

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Lactobacillus casei) | BDBM50022238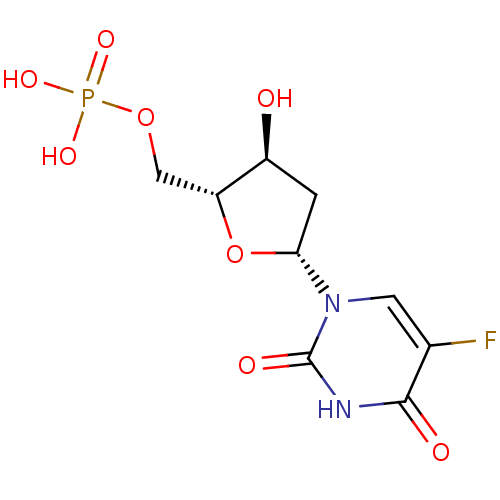 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010241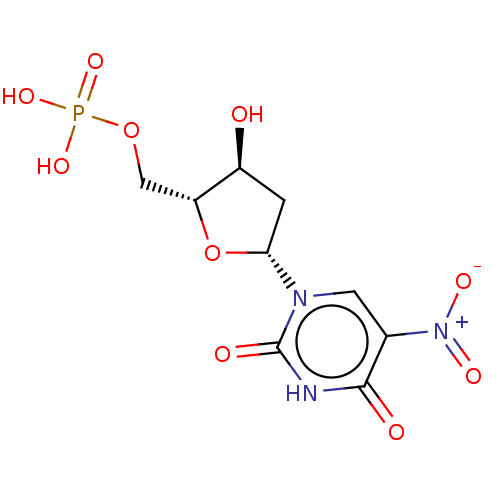 (CHEMBL1234672) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010236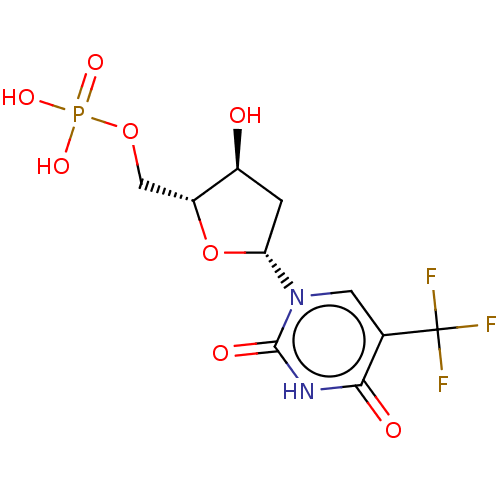 (CHEMBL3144200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50000038 (CHEMBL3228321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010239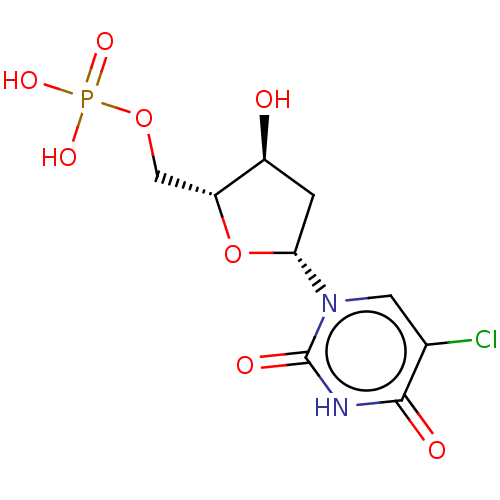 (CHEMBL1236538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023636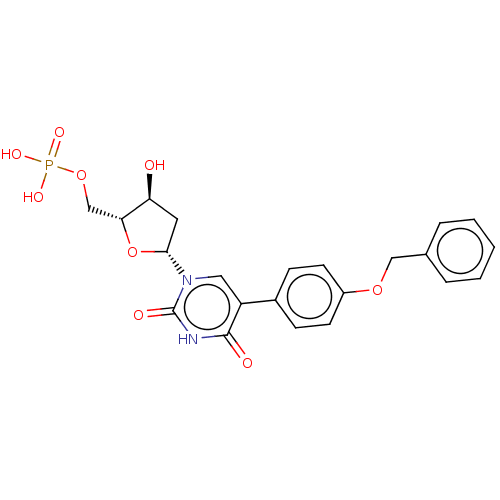 (CHEMBL3144190 | Phosphoric acid mono-{5-[5-(4-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50027919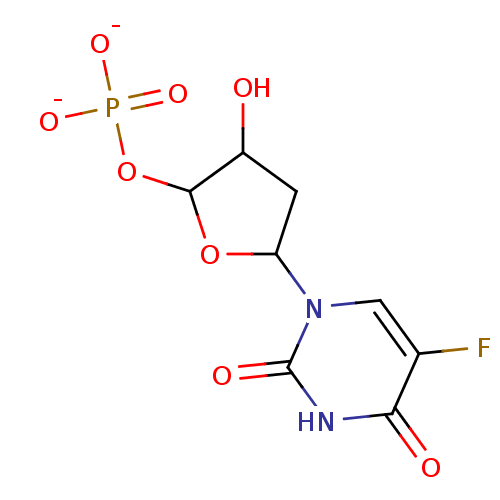 (5-fluoro-deoxyuridinemonophosphate | CHEMBL416879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inactivation of thymidylate synthetase measured asKi at 6.8 pH 30 degrees centigrade temp | J Med Chem 26: 1028-36 (1983) BindingDB Entry DOI: 10.7270/Q2VD6XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50404974 (5-NITRO-DUMP | CHEMBL2051758) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inactivation of thymidylate synthetase measured asKi at 6.8 pH 30 degrees centigrade temp | J Med Chem 26: 1028-36 (1983) BindingDB Entry DOI: 10.7270/Q2VD6XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010240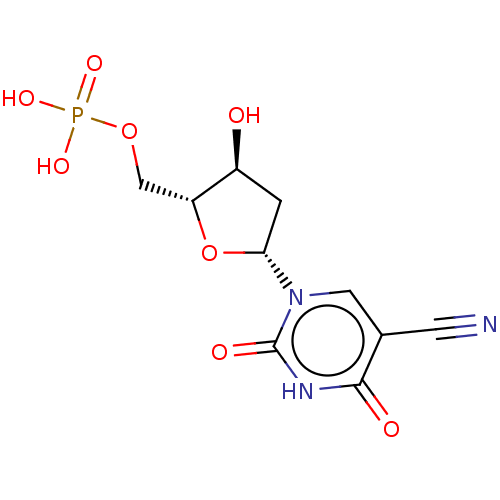 (CHEMBL3246102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50226709 (CHEMBL3143111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021750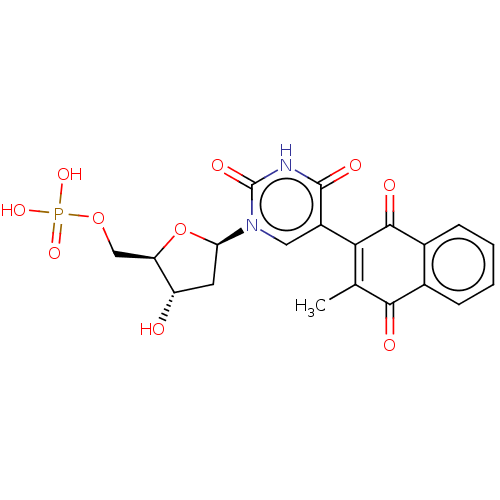 (CHEMBL3143104 | Phosphoric acid mono-{3-hydroxy-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023645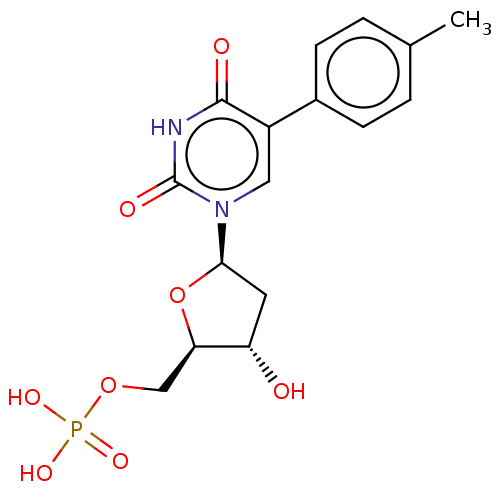 (CHEMBL3144187 | Phosphoric acid mono-[5-(2,4-dioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023647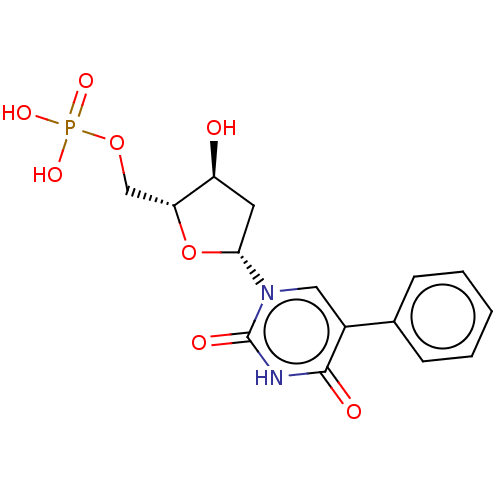 (CHEMBL3144186 | Phosphoric acid mono-[5-(2,4-dioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023637 (CHEMBL3144189 | Phosphoric acid mono-{5-[5-(4-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023640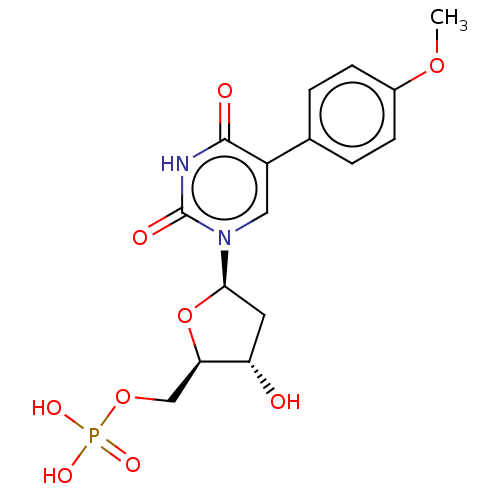 (CHEMBL3144188 | Phosphoric acid mono-{3-hydroxy-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021748 (CHEMBL3143138 | Phosphoric acid mono-{5-[5-(4,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021746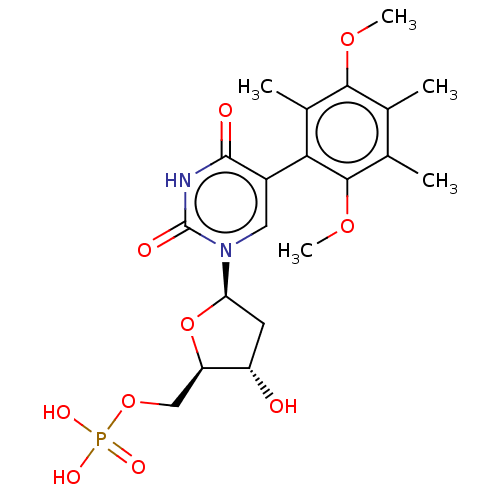 (CHEMBL3143107 | Phosphoric acid mono-{5-[5-(2,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010238 (CHEMBL1160593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023639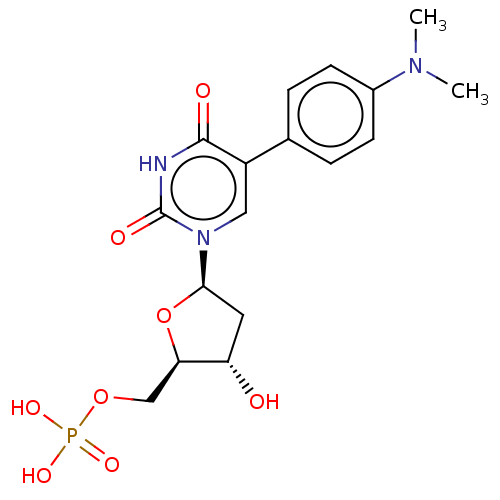 (CHEMBL3144192 | Phosphoric acid mono-{5-[5-(4-dime...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010242 (CHEMBL1160594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023641 (CHEMBL3144196 | Phosphoric acid mono-{5-[5-(4-brom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50021750 (CHEMBL3143104 | Phosphoric acid mono-{3-hydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from