,24H,10H2,1-3H3/b12-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50069689
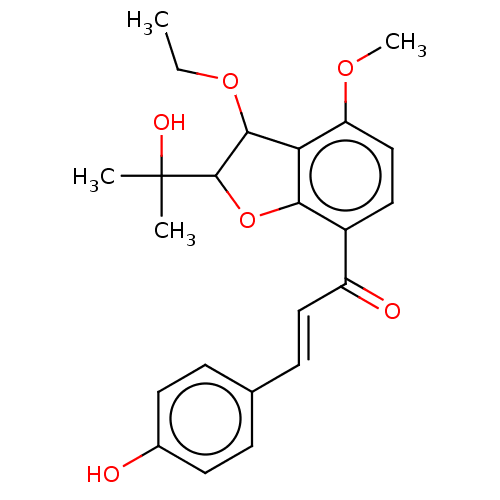
(CHEMBL3402550)Show SMILES CCOC1C(Oc2c1c(OC)ccc2C(=O)\C=C\c1ccc(O)cc1)C(C)(C)O Show InChI InChI=1S/C23H26O6/c1-5-28-21-19-18(27-4)13-11-16(20(19)29-22(21)23(2,3)26)17(25)12-8-14-6-9-15(24)10-7-14/h6-13,21-22,24,26H,5H2,1-4H3/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50352810

(CHEMBL1711961)Show SMILES COc1ccc(C(=O)\C=C\c2ccc(O)cc2)c(O)c1CC(O)C(C)=C Show InChI InChI=1S/C21H22O5/c1-13(2)19(24)12-17-20(26-3)11-9-16(21(17)25)18(23)10-6-14-4-7-15(22)8-5-14/h4-11,19,22,24-25H,1,12H2,2-3H3/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50067880
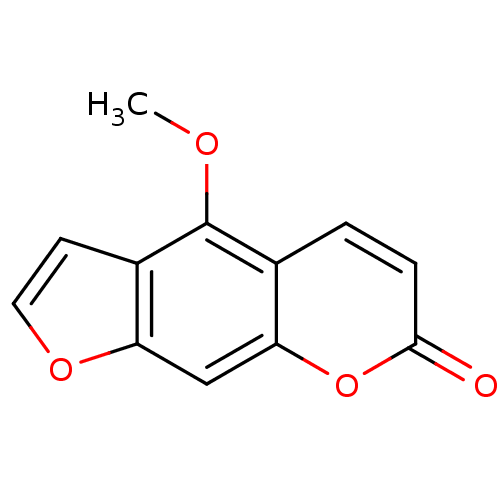
(4-methoxy-7H-furo[3,2-g][1]benzopyran-7-one | 4-me...)Show InChI InChI=1S/C12H8O4/c1-14-12-7-2-3-11(13)16-10(7)6-9-8(12)4-5-15-9/h2-6H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50069686
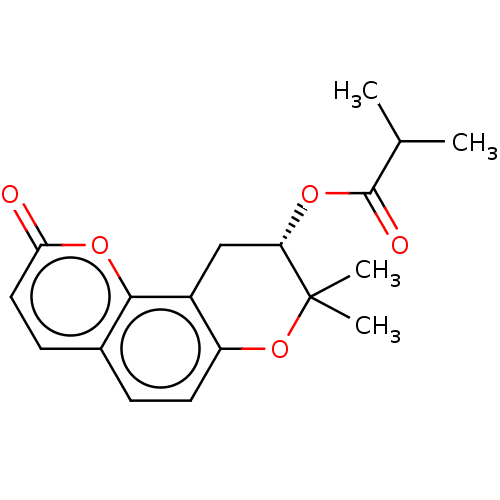
(CHEMBL3402554)Show SMILES CC(C)C(=O)O[C@H]1Cc2c(OC1(C)C)ccc1ccc(=O)oc21 |r| Show InChI InChI=1S/C18H20O5/c1-10(2)17(20)21-14-9-12-13(23-18(14,3)4)7-5-11-6-8-15(19)22-16(11)12/h5-8,10,14H,9H2,1-4H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50071370
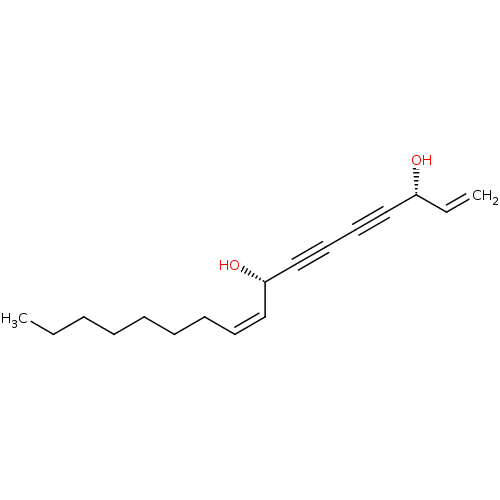
((3R,8S)-falcarindiol | (3R,8S,)-heptadeca-1,9-dien...)Show InChI InChI=1S/C17H24O2/c1-3-5-6-7-8-9-10-14-17(19)15-12-11-13-16(18)4-2/h4,10,14,16-19H,2-3,5-9H2,1H3/b14-10-/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50069685
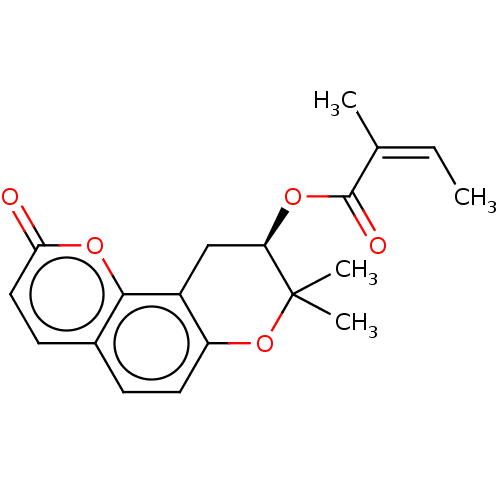
(Jatamansin)Show SMILES C\C=C(\C)C(=O)O[C@@H]1Cc2c(OC1(C)C)ccc1ccc(=O)oc21 |r| Show InChI InChI=1S/C19H20O5/c1-5-11(2)18(21)22-15-10-13-14(24-19(15,3)4)8-6-12-7-9-16(20)23-17(12)13/h5-9,15H,10H2,1-4H3/b11-5-/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50056315
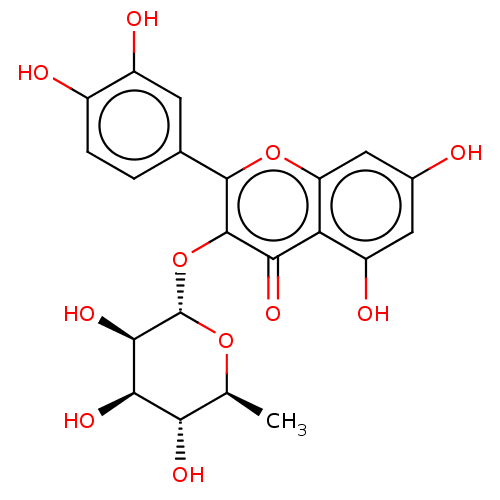
(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50069687

((+)-Cis-Khellactone | CHEMBL68727)Show SMILES CC1(C)Oc2ccc3ccc(=O)oc3c2[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H14O5/c1-14(2)13(17)11(16)10-8(19-14)5-3-7-4-6-9(15)18-12(7)10/h3-6,11,13,16-17H,1-2H3/t11-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data