murine leukemia L1210 cells was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50027920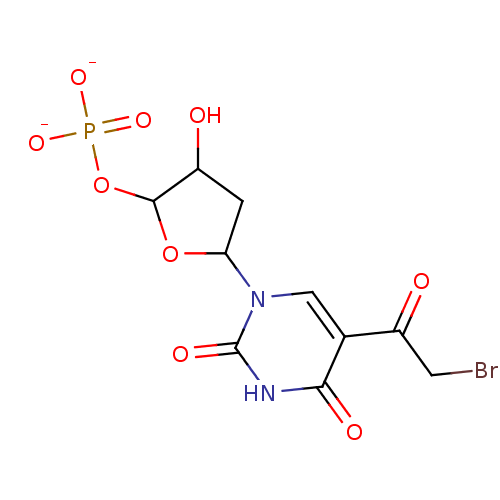 (5-quinonyl-deoxyuridinemonophosphate | CHEMBL8016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inactivation of thymidylate synthetase measured asKi at 6.8 pH 30 degrees centigrade temp | J Med Chem 26: 1028-36 (1983) BindingDB Entry DOI: 10.7270/Q2VD6XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021749 (CHEMBL3144392 | Phosphoric acid mono-{5-[5-(3,6-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50021749 (CHEMBL3144392 | Phosphoric acid mono-{5-[5-(3,6-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against thymidylateSynthase | J Med Chem 29: 1714-20 (1986) BindingDB Entry DOI: 10.7270/Q2N878SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50024666 ((1,4-Dimethoxy-3-methyl-naphthalen-2-yl)-phosphoni...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Binding affinity towards L. casei thymidylate synthase was determined | J Med Chem 30: 1705-6 (1987) BindingDB Entry DOI: 10.7270/Q23F4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021751 (CHEMBL3143106 | Phosphoric acid mono-{5-[5-(1,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50000019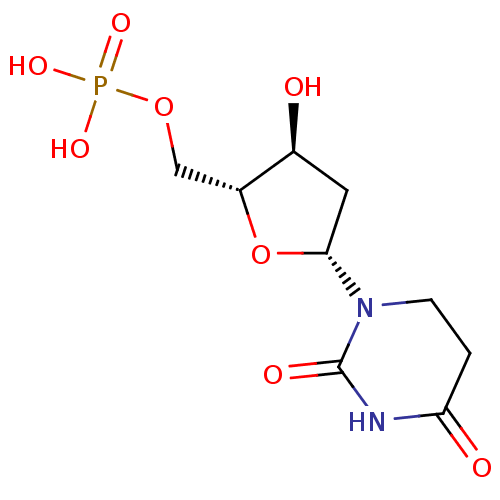 (CHEMBL3144338) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Lactobacillus casei thymidylate synthetase using dTMP as substrate assessed as release of water after 15 mins by double rec... | J Med Chem 22: 319-21 (1979) BindingDB Entry DOI: 10.7270/Q26111TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Bos taurus) | BDBM50010328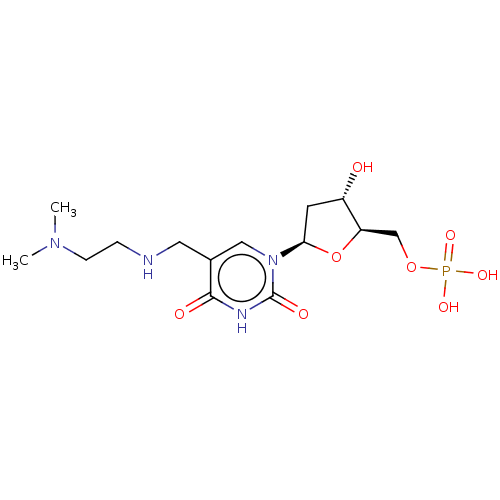 (CHEMBL3248468) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of purified calf thymus thymidylate synthetase using 5-[3H]-2'-deoxyuridine 5'-phosphate as substrate after 7 mins by double r... | J Med Chem 20: 669-73 (1977) BindingDB Entry DOI: 10.7270/Q2542Q4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021752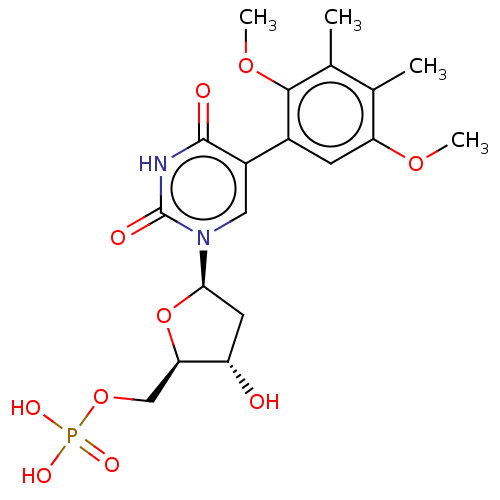 (CHEMBL3143105 | Phosphoric acid mono-{5-[5-(2,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant (Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023635 (4-[1-(4-Hydroxy-5-phosphonooxymethyl-tetrahydro-fu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50404975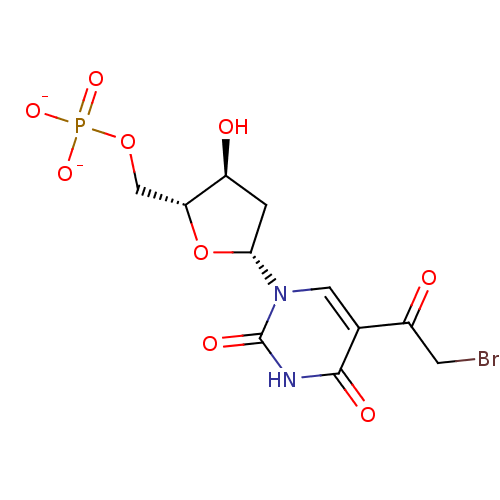 (CHEMBL2051976) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inactivation of thymidylate synthetase measured as Ki at 6.8 pH 30 degrees centigrade temp | J Med Chem 26: 1028-36 (1983) BindingDB Entry DOI: 10.7270/Q2VD6XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023638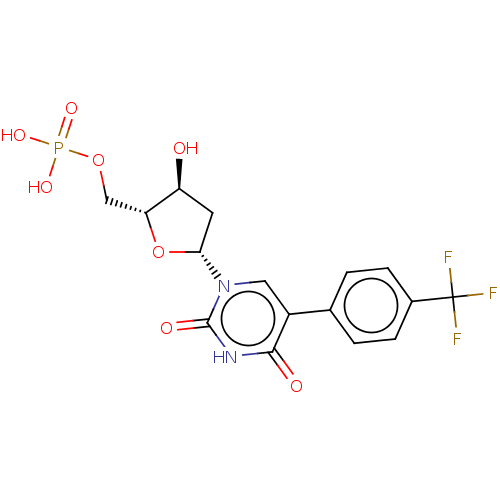 (CHEMBL3144194 | Phosphoric acid mono-{5-[2,4-dioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50021748 (CHEMBL3143138 | Phosphoric acid mono-{5-[5-(4,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from murine leukemia L1210 cells was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50023645 (CHEMBL3144187 | Phosphoric acid mono-[5-(2,4-dioxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023643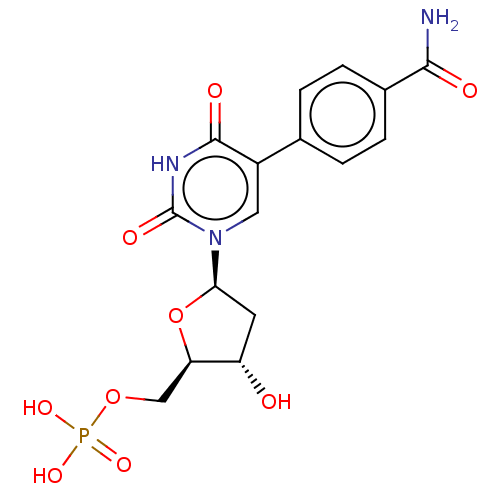 (CHEMBL3144195 | Phosphoric acid mono-{5-[5-(4-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50226709 (CHEMBL3143111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from murine leukemia L1210 cells was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50227388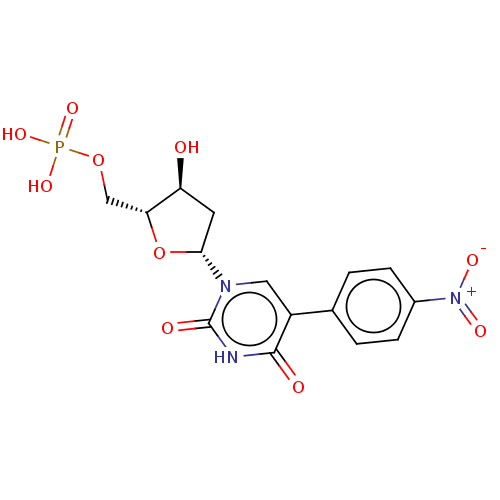 (CHEMBL3144092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50186267 (2'-deoxy-5'-uridylic acid | CHEMBL211312 | dUMP) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of purified mouse Ehrlich ascites tumor cell thymidylate synthetase using 5-[3H]-2'-deoxyuridine 5'-phosphate as substrate aft... | J Med Chem 20: 669-73 (1977) BindingDB Entry DOI: 10.7270/Q2542Q4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50023647 (CHEMBL3144186 | Phosphoric acid mono-[5-(2,4-dioxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023644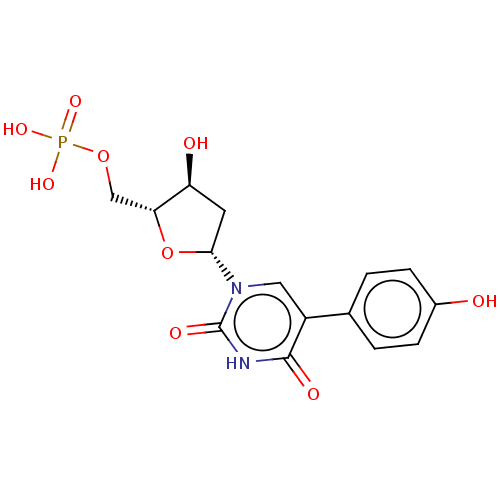 (CHEMBL3144191 | Phosphoric acid mono-{3-hydroxy-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Bos taurus) | BDBM50186267 (2'-deoxy-5'-uridylic acid | CHEMBL211312 | dUMP) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of purified calf thymus thymidylate synthetase using 5-[3H]-2'-deoxyuridine 5'-phosphate as substrate after 7 mins by double r... | J Med Chem 20: 669-73 (1977) BindingDB Entry DOI: 10.7270/Q2542Q4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50023645 (CHEMBL3144187 | Phosphoric acid mono-[5-(2,4-dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on murine leukemia (L1210) thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50023642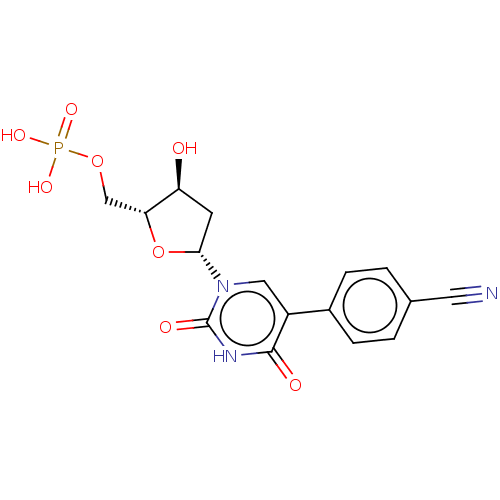 (CHEMBL3144193 | Phosphoric acid mono-{5-[5-(4-cyan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50023636 (CHEMBL3144190 | Phosphoric acid mono-{5-[5-(4-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on murine leukemia (L1210) thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50023637 (CHEMBL3144189 | Phosphoric acid mono-{5-[5-(4-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on murine leukemia (L1210) thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50023647 (CHEMBL3144186 | Phosphoric acid mono-[5-(2,4-dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on murine leukemia (L1210) thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50010328 (CHEMBL3248468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of purified mouse Ehrlich ascites tumor cell thymidylate synthetase using 5-[3H]-2'-deoxyuridine 5'-phosphate as substrate aft... | J Med Chem 20: 669-73 (1977) BindingDB Entry DOI: 10.7270/Q2542Q4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50023637 (CHEMBL3144189 | Phosphoric acid mono-{5-[5-(4-amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50023641 (CHEMBL3144196 | Phosphoric acid mono-{5-[5-(4-brom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase | J Med Chem 31: 1141-7 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 74 total ) | Next | Last >> |